Precursor A-Kinase Anchor Protein 4 as a Predictive Biomarker of Post-Thaw Semen Quality in Goats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Semen Collection and Initial Evaluation
2.2. Semen Cryopreservation
2.3. Post-Thaw Spermatological Analyses
2.3.1. Evaluation of Post-Thaw Semen Motility and Kinematics
2.3.2. Flow Cytometry Analysis
2.4. Determination of ProAKAP4 Concentration by ELISA
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKAP4 | A-kinase anchor protein 4 |
| proAKAP4 | Precursor a-kinase anchor protein 4 |
| DNA | Deoxyribonucleic acid |
| CASA | Computer-assisted sperm analysis |
| ELISA | Enzyme-linked immunosorbent assay |
| MOT | Total motility |
| PMOT | Progressive motility |
| VAP | Average path velocity |
| VSL | Straight-line velocity |
| VCL | Curvilinear velocity |
| ALH | Amplitude of lateral head displacement |
| STR | Straight-line velocity |
| LIN | Linearity |
| WOB | Wobble |
| CFDA | Carboxyfluorescein diacetate |
| PI | Propidium iodide |
| PNA | Peanut agglutinin |
| DMSO | Dimethyl sulfoxide |
| JC-1 | 1,1’,3,3’-Tetraethyl-5,5’,6,6’-tetrachloroimidacarbocyanine iodide |
| SPSS | Statistical package for the social sciences |
| ANOVA | Analysis of variance |
| HMMP | High mitochondrial membrane potential |
| MMP | Mitochondrial membrane potential |
| PKA | Protein kinase A |
| CAMP | Cyclic adenosine monophosphate |
References
- Nynca, J.; Arnold, G.J.; Fröhlich, T.; Ciereszko, A. Cryopreservation-induced alterations in protein composition of rainbow trout semen. Proteomics 2015, 15, 2643–2654. [Google Scholar] [CrossRef]
- Huang, C.; Tang, Y.; Hu, J.; Zhou, W.; Huang, Z.; Luo, X.; Li, Z.; Zhu, W. Update on techniques for cryopreservation of human spermatozoa. Asian J. Androl. 2022, 24, 563–569. [Google Scholar] [CrossRef]
- Niu, J.; Wang, X.; Liu, P.; Liu, H.; Li, R.; Li, Z.; He, Y.; Qi, J. Effects of cryopreservation on sperm with cryodiluent in viviparous black rockfish (Sebastes schlegelii). Int. J. Mol. Sci. 2022, 23, 3392. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Mansour, H.H.; Mahdy, A.B.; Amer, H.A.; Derbala, M.K.; Abdallah, A.A. Cryopreservation of stallion semen: A Review. Zagazig Vet. J. 2023, 51, 263–278. [Google Scholar] [CrossRef]
- Belala, R.; Bourahmoune, D.; Mimoune, N. The use of computer assisted sperm analysis (CASA) in domestic animal reproduction: A review. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 741–751. [Google Scholar] [CrossRef]
