Effect of an Ad Libitum Milk Supply During the First Three Weeks of Life of Dairy Calves on Heart Rate and Heart Rate Variability During Feeding and Rehousing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Calves, Management and Feeding
2.2. Body Weight
2.3. Heart Rate Variability
2.4. Statistical Evaluation
3. Results
3.1. Milk Intake and Body Weight
3.2. HR and HRV Before, During, and After Feeding
3.3. HRV During Rehousing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | ad libitum-fed calves |
| RES | restrictively fed calves |
| HR | heart rate |
| HRV | heart rate variability |
| SDNN | square root of variance of all R-R intervals |
| RMSSD | root mean square of successive interbeat interval differences |
| LF | low-frequency power |
| HF | high-frequency power |
| SD1 | standard deviation of instantaneous HRV measured from axis 1 in Poincaré plot |
References
- Maccari, P.; Wiedemann, S.; Kunz, H.J.; Piechotta, M.; Sanftleben, P.; Kaske, M. Effects of two different rearing protocols for Holstein bull calves in the first 3 weeks of life on health status, metabolism and subsequent performance. J. Anim. Physiol. Anim. Nutr. 2014, 99, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Jasper, J.; Weary, D.M. Effects of Ad Libitum Milk Intake on Dairy Calves. J. Dairy. Sci. 2002, 85, 3054–3058. [Google Scholar] [CrossRef]
- Appleby, M.C.; Weary, D.M.; Chua, B. Performance and feeding behaviour of calves on ad libitum milk from artificial teats. Appl. Anim. Behav. Sci. 2001, 74, 191–201. [Google Scholar] [CrossRef]
- Khan, M.A.; Weary, D.M.; von Keyserlingk, M.A.G. Invited review: Effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. J. Dairy. Sci. 2011, 94, 1071–1081. [Google Scholar] [CrossRef]
- Welk, A.; Otten, N.D.; Jensen, M.B. Invited review: The effect of milk feeding practices on dairy calf behavior, health, and performance-A systematic review. J. Dairy. Sci. 2023, 106, 5853–5879. [Google Scholar] [CrossRef] [PubMed]
- Scoley, G.; Gordon, A.; Morrison, S. Using Non-Invasive Monitoring Technologies to Capture Behavioural, Physiological and Health Responses of Dairy Calves to Different Nutritional Regimes during the First Ten Weeks of Life. Animals 2019, 9, 760. [Google Scholar] [CrossRef]
