The Effect of Renaltec on Serum Uremic Toxins in Cats with Experimentally Induced Chronic Kidney Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cats
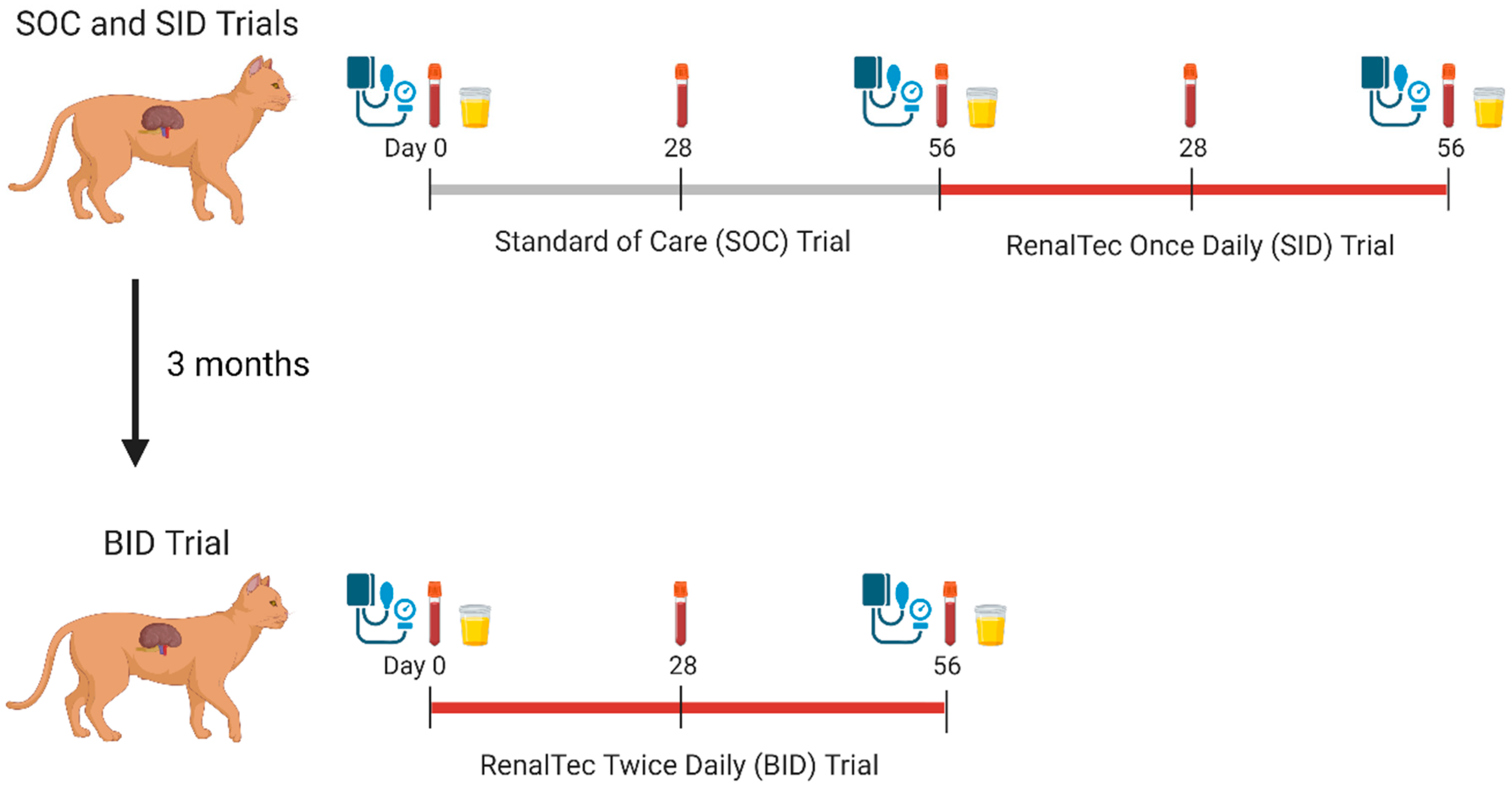
2.2. Study Design
2.3. SOC and SID Trials
2.4. BID Trial
2.5. Sample Collection and Processing
2.6. Statistical Analysis
3. Results
3.1. Weight, Body Condition Score, Muscle Condition Score
3.2. Clinicopathologic Parameters
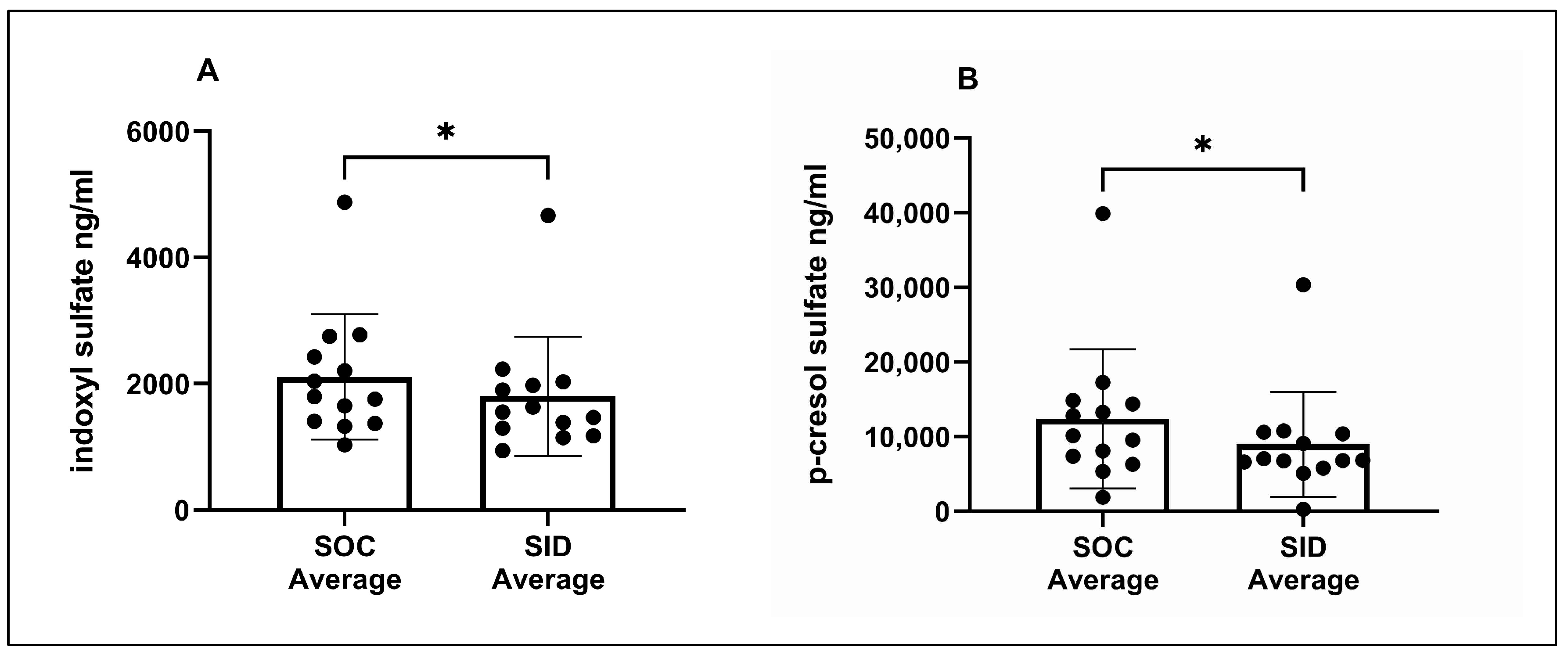
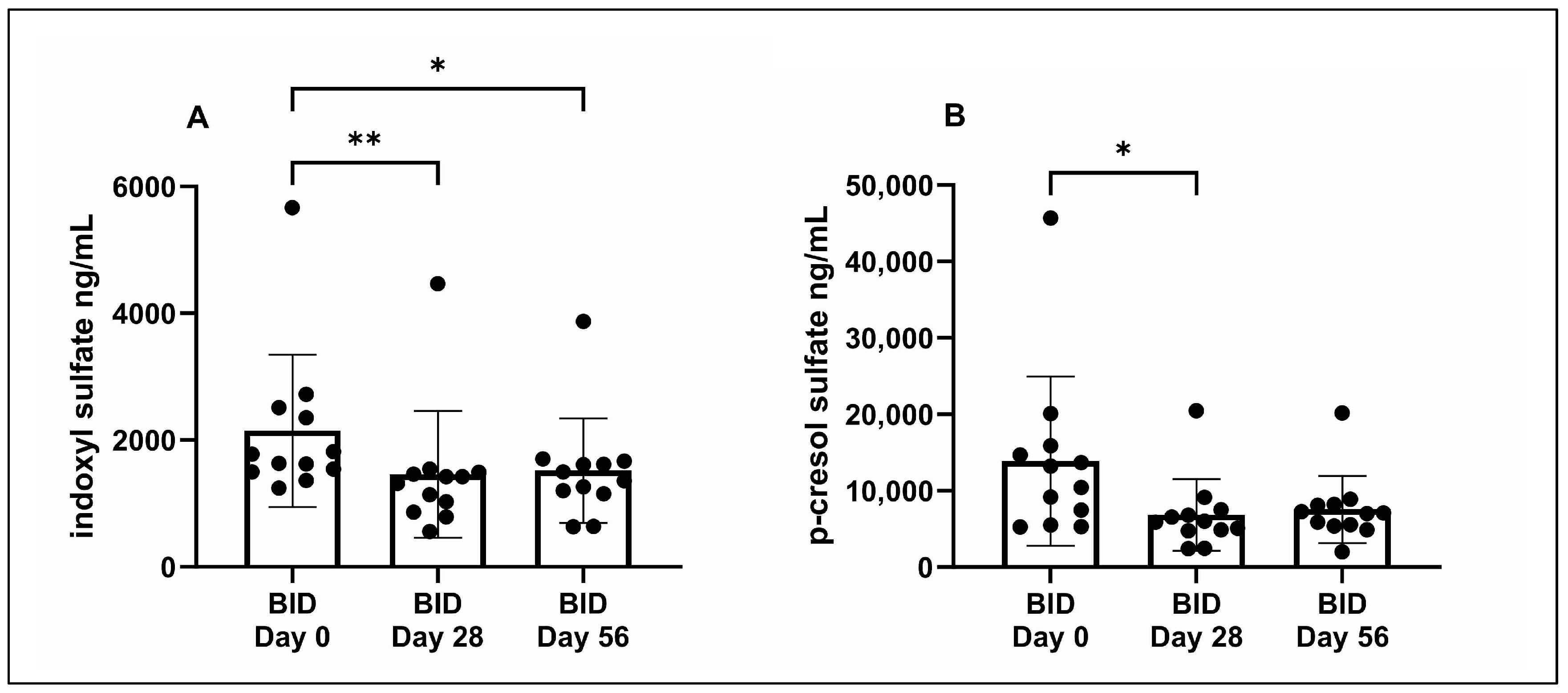
3.3. Serum IDS and pCS Concentrations
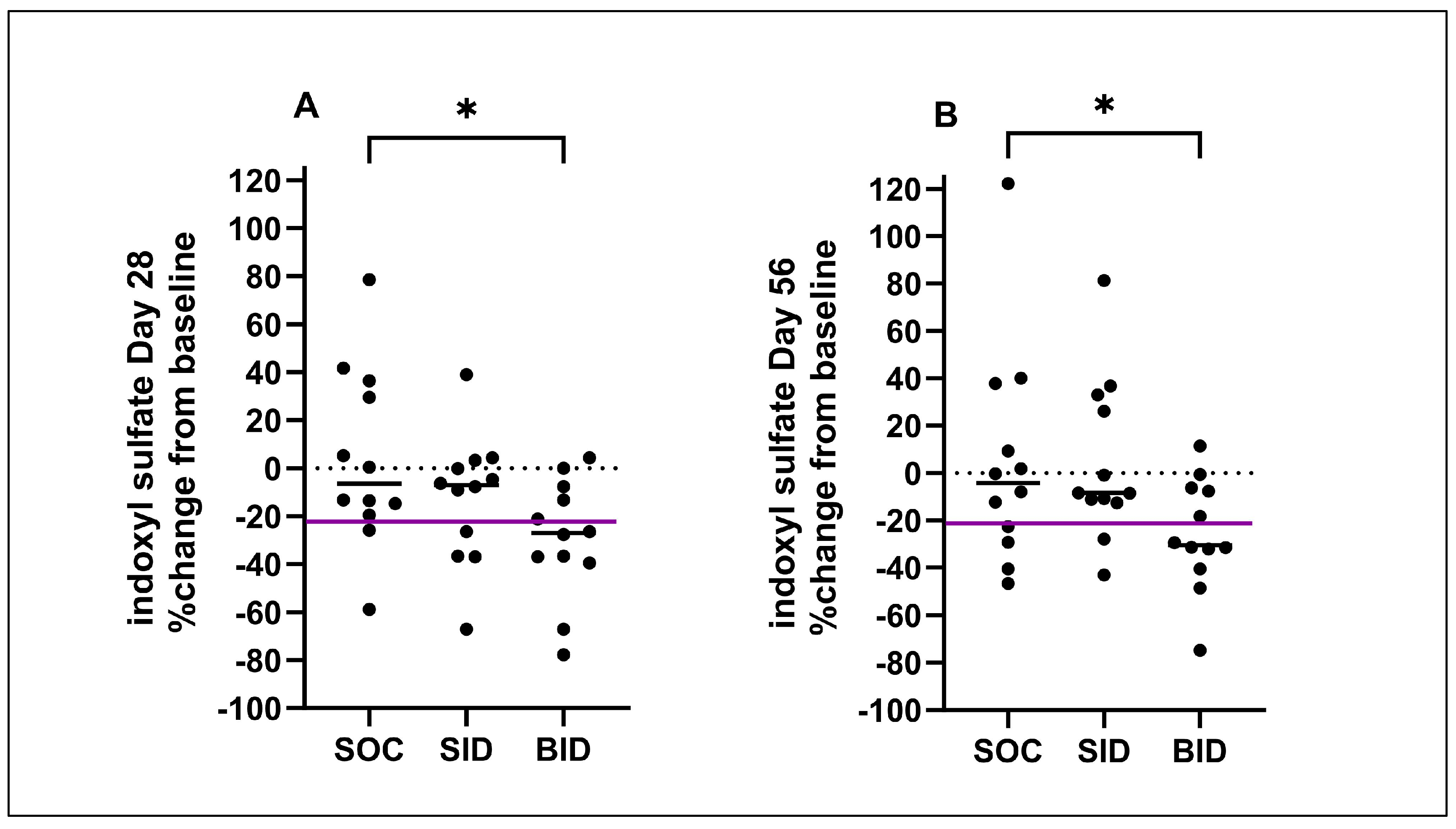
3.4. Percent Change in Uremic Toxins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulman, G.; Vanholder, R.; Niwa, T. AST-120 for the management of progression