- Daly, J.; Tiersch, T.R. Sources of variation in flow cytometric analysis of aquatic species sperm: The effect of cryoprotectants on flow cytometry scatter plots and subsequent population gating. Aquaculture 2012, 370–371, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Santiani, A.; Juárez, J.F.; Allauca, P.; Román, B.; Ugarelli, A.; Evangelista-Vargas, S. Cryopreservation effect on sperm viability, mitochondrial membrane potential, acrosome integrity and sperm capacitation of alpaca spermatozoa detected by imaging flow cytometry. Reprod. Domest. Anim. 2023, 58, 560–563. [Google Scholar] [CrossRef]
- Caballero, I.; Parrilla, I.; Almiñana, C.; Del Olmo, D.; Roca, J.; Martínez, E.A.; Vázquez, J.M. Seminal plasma proteins as modulators of the sperm function and their application in sperm biotechnologies. Reprod. Domest. Anim. 2012, 47, 12–21. [Google Scholar] [CrossRef]
- Peddinti, D.; Nanduri, B.; Kaya, A.; Feugang, J.M.; Burgess, S.C.; Memili, E. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst. Biol. 2008, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Aitken, R.J. Proteomic insights into spermatozoa: Critiques, comments and concerns. Expert Rev. Proteom. 2009, 6, 691–705. [Google Scholar] [CrossRef]
- Byrne, K.; Leahy, T.; McCulloch, R.; Colgrave, M.L.; Holland, M.K. Comprehensive mapping of the bull sperm surface proteome. Proteomics 2012, 12, 3559–3579. [Google Scholar] [CrossRef]
- Çil, B.; Akçay, E. The importance of seminal plasma proteins in bulls in terms of cryopreservation and fertility. Lalahan Hayv. Araş. Enst. Derg. 2017, 57, 118–126. [Google Scholar]
- D’Amours, O.; Frenette, G.; Bourassa, S.; Calvo, E.; Blondin, P.; Sullivan, R. Proteomic markers of functional sperm population in bovines: Comparison of low- and high-density spermatozoa following cryopreservation. J. Proteome Res. 2018, 17, 177–188. [Google Scholar] [CrossRef]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Arangasamy, A.; Kastelic, J.P. Sperm and seminal plasma proteomics of high-versus low-fertility Holstein bulls. Theriogenology 2019, 126, 41–48. [Google Scholar] [CrossRef]
- Bastan, I.; Akcay, E. Quality assessment of frozen bull semen with the precursor A-kinase anchor protein 4 biomarker. Andrologia 2021, 53, e14164. [Google Scholar] [CrossRef]