- Hammell, K.L.; Metz, J.H.M.; Mekking, P. Sucking behaviour of dairy calves fed milk ad libitum by bucket or teat. Appl. Anim. Behav. Sci. 1988, 20, 275–285. [Google Scholar] [CrossRef]
- Buckham-Sporer, K.; Earley, B.; Marti, S. Current Knowledge on the Transportation by Road of Cattle, including Unweaned Calves. Animals 2023, 13, 3393. [Google Scholar] [CrossRef]
- Kovács, L.; Kézér, F.L.; Ruff, F.; Jurkovich, V.; Szenci, O. Heart rate, cardiac vagal tone, respiratory rate, and rectal temperature in dairy calves exposed to heat stress in a continental region. Int. J. Biometeorol. 2018, 62, 1791–1797. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, C.; Zhao, X.; Miao, E.; Wang, Z.; Xu, Y.; Bai, X.; Bao, J. Effect of group size and regrouping on physiological stress and behavior of dairy calves. J. Integr. Agric. 2023, 22, 844–852. [Google Scholar] [CrossRef]
- Silva, F.G.; Conceição, C.; Pereira, A.M.F.; Cerqueira, J.L.; Silva, S.R. Literature Review on Technological Applications to Monitor and Evaluate Calves’ Health and Welfare. Animals 2023, 13, 1148. [Google Scholar] [CrossRef]
- Kovács, L.; Jurkovich, V.; Bakony, M.; Szenci, O.; Póti, P.; Tőzsér, J. Welfare implication of measuring heart rate and heart rate variability in dairy cattle: Literature review and conclusions for future research. Animal 2014, 8, 316–330. [Google Scholar] [CrossRef]
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—a review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Mohr, E.; Langbein, J.; Nürnberg, G. Heart rate variability: A noninvasive approach to measure stress in calves and cows. Physiol. Behav. 2002, 75, 251–259. [Google Scholar] [CrossRef]
- Prokop, L. Effects of an Ad Libitum Milk Supply During Early Life of Calves on Shortterm Stress as Well as Long-Term Development of the Endocrine Pancreas and Assessment of Milk Quality Aspects; Selbstverlag: Kiel, Germany, 2016. [Google Scholar]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Günter, B.; Cerutti, S.; Choen, R. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Hegerová, T.; Juhás, P.; Dóbi, J.; Vavrišínová, K. Evaluation of changes in behavior, heart rate and rectal body temperature in calves during two hours after birth. J. Cent. Eur. Agric. 2022, 23, 261–266. [Google Scholar] [CrossRef]
