Punicalagin Inhibits African Swine Fever Virus Replication by Targeting Early Viral Stages and Modulating Inflammatory Pathways
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Virus, and Regents
2.2. Cell Viability Assay
2.3. Screening of the Antiviral Activity of the Compound Library
2.4. Anti-ASFV Activity Assay
2.5. Time-of-Addition Assay
2.6. Virucidal Assay
2.7. Virus Entry Assay
2.8. Fluorescence Imaging
2.9. Western Blotting
2.10. qPCR and RT-qPCR
2.11. Statistical Analysis
3. Results
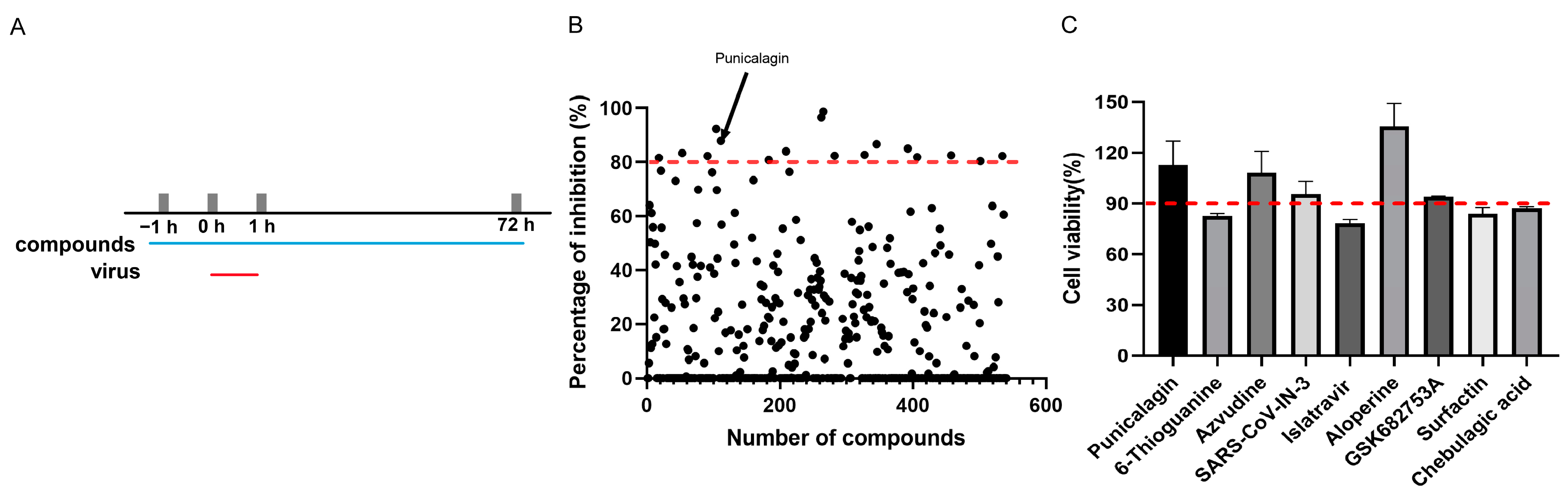
3.1. Screening of Compounds against ASFV
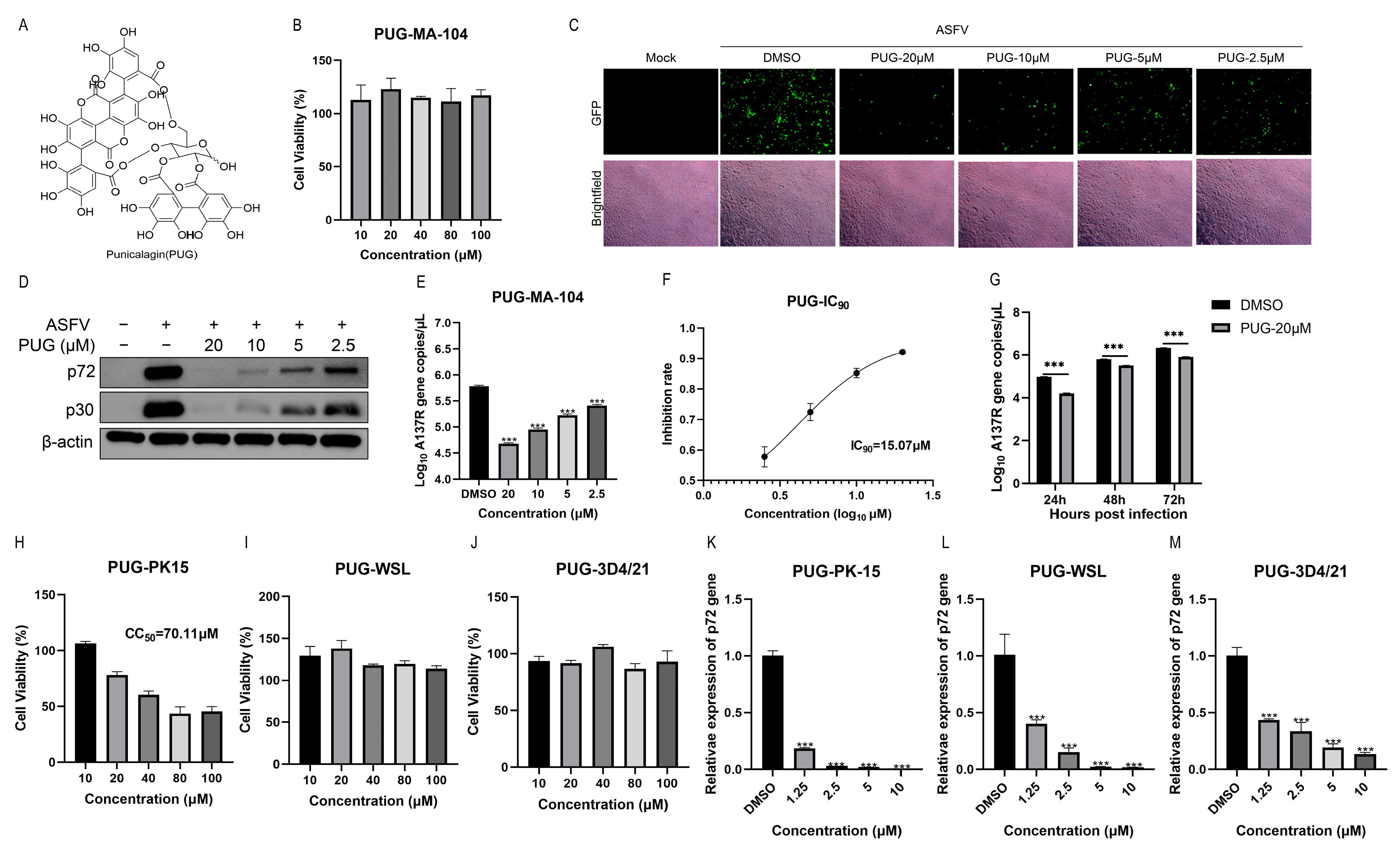
3.2. Punicalagin Could Inhibit ASFV Replication In Vitro
3.3. Punicalagin Affected the Early Stage of ASFV Infection
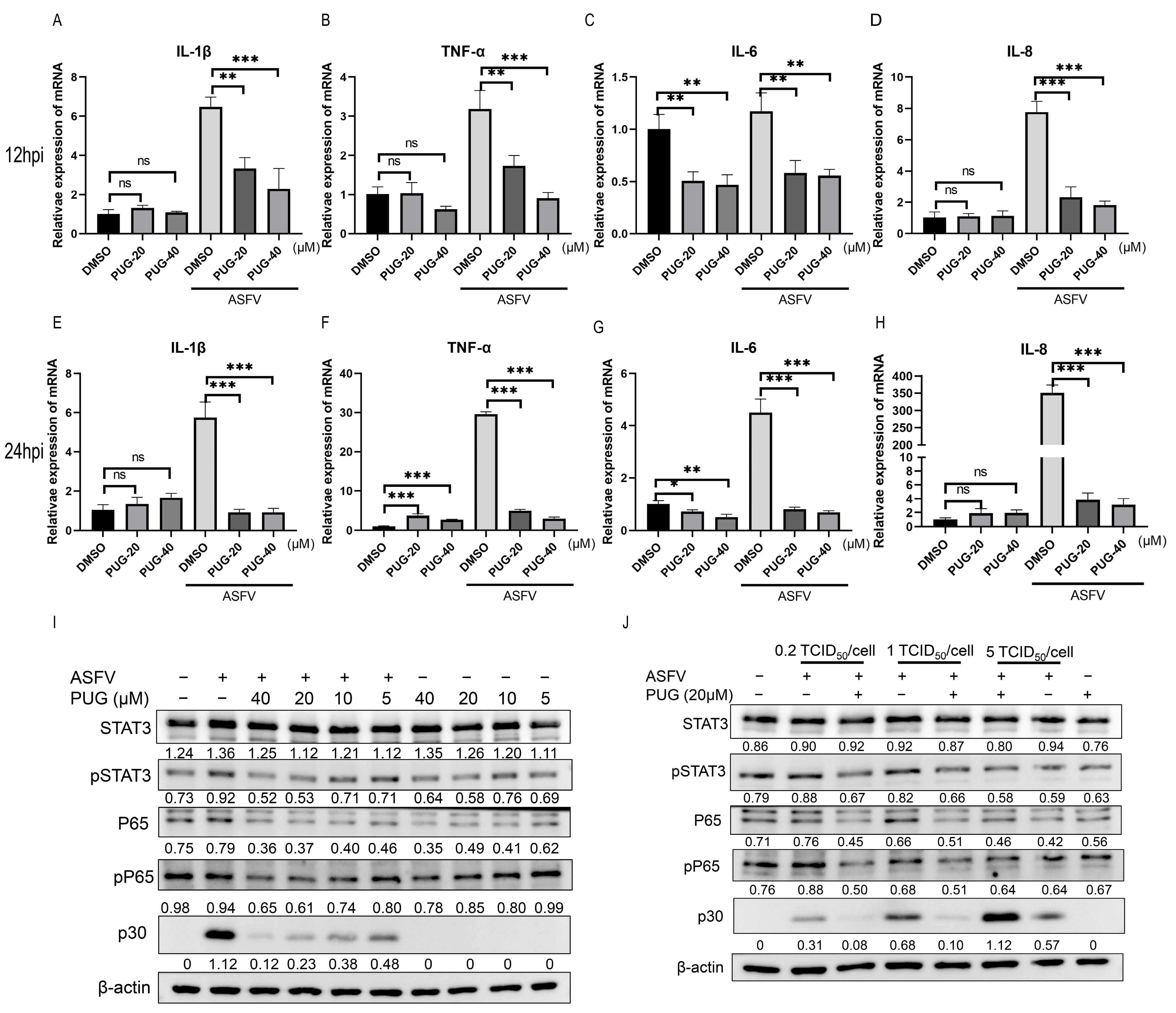
3.4. Punicalaginsuppressed the ASFV-Induced NF-κB/STAT3 Pathway
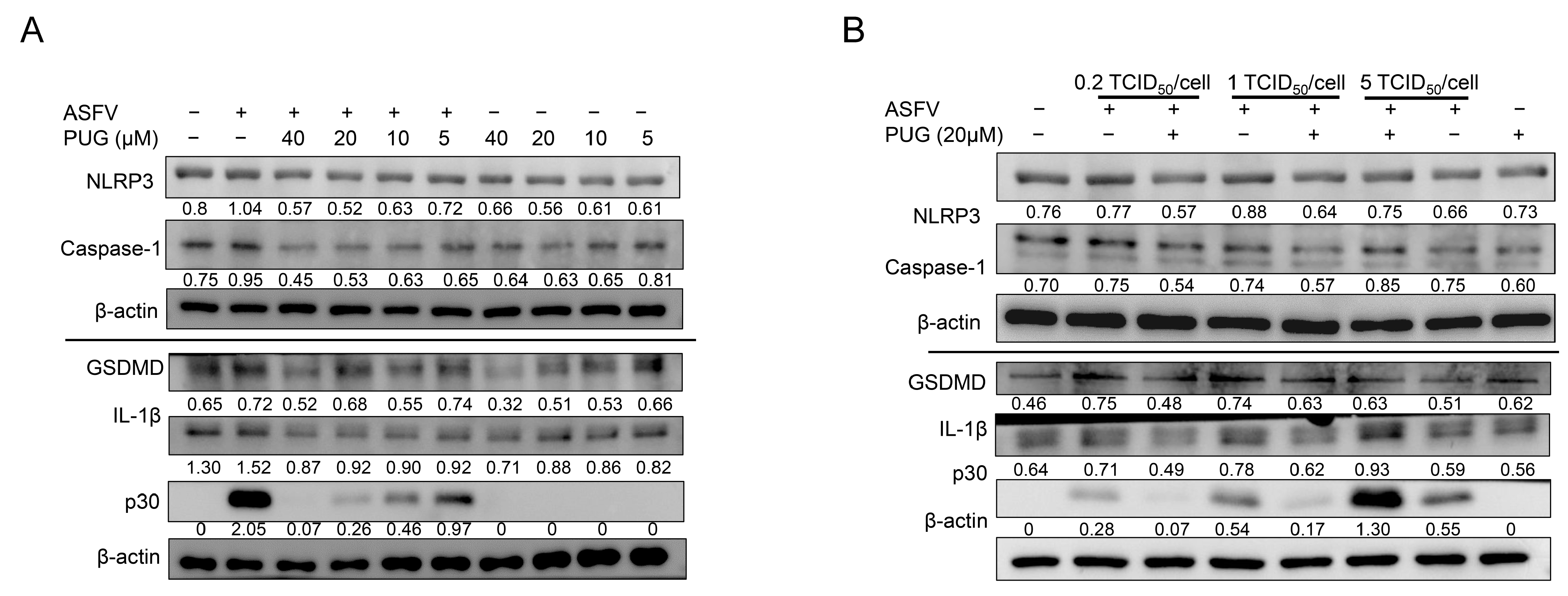
3.5. Punicalagin Inhibited NLRP3 Inflammasome Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, Y.; Qiu, H.-J. African swine fever: An unprecedented disaster and challenge to China. Infect. Dis. Poverty 2018, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, S.; Zhang, H.; Qin, Z.; Shan, H.; Cai, X. Vaccines for African swine fever: An update. Front. Microbiol. 2023, 14, 1139494. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pérez, C.; Jurado, C.; Sánchez-Vizcaíno, J.M. African swine fever vaccine: Turning a dream into reality. Transbound. Emerg. Dis. 2021, 68, 2657–2668. [Google Scholar] [CrossRef]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African swine fever virus vaccine candidate ASFV-G-ΔI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound. Emerg. Dis. 2022, 69, e497–e504. [Google Scholar] [CrossRef]