- Dordas-Perpinyà, M.; Yánez-Ortiz, I.; Sergeant, N.; Mevel, V.; Catalán, J.; Bruyas, J.F.; Miró, J.; Briand-Amirat, L. ProAKAP4 as indicator of long-lasting motility marker in post-thaw conditions in stallions. Animals 2024, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- Pardede, B.; Setyawan, E.; Said, S.; Kusumawati, A.; Purwantara, B.; Pangestu, M.; Memili, E. A-kinase anchor protein 4 (proAKAP4): Protein molecule–based fertility marker of Indonesian dairy bull and its correlation with frozen-thawed sperm quality. Vet. Med. Int. 2025, 1, 8367714. [Google Scholar] [CrossRef]
- Siena, G.; Fontbonne, A.; Contiero, B.; Maenhoudt, C.; Robiteau, G.; Slimani, S.; Sergeant, N.; Tiret, L.; Milani, C. Stability over time of the sperm motility biomarker proAKAP4 in repeated dog ejaculates. Animals 2025, 15, 1160. [Google Scholar] [CrossRef]
- Riesco, M.F.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Alvarez, M.; de Paz, P.; Anel, L. ProAKAP4 as novel molecular marker of sperm quality in ram: An integrative study in fresh, cooled and cryopreserved sperm. Biomolecules 2020, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Fatet, A.; Sergeant, N.; Dordas-Perpinyà, M.; Drouet, B.; Ponthoreau, O.; Carracedo, S.; Bruyas, J.; Thorin, C.; Delehedde, M.; Briand-Amirat, L. Sperm-specific protein proAKAP4 as a marker to evaluate sperm quality and fertility of Alpine and Saanen bucks. In Reproduction in Domestic Animals: Volume 57, Issue S4, Proceedings of the 25th Annual Conference of the European Society for Domestic Animal Reproduction (ESDAR), Thessaloniki, Greece, 27 September–2 October 2022; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Ungerfeld, R.; Viera, M.N.; Freitas-de-Melo, A.; Giriboni, J.; Casuriaga, D.; Silveira, P. Seasonality of the stress response in goat bucks when there is use of electroejaculation for semen collection. Anim. Reprod. Sci. 2021, 226, 106719. [Google Scholar] [CrossRef] [PubMed]
- Alcay, S.; Aktar, A.; Koca, D.; Kilic, M.A.; Akkasoglu, M.; Sagirkaya, H. Positive effect of autologous platelet rich plasma on Saanen buck semen cryopreservation in non-breeding season. Cryobiology 2021, 103, 45–48. [Google Scholar] [CrossRef]
- Toker, M.; Alcay, S. Comprehensive effects of fetal calf serum in soybean-lecithin based goat semen cryopreservation extenders and impacts on incubation resilience. Kafkas Univ. Vet. Fak. Derg. 2022, 28, 455–460. [Google Scholar] [CrossRef]