of chronic kidney disease. Int. J. Nephrol. Renov. Dis. 2014, 7, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.C.; Quimby, J.M.; Isaiah, A.; Suchodolski, J.S.; Lunghofer, P.J.; Gustafson, D.L. The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease. J. Vet. Intern. Med. 2019, 33, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.P.; Hsieh, M.J.; Chou, C.C.; Hsu, W.L.; Lee, Y.J. Detection of indoxyl sulfate levels in dogs and cats suffering from naturally occurring kidney diseases. Vet. J. 2015, 205, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Chou, C.C.; Tsai, P.S.J.; Lee, Y.J. Plasma indoxyl sulfate concentration predicts progression of chronic kidney disease in dogs and cats. Vet. J. 2018, 232, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Villain, C.; Massy, Z.A. Protein-bound toxins: Has the Cinderella of uraemic toxins turned into a princess? Clin. Sci. 2016, 130, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018, 132, 509–522. [Google Scholar] [CrossRef]
- Niwa, T.; Shimizu, H. Indoxyl sulfate induces nephrovascular senescence. J. Ren. Nutr. 2012, 22, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Tomino, Y.; Lu, K.C. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The gut as a source of inflammation in chronic kidney disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef]
- Asai, M.; Kumakura, S.; Kikuchi, M. Review of the efficacy of AST-120 (KREMEZIN((R))) on renal function in chronic kidney disease patients. Ren. Fail. 2019, 41, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Su, P.Y.; Lee, Y.H.; Kuo, L.N.; Chen, Y.C.; Chen, C.; Kang, Y.N.; Chang, E.H. Efficacy of AST-120 for Patients with Chronic Kidney Disease: A Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 676345. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.H.; Krebber, R.; Murphy, M.; Van der Staay, F.J. AST-120 attenuates serum levels of the uremic toxin, indoxyl sulfate, in cats with decreased renal mass. J. Vet. Intern. Med. 2012, 26, 800. [Google Scholar]
- C230186-Porus One Veterinary Detailer v3-2. 2023. Available online: https://www.dechra-us.com/our-products/us/companion-animal/cat/non-prescription/porus-one (accessed on 15 December 2022).