- Li, T.; Zheng, J.; Huang, T.; Wang, X.; Li, J.; Jin, F.; Wei, W.; Chen, X.; Liu, C.; Bao, M. Identification of several African swine fever virus replication inhibitors by screening of a library of FDA-approved drugs. Virology 2024, 593, 110014. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Liu, Z.; Wang, D.; Liu, H.; Li, L.; Chen, Q.; Yang, D.; Liu, Q.; Guo, H.; et al. Brincidofovir is a robust replication inhibitor against African swine fever virus in vivo and in vitro. Emerg. Microbes Infect. 2023, 12, 2220572. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Z.; Chang, H.; Guo, Y.; Wei, Z.; Sun, Y.; Gong, L.; Zheng, Z.; Zhang, G. Dihydromyricetin inhibits African swine fever virus replication by downregulating toll-like receptor 4-dependent pyroptosis in vitro. Vet. Res. 2023, 54, 58. [Google Scholar] [CrossRef]
- Arabyan, E.; Hakobyan, A.; Hakobyan, T.; Grigoryan, R.; Izmailyan, R.; Avetisyan, A.; Karalyan, Z.; Jackman, J.A.; Ferreira, F.; Elrod, C.C.; et al. Flavonoid Library Screening Reveals Kaempferol as a Potential Antiviral Agent Against African Swine Fever Virus. Front. Microbiol. 2021, 12, 736780. [Google Scholar] [CrossRef]
- Xu, J.; Cao, K.; Liu, X.; Zhao, L.; Feng, Z.; Liu, J. Punicalagin Regulates Signaling Pathways in Inflammation-Associated Chronic Diseases. Antioxidants 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Venusova, E.; Kolesarova, A.; Horky, P.; Slama, P. Physiological and Immune Functions of Punicalagin. Nutrients 2021, 13, 2150. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Chen, T.-Y.; Lin, S.-C.; Chung, C.-Y.; Lin, T.-C.; Wang, G.-H.; Anderson, R.; Lin, C.-C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Chen, T.-Y.; Chung, C.-Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.-C.; Wang, G.-H.; Lin, C.