- Tekin, K.; Daşkın, A. Effect of different extenders on motility and some sperm kinematics parameters in Norduz goat semen. Turk. J. Vet. Anim. Sci. 2016, 40, 490–495. [Google Scholar] [CrossRef]
- Câmara, D.R.; Silva, S.V.; Almeida, F.C.; Nunes, J.F.; Guerra, M.M.P. Effects of antioxidants and duration of pre-freezing equilibration on frozen-thawed ram semen. Theriogenology 2011, 76, 342–350. [Google Scholar] [CrossRef]
- Marco-Jiménez, F.; Puchades, S.; Gadea, J.; Vicente, J.S.; Viudes-de-Castro, M.P. Effect of semen collection method on pre- and post-thaw Guirra ram spermatozoa. Theriogenology 2005, 64, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Ball, B.A.; Squires, E.L. A new method for evaluating stallion sperm viability and mitochondrial membrane potential in fixed semen samples. Cytom. B Clin. Cytom. 2018, 94, 302–311. [Google Scholar] [CrossRef]
- Dariush, G.; Gholamhossein, R.; Rouhollah, F.; Mahmood, G.S.; Abdolhossein, S.; Mohsen, S.; Loghman, A. The application of ultrasonic vibration in human sperm cryopreservation as a novel method for the modification of physicochemical characteristics of freezing media. Sci. Rep. 2019, 9, 10066. [Google Scholar] [CrossRef]
- Syifa, N.; Yang, J.-T.; Wu, C.-S.; Lin, M.-H.; Wu, W.-L.; Lai, C.-W.; Ku, S.-H.; Liang, S.-Y.; Hung, Y.-C.; Chou, C.-T.; et al. Phosphoproteomics and Bioinformatics Analyses Reveal Key Roles of GSK-3 and AKAP4 in Mouse Sperm Capacitation. Int. J. Mol. Sci. 2020, 21, 7283. [Google Scholar] [CrossRef]
- Jumeau, F.; Sigala, J.; Dossou-Gbete, F.; Frimat, K.; Barbotin, A.; Buée, L.; Béhal, H.; Sergeant, N.; Mitchell, V. A-kinase anchor protein 4 precursor (pro-AKAP4) in human spermatozoa. Andrology 2018, 6, 854–859. [Google Scholar] [CrossRef]
- Dordas-Perpinyà, M.; Sergeant, N.; Ruelle, I.; Bruyas, J.F.; Charreaux, F.; Michaud, S.; Carracedo, S.; Catalán, J.; Miró, J.; Delehedde, M.; et al. ProAKAP4 semen concentrations as a valuable marker protein of post-thawed semen quality and bull fertility: A retrospective study. Vet. Sci. 2022, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Jouy, N.; Mitchell, V.; Franck, T.; Serteyn, D. Expression, localization, and concentration of A-kinase anchor protein 4 (AKAP4) and its precursor (proAKAP4) in equine semen: Promising marker correlated to the total and progressive motility in thawed spermatozoa. Theriogenology 2019, 131, 52–60. [Google Scholar] [CrossRef]