- Santos, F.; Santarosa, B.P.; Dal Más, F.E.; Silva, K.N.; Guirro, E.; Gomes, V. Clinical physiological parameters of Holstein calves in the first month of life. Anim.-Open Space 2023, 2, 100036. [Google Scholar] [CrossRef]
- von Keyserlingk, M.A.G.; Wolf, F.; Hötzel, M.; Weary, D.M. Effects of Continuous Versus Periodic Milk Availability on Behavior and Performance of Dairy Calves. J. Dairy. Sci. 2006, 89, 2126–2131. [Google Scholar] [CrossRef]
- Rietmann, T.R.; Stuart, A.E.A.; Bernasconi, P.; Stauffacher, M.; Auer, J.A.; Weishaupt, M.A. Assessment of mental stress in warmblood horses: Heart rate variability in comparison to heart rate and selected behavioural parameters. Appl. Anim. Behav. Sci. 2004, 88, 121–136. [Google Scholar] [CrossRef]
- Tahara, Y.; Aoyama, S.; Shibata, S. The mammalian circadian clock and its entrainment by stress and exercise. J. Physiol. Sci. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Ohara, K.; Okita, Y.; Kouda, K.; Mase, T.; Miyawaki, C.; Nakamura, H. Cardiovascular response to short-term fasting in menstrual phases in young women: An observational study. BMC Women’s Health 2015, 15, 67. [Google Scholar] [CrossRef]
- Mazurak, N.; Gunther, A.; Grau, F.S.; Muth, E.R.; Pustovoyt, M.; Bischoff, S.C.; Zipfel, S.; Enck, P. Effects of a 48-h fast on heart rate variability and cortisol levels in healthy female subjects. Eur. J. Clin. Nutr. 2013, 67, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, D.; Lourenço, M.; Bolaños, C.; Takahira, R.K.; Oba, E.; Alfonso, A.; Chiacchio, S.B. Association between heart rate, heart rate variability, cortisol, glucose and electrolytes in healthy newborn calves. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1922–1928. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, X.; Su, H.; Wang, Z.; Wang, C.; Li, J.-H.; Li, X.; Zhang, R.-X.; Bao, J. Effects of group size on the behaviour, heart rate, immunity, and growth of Holstein dairy calves. Appl. Anim. Behav. Sci. 2021, 241, 105378. [Google Scholar] [CrossRef]
- Hofer, M.A. Nutrient control of cardiac rate in the infant rat: Alpha-adrenergic mechanisms. Physiol. Behav. 1986, 36, 557–565. [Google Scholar] [CrossRef]