-C.; Richardson, C.D. Hydrolyzable Tannins (Chebulagic Acid and Punicalagin) Target Viral Glycoprotein-Glycosaminoglycan Interactions To Inhibit Herpes Simplex Virus 1 Entry and Cell-to-Cell Spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Cooper, L.; Chen, Z.; Lee, H.; Rong, L.; Cui, Q. Discovery of chebulagic acid and punicalagin as novel allosteric inhibitors of SARS-CoV-2 3CLpro. Antiviral Res. 2021, 190, 105075. [Google Scholar] [CrossRef]
- Yin, D.; Shi, B.; Geng, R.; Liu, Y.; Gong, L.; Shao, H.; Qian, K.; Chen, H.; Qin, A. Function investigation of p11.5 in ASFV infection. Virol. Sin. 2024, 39, 469–477. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Xie, Z.; Ao, Q.; Di, D.; Yu, W.; Lv, L.; Zhong, Q.; Song, Y.; Liao, X.; et al. Development and in vivo evaluation of MGF100-1R deletion mutant in an African swine fever virus Chinese strain. Vet. Microbiol. 2021, 261, 109208. [Google Scholar] [CrossRef]
- Yin, D.; Geng, R.; Shao, H.; Ye, J.; Qian, K.; Chen, H.; Qin, A. Identification of novel linear epitopes in P72 protein of African swine fever virus recognized by monoclonal antibodies. Front. Microbiol. 2022, 13, 1055820. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Yin, D.; Geng, R.; Lv, H.; Bao, C.; Shao, H.; Ye, J.; Qian, K.; Qin, A. Development of Real-Time PCR Based on A137R Gene for the Detection of African Swine Fever Virus. Front. Vet. Sci. 2021, 8, 753967. [Google Scholar] [CrossRef]
- Goulding, L.V.; Kiss, E.; Goatley, L.; Vrancken, R.; Goris, N.E.; Dixon, L. In vitro and in vivo antiviral activity of nucleoside analogue cHPMPC against African swine fever virus replication. Antiviral Res. 2022, 208, 105433. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ma, X.; Wubshet, A.K.; Li, Q.; Shang, X.; Luo, Z.; Liu, J.; Li, Z.; Li, M.; Song, Y. The Accumulation of Phenyllactic Acid Impairs Host Glutamine Metabolism and Inhibits African Swine Fever Virus Replication: A Novel Target for the Development of Anti-ASFV Drugs. Viruses 2024, 16, 449. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, A.; Valdeira, M. Effect of chloroquine on African swine fever virus infection. J. Gen. Virol. 1985, 66, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, R.; Arabyan, E.; Izmailyan, R.; Karalyan, Z.; Jordão, N.; Ferreira, F.; Zakaryan, H. Antiviral activity of brequinar against African swine fever virus infection in vitro. Virus Res. 2022, 317, 198826. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, Y.; Wang, W.; Gao, Q.; Gong, T.; Feng, Y.; Wu, D.; Zheng, X.; Zhang, G.; Wang, H. Aloe-emodin inhibits African swine fever virus replication by promoting apoptosis via regulating NF-κB signaling pathway. Virol. J. 2023, 20, 158. [Google Scholar] [CrossRef]
- Feng, X.; Yang, Q.; Wang, C.; Tong, W.; Xu, W. Punicalagin Exerts Protective Effects against Ankylosing Spondylitis by Regulating NF-κB-TH17/JAK2/STAT3 Signaling and Oxidative Stress. BioMed. Res. Int. 2020, 2020, 4918239. [Google Scholar] [CrossRef]
- Huang, M.; Wu, K.; Zeng, S.; Liu, W.; Cui, T.; Chen, Z.; Lin, L.; Chen, D.; Ouyang, H. Punicalagin Inhibited Inflammation and Migration of Fibroblast-Like Synoviocytes Through NF-κB Pathway in the Experimental Study of Rheumatoid Arthritis. J. Inflamm. Res. 2021, 14, 1901–1913. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Kang, L.; Huang, L.; Zhou, S.; Hu, L.; Zheng, J.; Li, C.; Zhang, X.; He, X. pMGF505-7R determines pathogenicity of African swine fever virus infection by inhibiting IL-1β and type I IFN production. PLoS Pathog. 2021, 17, e1009733. [Google Scholar] [CrossRef]
- Li, S.; Song, J.; Zhou, S.; Zhao, G.; Li, T.; Huang, L.; Li, J.; Weng, C. African swine fever virus infection regulates pyroptosis by cleaving gasdermin A (GSDMA) via active caspase-3 and caspase-4. J. Biol. Chem. 2024, 300, 107307. [Google Scholar] [CrossRef]