- Dordas-Perpinyà, M.; Yánez-Ortiz, I.; Sergeant, N.; Mevel, V.; Bruyas, J.; Catalán, J.; Delehedde, M.; Briand-Amirat, L.; Miró, J. ProAKAP4 concentration is related to sperm motility and motile sperm subpopulations in frozen–thawed horse semen. Animals 2022, 12, 3417. [Google Scholar] [CrossRef]
- de Almeida, A.B.M.; Hidalgo, M.M.T.; Trautwein, L.G.C.; de Fátima Schnitzer, J.; Silva, L.A.S.; de Moraes, F.L.Z.; Rizzoto, G.; Ferreira, M.I.M. Is the proAKAP4 a suitable biomarker of X-sorted sperm quality from Nelore and Gir bulls? Semin. Cienc. Agrar. 2024, 45, 1413–1422. [Google Scholar] [CrossRef]
- Dcunha, R.; Hussein, R.; Ananda, H.; Kumari, S.; Adiga, S.; Kannan, N.; Zhao, Y.; Kalthur, G. Current insights and latest updates in sperm motility and associated applications in assisted reproduction. Reprod. Sci. 2020, 29, 7–25. [Google Scholar] [CrossRef]
- Ruelle, I.; Sergeant, N.; Bencharif, D.; Charreaux, F.; Thorin, C.; Michaud, S.; Briand-Amirat, L. ProAKAP4 concentrations in semen as a predictive tool of bull fertility: A preliminary study. Reprod. Fertil. Dev. 2020, 32, 199. [Google Scholar] [CrossRef]
- de Almeida, A.B.M.; Hidalgo, M.M.T.; de Moraes, F.L.Z.; Trautwein, L.G.C.; de Fátima Schnitzer, J.; dos Santos Silva, L.A.; Rizzoto, G.; Ferreira, J.C.P.; Martins, M.I.M. The proAKAP4 concentrations in Nelore bull sperm and their relation to FTAI conception rate results. Anim. Reprod. Sci. 2022, 247, 107156. [Google Scholar] [CrossRef]
- Kudratullah; Arifiantini, R.I.; Yuliani, E.; Pardede, B.P.; Said, S.; Purwantara, B. Semen characteristics, freezability, and application of motility-based protein markers (proAKAP4) in assessing the suitability of superior Bali bulls (Bos sondaicus) at the Regional AI Center. Reprod. Breed. 2024, 4, 279–286. [Google Scholar] [CrossRef]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Donnay, I.; Lejeune, J.P.; Franck, T.; Serteyn, D. First results about ProAKAP4 concentration in stallion semen after cryopreservation in two different freezing media. Cryobiology 2021, 102, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Dordas-Perpinyà, M.; Sergeant, N.; Yánez-Ortiz, I.; Mevel, V.; Catalán, J.; Bruyas, J.F.; Briand-Amirat, L.; Miró, J. ProAKAP4 as a motility long-lasting marker in Catalan donkey spermatozoa. Anim. Reprod. Sci. 2024, 262, 107427. [Google Scholar] [CrossRef] [PubMed]