- Knight, W.D.; Witte, M.M.; Parsons, A.D.; Gierach, M.; Overton, J.M. Long-term caloric restriction reduces metabolic rate and heart rate under cool and thermoneutral conditions in FBNF1 rats. Mech. Ageing Dev. 2011, 132, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Brosh, A.; Aharoni, Y.; Holzer, Z. Energy expenditure estimation from heart rate: Validation by long-term energy balance measurement in cows. Livest. Prod. Sci. 2002, 77, 287–299. [Google Scholar] [CrossRef]
- Savory, C.J.; Kostal, L.; Nevison, I.M. Circadian variation in heart rate, blood pressure, body temperature and EEG of immature broiler breeder chickens in restricted-fed and ad libitum-fed states. Br. Poult. Sci. 2006, 47, 599–606. [Google Scholar] [CrossRef]
- Puchala, R.; Tovar-Luna, I.; Sahlu, T.; Freetly, H.C.; Goetsch, A.L. Technical Note: The relationship between heart rate and energy expenditure in growing crossbred Boer and Spanish wethers. J. Anim. Sci. 2009, 87, 1714–1721. [Google Scholar] [CrossRef]
- Brosh, A. Heart rate measurements as an index of energy expenditure and energy balance in ruminants: A review. J. Anim. Sci. 2007, 85, 1213–1227. [Google Scholar] [CrossRef]
- Hammon, H.M.; Schiessler, G.; Nussbaum, A.; Blum, J.W. Feed intake patterns, growth performance, and metabolic and endocrine traits in calves fed unlimited amounts of colostrum and milk by automate, starting in the neonatal period. J. Dairy. Sci. 2002, 85, 3352–3362. [Google Scholar] [CrossRef] [PubMed]
- Veissier, I.; Boissy, A.; dePassille, A.M.; Rushen, J.; van Reenen, C.G.; Roussel, S.; Andnason, S.; Pradel, P. Calves’ responses to repeated social regrouping and relocation. J. Anim. Sci. 2001, 79, 2580–2593. [Google Scholar] [CrossRef]
- Machado, M.; Silveira, R.M.F.; Bittar, C.M.M.; Lobos, C.M.V.; Da Silva, I.J.O. Can the emotional state of calves be noticed by their facial expression and heart rate? Appl. Anim. Behav. Sci. 2023, 260, 105874. [Google Scholar] [CrossRef]
- Visser, E.K.; van Reenen, C.G.; van der Werf, J.T.N.; Schilder, M.B.H.; Knaap, J.H.; Barneveld, A.; Blokhuis, H.J. Heart rate and heart rate variability during a novel object test and a handling test in young horses. Physiol. Behav. 2002, 76, 289–296. [Google Scholar] [CrossRef]
- Sztajzel, J. Heart rate variability: A noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med. Wkly. 2004, 134, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Camm, A.J. Heart Rate Variability; Futura Publishing Company: Armonk, NY, USA, 1995; ISBN 087993607X. [Google Scholar]