- Mottet, J.; Kowollik, N. Renaltec attenuates serum levels of indoxyl sulfate in geriatic cats. In Proceedings of the BSAVA Congress, Birmingham, UK, 4–6 April 2019. [Google Scholar]
- Elzenbeck, I.; Teichmann-Knorrn, S.; Hartmann, K.; Zablotski, Y.; Suchodolski, J.; Dorsch, R. Evaluierung des Effekts von Renaltec bei Katzen mit chronischer Nierenerkrankung im IRIS-Stage 2 und 3. In Proceedings of the Inn Lab-Goettingen, Hannover, Germany, 13–15 June 2024. [Google Scholar]
- Schmiedt, C.W.; Beita, K.G.; Lourenco, B.N.; Erickson, M.C.M.; Herrera, C.T.; Rissi, D.R.; Brown, C.A.; Brown, S.A. A remnant kidney surgery model in cats induces acute and chronic outcomes that mimic spontaneous chronic kidney disease. Am. J. Vet. Res. 2023, 84, 1–11. [Google Scholar] [CrossRef]
- International Renal Interest Society. Available online: http://iris-kidney.com/education/education/index.html (accessed on 26 May 2024).
- Summers, S.; Quimby, J.; Yao, L.; Hess, A.; Broeckling, C.; Lappin, M. Biological variation of major gut-derived uremic toxins in the serum of healthy adult cats. J. Vet. Intern. Med. 2021, 35, 902–911. [Google Scholar] [CrossRef]
- Sato, E.; Mori, T.; Mishima, E.; Suzuki, A.; Sugawara, S.; Kurasawa, N.; Saigusa, D.; Miura, D.; Morikawa-Ichinose, T.; Saito, R.; et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci. Rep. 2016, 6, 36618. [Google Scholar] [CrossRef]
- Velenosi, T.J.; Hennop, A.; Feere, D.A.; Tieu, A.; Kucey, A.S.; Kyriacou, P.; McCuaig, L.E.; Nevison, S.E.; Kerr, M.A.; Urquhart, B.L. Untargeted plasma and tissue metabolomics in rats with chronic kidney disease given AST-120. Sci. Rep. 2016, 6, 22526. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Lachaud, M.P.; Matthews, S.; Rhodes, L.; Zollers, B. Evaluation of weight loss over time in cats with chronic kidney disease. J. Vet. Intern. Med. 2016, 30, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L. Metabolic Acidosis in CKD: Pathogenesis, Adverse Effects, and Treatment Effects. Int. J. Mol. Sci. 2024, 25, 5187. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.E.; Castro, R.A.; Griffin, F.C.; Mandese, W.W.; Rooks, J.K.; Stone, A.E.; Wuerz, J.A.; Cooke, K.L.; Specht, A.J.; Harris, A.N. Development of a reference interval for urinary ammonia-to-creatinine ratio in feline patients. Vet. Clin. Pathol. 2024, 53, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Jewell, D.E.; Ephraim, E. Feeding cats with chronic kidney disease food supplemented with betaine and prebiotics increases total body mass and reduces uremic toxins. PLoS ONE 2022, 17, e0268624. [Google Scholar] [CrossRef] [PubMed]