- Bencharif, D.; Belala, R.; Mimoune, N.; Le Couazer, D.; Farnia, H. ProAKAP4 concentration in fresh canine semen and its correlation with motility parameters. Vet. Anim. Sci. 2025, 28, 100455. [Google Scholar] [CrossRef] [PubMed]
- Prochowska, S.; Eberhardt, M.; Niżański, W. Evaluation of a commercial proAKAP4 kit for the assessment of fresh and frozen–thawed feline spermatozoa. Reprod. Domest. Anim. 2024, 59, e14547. [Google Scholar] [CrossRef]
- Battut, I.; Kempfer, A.; Becker, J.S.; Lebailly, L.; Camugli, S.; Chevrier, L. Development of a new fertility prediction model for stallion semen, including flow cytometry. Theriogenology 2016, 86, 1111–1131. [Google Scholar] [CrossRef]
- Farhan, T.M. Correlative study of sperm motility and mitochondrial membrane potential by fluorescent staining: First application in Iraqi centers. Al-Anbar Med. J. 2024, 20, 31–35. [Google Scholar] [CrossRef]
- Giaccagli, M.M.; Gómez-Elías, M.D.; Herzfeld, J.D.; Marín-Briggiler, C.I.; Cuasnicú, P.S.; Cohen, D.J.; Da Ros, V.G. Capacitation-induced mitochondrial activity is required for sperm fertilizing ability in mice by modulating hyperactivation. Front. Cell Dev. Biol. 2021, 9, 767161. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.B.; Gerton, G.L. A-kinase anchor proteins in endocrine systems and reproduction. Trends Endocrinol. Metab. 2001, 12, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kan, F. Human OVGP1 enhances tyrosine phosphorylation of proteins in the fibrous sheath involving AKAP3 and increases sperm–zona binding. J. Assist. Reprod. Genet. 2019, 36, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, X.H.; Wu, J.; Wang, N.; Li, G.; Hao, G.M.; Cao, J.F. Decreased AKAP4/PKA signaling pathway in high DFI sperm affects sperm capacitation. Asian J. Androl. 2024, 26, 25–33. [Google Scholar] [CrossRef] [PubMed]
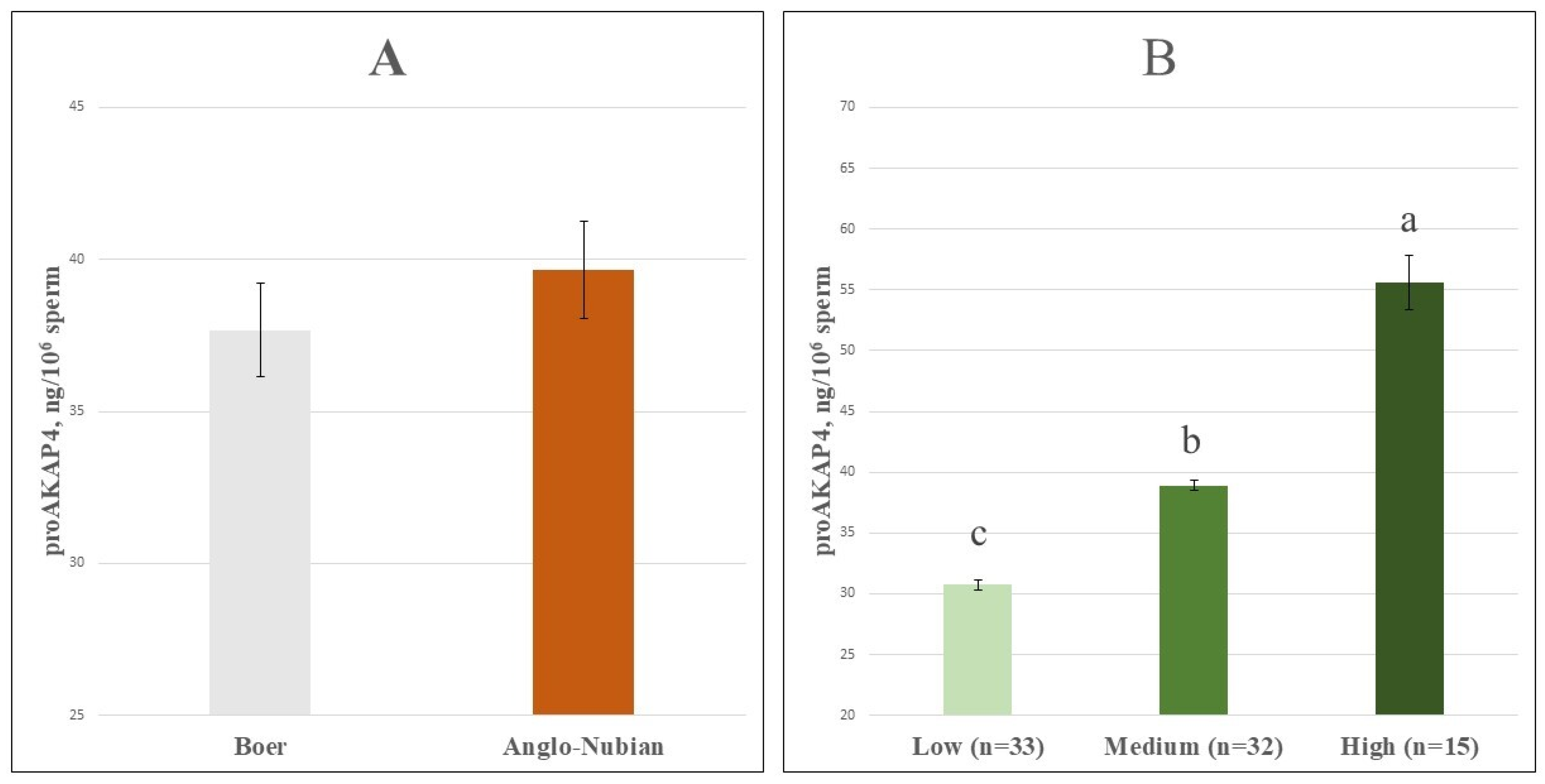
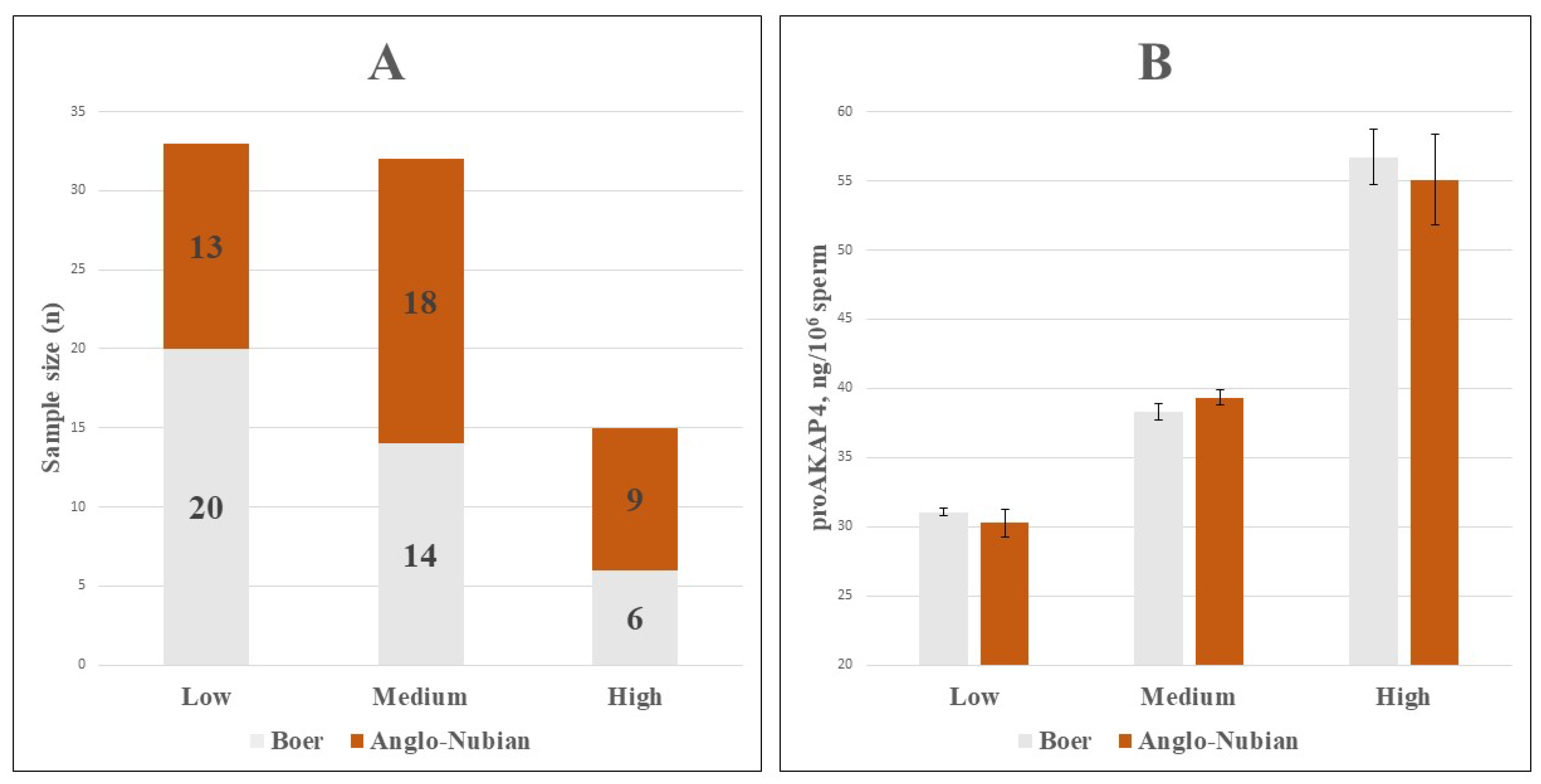

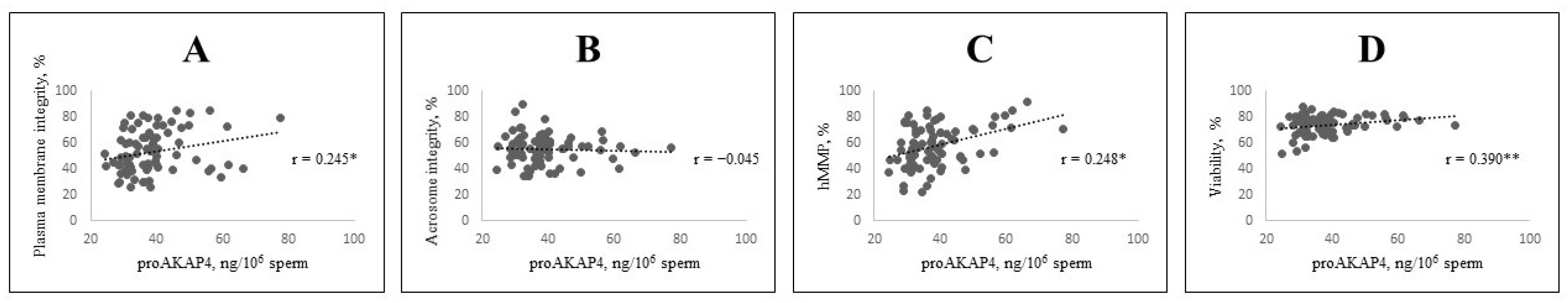
| CASA Parameters | proAKAP4 Groups | ||||
|---|---|---|---|---|---|
| Low (n = 33) | Medium (n = 32) | High (n = 15) | F | p-Value | |
| MOT, % | 53.08 ± 2.44 c | 63.05 ± 2.74 b | 81.86 ± 3.32 a | 20.442 | <0.001 |