- Houle, M.S.; Billman, G.E. Low-frequency component of the heart rate variability spectrum: A poor marker of sympathetic activity. Am. J. Physiol.-Heart Circ. Physiol. 1999, 276, H215–H223. [Google Scholar] [CrossRef]
- Öri, Z.; Monir, G.; Weiss, J.; Sayhouni, X.; Singer, D.H. Heart rate variability. Frequency domain analysis. Cardiol. Clin. 1992, 10, 499–537. [Google Scholar]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef]
- Friedman, B.H.; Thayer, J.F. Autonomic balance revisited: Panic anxiety and heart rate variability. J. Psychosom. Res. 1998, 44, 133–151. [Google Scholar] [CrossRef]
- Sgoifo, A.; de Boer, S.F.; Westenbroek, C.; Maes, F.W.; Beldhuis, H.; Suzuki, T.; Koolhaas, J.M. Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am. J. Physiol.-Heart Circ. Physiol. 1997, 273, H1754–H1760. [Google Scholar] [CrossRef]
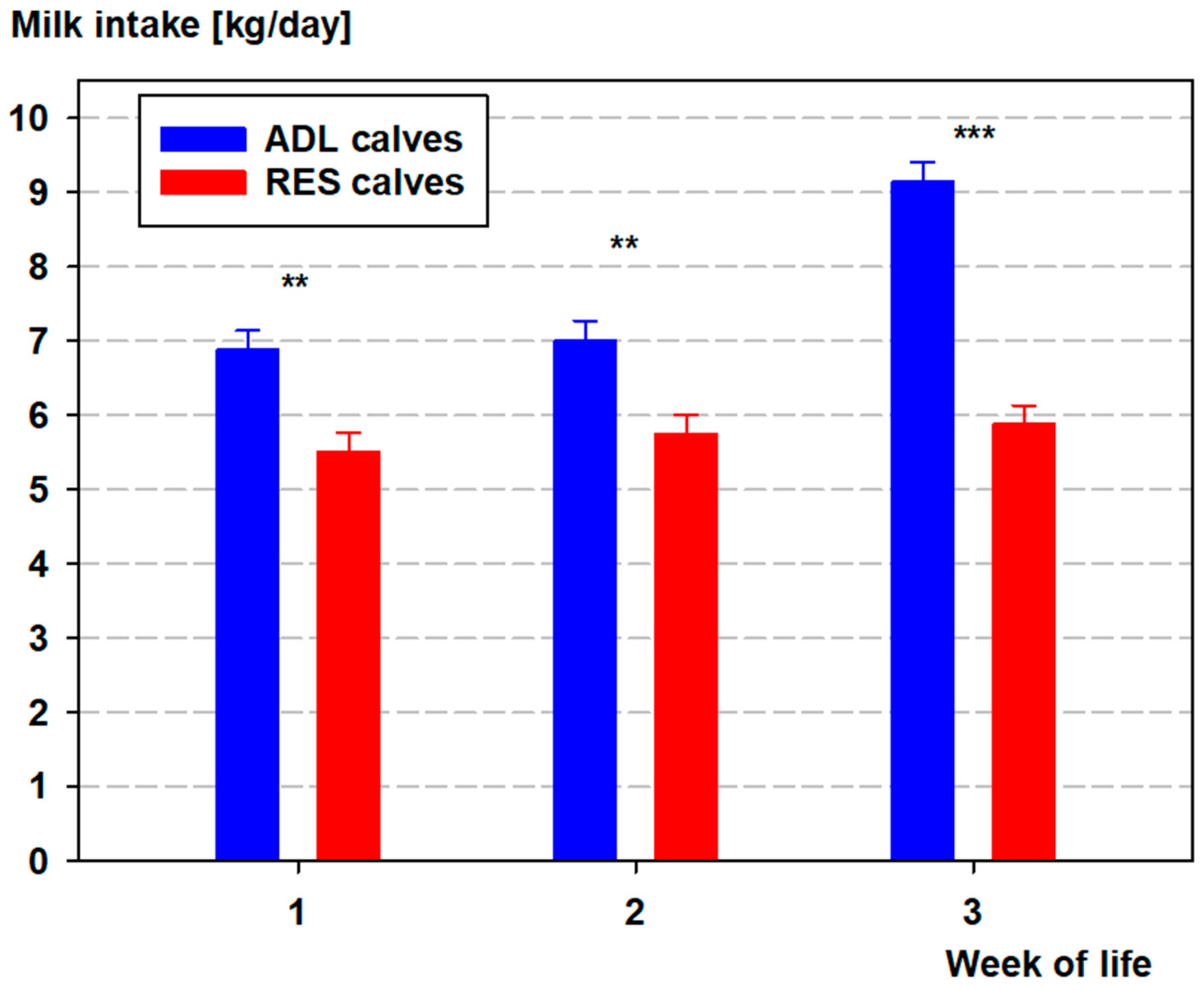
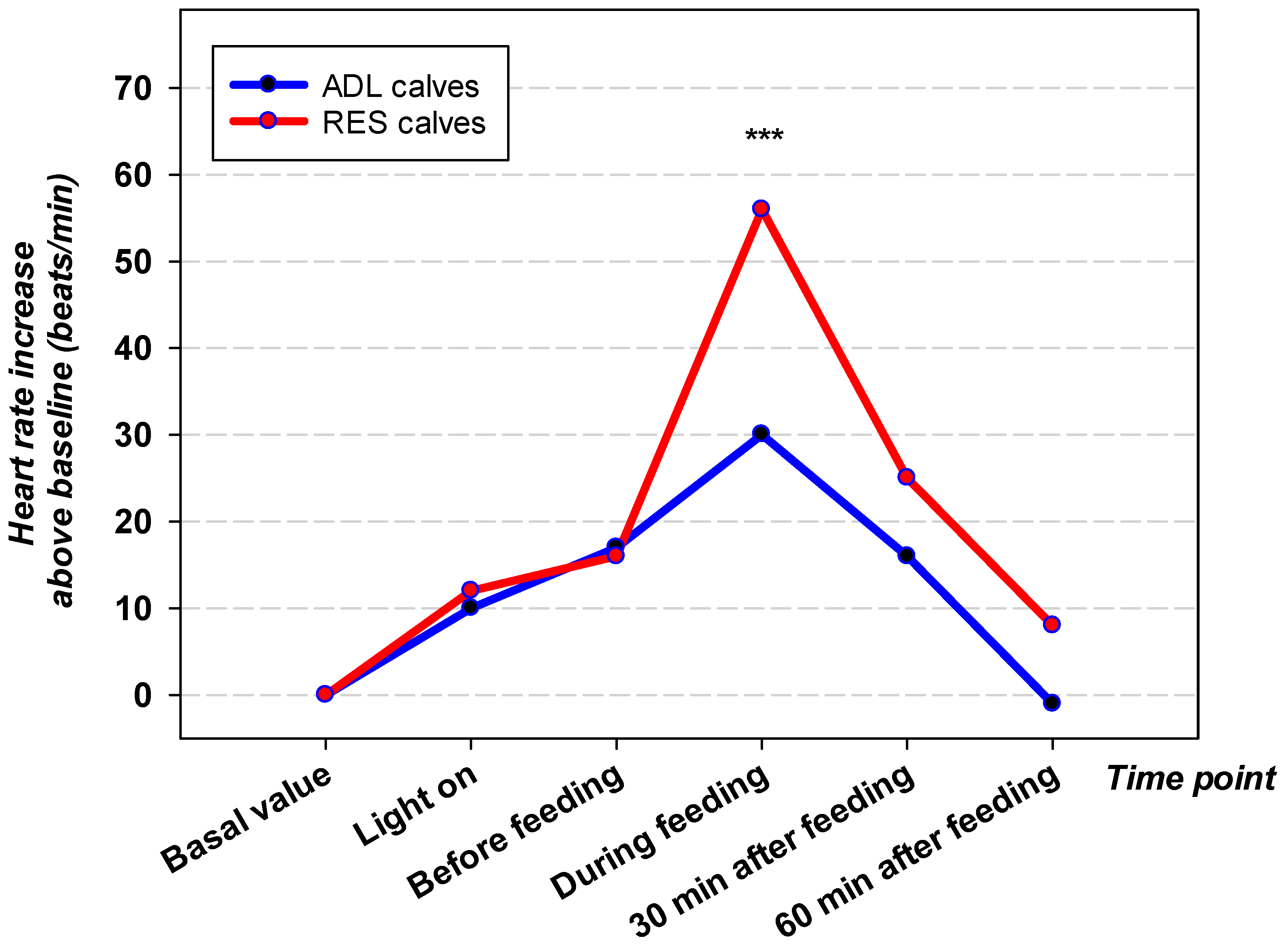
| Parameter | ADL Male | ADL Female | RES Male | RES Female |
|---|---|---|---|---|
| Birth weight, kg | 42.1 ± 1.4 a,b | 43.1 ± 1.3 a,b | 45.5 ± 1.4 b | 39.6 ± 1.3 a |
| Weight on d 23, kg | 57.6 ± 1.8 a,b | 59.8 ± 1.6 a | 54.9 ± 1.7 a,b | 53.2 ± 1.6 b |
| Ø daily weight gain, d 1–23, g/d | 725 ± 83 | 778 ± 76 | 568 ± 81 | 524 ± 77 |
| Parameter | Group | Resting Value | Activity of Personnel | Time Window in Relation to Feed Supply | |||
|---|---|---|---|---|---|---|---|
| 05.00 a.m. | Light on | 15 min Before | During | 15 min After | 1 h After | ||
| HR (bpm) | ADL | 120 b | 131 b | 137 ab | 151 a | 136 ab | 119 b |
| RES | 96 c | 108 bc | 112 bc | 152 a | 121 b | 103 c | |
| SDNN (ms) | ADL | 41.4 | 46.7 | 41.2 | 35.5 | 34.2 | 39.9 |
| RES | 35.3 ab | 41.4 ab | 37.9 ab | 27.4 b | 43.1 ab | 47.9 a | |
| RMSSD (ms) | ADL | 40.2 | 39.4 | 33.0 | 34.4 | 36.4 | 37.7 |
| RES | 41.2 | 37.5 | 38.9 | 35.8 | 39.4 | 39.9 | |
| LFnorm (nu) | ADL | 76.3 ab | 81.9 a | 78.2 ab | 79.4 ab | 69.3 ab | 65.1 b |
| RES | 61.3 b | 76.7 a | 73.6 ab | 77.9 ab | 76.1 ab | 67.9 ab | |
| HFnorm (nu) | ADL | 23.7 ab | 18.1 b | 21.7 ab | 20.6 ab | 30.7 ab | 34.8 a |
| RES | 38.7 a | 23.3 b | 26.4 ab | 23.8 ab | 29.2 ab | 32.0 ab | |
| LF:HF ratio | ADL | 7.7 b | 28.1 a | 5.6 b | 7.4 b | 8.7 b | 2.3 b |
| RES | 2.2 | 7.7 | 4.1 | 6.9 | 5.0 | 5.8 | |