- Rai, A.; Pruitt, S.; Ramirez-Medina, E.; Vuono, E.A.; Silva, E.; Velazquez-Salinas, L.; Carrillo, C.; Borca, M.V.; Gladue, D.P. Detection and Quantification of African Swine Fever Virus in MA-104 Cells. Bio Protoc. 2021, 11, e3955. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Li, T.; Liu, C.; Bao, M.; Wang, X.; Li, X.; Li, J.; Huang, L.; Zhang, Z.; et al. Coreceptor AXL Facilitates African Swine Fever Virus Entry via Apoptotic Mimicry. J. Virol. 2023, 97, e0061623. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Pruitt, S.; Ramirez-Medina, E.; Vuono, E.A.; Silva, E.; Velazquez-Salinas, L.; Carrillo, C.; Borca, M.V.; Gladue, D.P. Identification of a Continuously Stable and Commercially Available Cell Line for the Identification of Infectious African Swine Fever Virus in Clinical Samples. Viruses 2020, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Shao, H.; Qian, K.; Chen, H.; Qin, A. Aloperine Inhibits ASFV via Regulating PRLR/JAK2 Signaling Pathway In Vitro. Int. J. Mol. Sci. 2024, 25, 9083. [Google Scholar] [CrossRef] [PubMed]
- WuDunn, D.; Spear, P.G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 1989, 63, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef]
- García-Villalón, D.; Gil-Fernández, C. Antiviral activity of sulfated polysaccharides against African swine fever virus. Antiviral Res. 1991, 15, 139–148. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Zhang, Y.; Yang, J.; Wang, L.; Qi, Y.; Han, X.; Zhou, X.; Miao, F.; Chen, T.; et al. Cytokine Storm in Domestic Pigs Induced by Infection of Virulent African Swine Fever Virus. Front. Vet. Sci. 2021, 7, 601641. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Y.; Feng, Y.; Quan, W.; Luo, Y.; Wang, H.; Zheng, J.; Chen, X.; Huang, Z.; Chen, X.; et al. Effects of the NF-κB Signaling Pathway Inhibitor BAY11-7082 in the Replication of ASFV. Viruses 2022, 14, 297. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Y.; Luo, Y.; Chen, X.; Gong, T.; Wu, D.; Feng, Y.; Zheng, X.; Wang, H.; Zhang, G.; et al. African Swine Fever Virus Envelope Glycoprotein CD2v Interacts with Host CSF2RA to Regulate the JAK2-STAT3 Pathway and Inhibit Apoptosis to Facilitate Virus Replication. J. Virol. 2023, 97, e0188922. [Google Scholar] [CrossRef]
- Martin-Sanchez, F.; Diamond, C.; Zeitler, M.; Gomez, A.I.; Baroja-Mazo, A.; Bagnall, J.; Spiller, D.; White, M.; Daniels, M.J.; Mortellaro, A.; et al. Inflammasome-dependent IL-1beta release depends upon membrane permeabilisation. Cell Death Differ. 2016, 23, 1219–1231. [Google Scholar] [CrossRef]
- Jackman, J.A.; Hakobyan, A.; Grigoryan, R.; Izmailyan, R.; Elrod, C.C.; Zakaryan, H. Antiviral screening of natural, anti-inflammatory compound library against African swine fever virus. Virol. J. 2024, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guo, Z.; Zhou, B. Neuroprotective Potential of Punicalagin, a Natural Component of Pomegranate Polyphenols: A Review. J. Integr. Neurosci. 2023, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation strategies to improve oral bioavailability of ellagic acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
- Siddiqui, N.; Saifi, A.; Chaudhary, A.; Tripathi, P.N.; Chaudhary, A.; Sharma, A. Multifaceted Neuroprotective Role of Punicalagin: A Review. Neurochem. Res. 2023, 49, 1427–1436. [Google Scholar] [CrossRef]

| Target | Sequence (5′–3′) |
|---|---|
| ASFV-A137R-F | GGACATCGAGTGGTATTAAAAGG |
| ASFV-A137R-R | TGGCCTGAAAGTCAACATTGA |
| β-actin (monkey)-F | TCGATCATGAAGTGCGACGTG |
| β-actin (monkey)-R | GTGATCTCCTTCTGCATCCTGTC |