| pMOT, % | 17.73 ± 1.33 c | 22.31 ± 1.55 b | 29.14 ± 1.50 a | 11.036 | <0.001 |
| VAP, µm/s | 38.30 ± 1.60 b | 40.86 ± 1.73 b | 52.90 ± 2.05 a | 13.326 | <0.001 |
| VSL, µm/s | 26.97 ± 1.37 b | 30.29 ± 1.43 b | 38.33 ± 1.55 a | 11.282 | <0.001 |
| VCL, µm/s | 70.67 ± 2.94 b | 76.44 ± 3.07 b | 95.49 ± 4.74 a | 10.662 | <0.001 |
| ALH, µm | 1.82 ± 0.06 b | 1.89 ± 0.06 b | 2.28 ± 0.10 a | 7.334 | 0.001 |
| BCF, Hz | 8.43 ± 0.43 c | 9.94 ± 0.46 b | 12.45 ± 0.38 a | 14.441 | <0.001 |
| STR, % | 58.72 ± 1.03 b | 60.98 ± 1.00 ab | 62.83 ± 1.18 a | 3.060 | 0.053 |
| LIN, % | 34.85 ± 1.20 | 36.66 ± 1.06 | 37.38 ± 1.21 | 1.110 | 0.335 |
| WOB, % | 54.00 ± 0.96 | 55.38 ± 0.87 | 54.57 ± 1.38 | 0.563 | 0.572 |
| Flow Cytometry Parameters | proAKAP4 Groups | ||||
|---|---|---|---|---|---|
| Low (n = 33) | Medium (n = 32) | High (n = 15) | F | p-Value | |
| Membrane integrity, % | 48.74 ± 2.50 b | 53.78 ± 2.86 ab | 60.09 ± 4.93 a | 2.656 | 0.077 |
| Acrosome integrity, % | 55.28 ± 2.29 | 54.11 ± 1.78 | 54.72 ± 2.17 | 0.087 | 0.916 |
| Viability, % | 72.41 ± 1.54 b | 74.14 ± 1.05 ab | 77.14 ± 0.94 a | 3.741 | 0.028 |
| hMMP, % | 52.93 ± 2.70 b | 59.11 ± 2.52 ab | 65.47 ± 4.16 a | 2.343 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eser, A.; Alakuş, A.; Bağcı, K.; Cihangiroğlu, A.Ç.; Yağcıoğlu, S.; Arıcı, R.; Demir, K. Precursor A-Kinase Anchor Protein 4 as a Predictive Biomarker of Post-Thaw Semen Quality in Goats. Vet. Sci. 2025, 12, 1003. https://doi.org/10.3390/vetsci12101003
Eser A, Alakuş A, Bağcı K, Cihangiroğlu AÇ, Yağcıoğlu S, Arıcı R, Demir K. Precursor A-Kinase Anchor Protein 4 as a Predictive Biomarker of Post-Thaw Semen Quality in Goats. Veterinary Sciences. 2025; 12(10):1003. https://doi.org/10.3390/vetsci12101003
Chicago/Turabian StyleEser, Ahmet, Abdurrahman Alakuş, Kemal Bağcı, Aslıhan Çakır Cihangiroğlu, Selin Yağcıoğlu, Ramazan Arıcı, and Kamber Demir. 2025. "Precursor A-Kinase Anchor Protein 4 as a Predictive Biomarker of Post-Thaw Semen Quality in Goats" Veterinary Sciences 12, no. 10: 1003. https://doi.org/10.3390/vetsci12101003
APA StyleEser, A., Alakuş, A., Bağcı, K., Cihangiroğlu, A. Ç., Yağcıoğlu, S., Arıcı, R., & Demir, K. (2025). Precursor A-Kinase Anchor Protein 4 as a Predictive Biomarker of Post-Thaw Semen Quality in Goats. Veterinary Sciences, 12(10), 1003. https://doi.org/10.3390/vetsci12101003