| SD1 (ms) | ADL | 28.5 | 27.9 | 23.3 | 24.4 | 25.8 | 26.6 |
| RES | 29.1 | 26.5 | 27.5 | 25.4 | 27.9 | 28.2 | |
| Parameter | Group | Resting Value | Time Window in Relation to Rehousing | |||
|---|---|---|---|---|---|---|
| 05.00 a.m. | 1 H Before | During | 30 min After | 1 h After | ||
| HR (bpm) | ADL | 122 d | 127 cd | 177 a | 155 b | 143 cb |
| RES | 98 c | 111 c | 171 a | 138 b | 130 b | |
| SDNN (ms) | ADL | 29.3 | 92.6 | 71.1 | 52.5 | 35.1 |
| RES | 30.6 b | 151.0 a | 70.3 ab | 47.9 b | 58.3 ab | |
| RMSSD (ms) | ADL | 31.3 b | 50.7 ab | 59.2a | 36.5 ab | 26.2 b |
| RES | 40.0 abc | 56.6 ab | 57.0a | 28.9 c | 34.6 bc | |
| LFnorm (nu) | ADL | 76.0 | 70.5 | 65.2 | 75.7 | 76.4 |
| RES | 59.9 b | 71.3 ab | 64.1 ab | 76.7 a | 75.2 ab | |
| HFnorm (nu) | ADL | 24.0 | 29.4 | 34.7 | 24.2 | 23.6 |
| RES | 40.1 a | 28.7 ab | 35.8 ab | 23.2 b | 24.8 ab | |
| LF:HF ratio | ADL | 7.0 | 8.0 | 2.9 | 11.2 | 12.8 |
| RES | 1.5 | 15.4 | 2.1 | 11.7 | 17.9 | |
| SD1 (ms) | ADL | 22.1 b | 35.9 ab | 41.9 a | 25.8 ab | 18.5 b |
| RES | 28.3 ab | 40.3 a | 40.3 a | 20.4 b | 24.5 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokop, L.; Hoffmann, G.; Kaske, M.; Wiedemann, S. Effect of an Ad Libitum Milk Supply During the First Three Weeks of Life of Dairy Calves on Heart Rate and Heart Rate Variability During Feeding and Rehousing. Vet. Sci. 2025, 12, 1009. https://doi.org/10.3390/vetsci12101009
Prokop L, Hoffmann G, Kaske M, Wiedemann S. Effect of an Ad Libitum Milk Supply During the First Three Weeks of Life of Dairy Calves on Heart Rate and Heart Rate Variability During Feeding and Rehousing. Veterinary Sciences. 2025; 12(10):1009. https://doi.org/10.3390/vetsci12101009
Chicago/Turabian StyleProkop, Luise, Gundula Hoffmann, Martin Kaske, and Steffi Wiedemann. 2025. "Effect of an Ad Libitum Milk Supply During the First Three Weeks of Life of Dairy Calves on Heart Rate and Heart Rate Variability During Feeding and Rehousing" Veterinary Sciences 12, no. 10: 1009. https://doi.org/10.3390/vetsci12101009
APA StyleProkop, L., Hoffmann, G., Kaske, M., & Wiedemann, S. (2025). Effect of an Ad Libitum Milk Supply During the First Three Weeks of Life of Dairy Calves on Heart Rate and Heart Rate Variability During Feeding and Rehousing. Veterinary Sciences, 12(10), 1009. https://doi.org/10.3390/vetsci12101009