| IL-1β (monkey)-F | TAGACCTCTGCCCTCTGGAT |
| IL-1β (monkey)-R | CTCCATGGCTACAACAACCG |
| TNF-α (monkey)-F | CTGCACTTTGGAGTGATCGG |
| TNF-α (monkey)-R | GCTACAGGCTTGTCACTTGG |
| IL-6 (monkey)-F | GGAACGAAAGAGAAGCTCTA |
| IL-6 (monkey)-R | CTTGTGGAGACGGAGTTCA |
| IL-8 (monkey)-F | AGCTCTGTGTGAAGGTGCAG |
| IL-8 (monkey)-R | CAGAGCTCTCTTCCATCAGAAA |
| GAPDH (pig)-F | CAAGGCTGTGGGCAAGGTCATC |
| GAPDH (pig)-R | CACGAGGAAGCAAGCAGAGTCAG |
| ASFV-p72-F | CTGCTCATGGTATCAATCTTATCGA |
| ASFV-p72-R | GATACCACAAGATCAGCCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, R.; Yin, D.; Liu, Y.; Lv, H.; Zhou, X.; Bao, C.; Gong, L.; Shao, H.; Qian, K.; Chen, H.; et al. Punicalagin Inhibits African Swine Fever Virus Replication by Targeting Early Viral Stages and Modulating Inflammatory Pathways. Vet. Sci. 2024, 11, 440. https://doi.org/10.3390/vetsci11090440
Geng R, Yin D, Liu Y, Lv H, Zhou X, Bao C, Gong L, Shao H, Qian K, Chen H, et al. Punicalagin Inhibits African Swine Fever Virus Replication by Targeting Early Viral Stages and Modulating Inflammatory Pathways. Veterinary Sciences. 2024; 11(9):440. https://doi.org/10.3390/vetsci11090440
Chicago/Turabian StyleGeng, Renhao, Dan Yin, Yingnan Liu, Hui Lv, Xiaoyu Zhou, Chunhui Bao, Lang Gong, Hongxia Shao, Kun Qian, Hongjun Chen, and et al. 2024. "Punicalagin Inhibits African Swine Fever Virus Replication by Targeting Early Viral Stages and Modulating Inflammatory Pathways" Veterinary Sciences 11, no. 9: 440. https://doi.org/10.3390/vetsci11090440
APA StyleGeng, R., Yin, D., Liu, Y., Lv, H., Zhou, X., Bao, C., Gong, L., Shao, H., Qian, K., Chen, H., & Qin, A. (2024). Punicalagin Inhibits African Swine Fever Virus Replication by Targeting Early Viral Stages and Modulating Inflammatory Pathways. Veterinary Sciences, 11(9), 440. https://doi.org/10.3390/vetsci11090440