| Diet | kcal/cup | Na (mg) | K (mg) | Protein (g) | Fat (g) | Phos (mg) | Ca (mg) | Crude Fiber (g) | TDF (g) | EPA + DHA (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hill’s Prescription Diet k/d w/Chicken (dry) | 534 | 57 | 171 | 6.7 | 5.4 | 119 | 183 | 0.7 | 1.6 | 100 |
| Hill’s k/d ACTIVBIOME+ Kidney Defense w/Oceanfish (dry) | 498 | 59 | 172 | 6.8 | 5.2 | 118 | 183 | 0.7 | 1.5 | 135 |
| Laboratory Reference Range | SOC Day 0 | SOC Day 28 | SOC Day 56 (SID Day 0) | SID Day 28 | SID Day 56 | |
|---|---|---|---|---|---|---|
| Weight a,b (kg) | - | 4.0 (3.3–4.6) | 3.9 (3–4.6) | 3.9 (3.1–4.5) | 3.9 (3–4.5) | 3.8 (3.1–4.4) |
| BCS | - | 6.0 (5–7) | 5 (5–7) | 5 (5–6) | 5 (5–7) | 5 (5–7) |
| MCS | - | 1 (0–2) | 1 (0–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| HCT (%) | 33.6–49 | 35.7 (28.2–43.6) | - | 36.5 (27.2–40.1) | - | 35.7 (27.4–39.5) |
| BUN b,d (mg/dL) | 18–35 | 22 (18–35) | - | 22 (17–37) | - | 24 (20–44) |
| Creatinine b,d (mg/dL) | 0.7–1.8 | 1.9 (1.5–4.0) | - | 2.0 (1.6–4.0) | - | 2.1 (1.6–4.7) |
| Phosphorous (mg/dL) | 2.8–5.9 | 3.6 (2.8–4.4) | - | 3.5 (2.4–4.5) | - | 3.4 (2.4–4.6) |
| Bicarbonate (mmol/L) | 15–23 | 16 (12–20) | - | 16 (10–17) | - | 17 (14–19) |
| Anion gap b,d (mmol/L) | 19–29 | 22 (18–26) | - | 23 (18–30) | - | 20 (17–24) |
| Potassium (mmol/L) | 3.6–5.3 | 4.4 (3.6–4.9) | - | 4.6 (3.7–5.2) | - | 4.6 (3.7–5.0) |
| UPC | <0.20 | 0.12 (0.02–0.20) | - | 0.11 (0.05–0.30) | - | 0.10 (0.02–0.50) |
| BP (mmHg) | 100–160 | 156 (107–184) | - | 142 (116–202) | - | 137 (108–170) |
| IDS (ng/mL) c | - | 2012 (617–5827) | 1780 (1015–4682) | 1652 (1257–4119) | 1417 (664–3856) | 1513 (1157–5479) |
| pCS (ng/mL) c | - | 10,422 (5143–47,316) | 8535 (308–37,815) | 12,720 (268–34,609) | 7604 (263–26,575) | 8520 (290–34,114) |
| Average of SOC Days 0, 28, 56 | Average of SID Days 28, 56 | |||||
| IDS (ng/mL) | - | 1800 (1030–4876) | 1592 (944–4669) | |||
| pCS (ng/mL) | - | 10,141 (1906–39,913) | 6861 (277–30,345) | |||
| Laboratory Reference Range | BID Day 0 | BID Day 28 | BID Day 56 | |
|---|---|---|---|---|
| Weight (kg) | - | 3.8 (3.1–4.3) | 3.9 (3.2–4.3) | 3.9 (3.0–4.3) |
| BCS | - | 5 (4–7) | 5 (4–6) | 5 (4–6) |
| MCS | - | 2 (1–3) | 2 (1–4) | 2 (1–4) |
| HCT (%) b | 33.6–49.0 | 35.6 (28.2–41.1) | - | 31.3 (25.5–37.4) |
| BUN (mg/dL) b | 18–35 | 24 (18–40) | - | 26 (22–46) |
| Creatinine (mg/dL) | 0.7–1.8 | 2.1 (1.5–4.4) | - | 2.2 (1.5–4.2) |
| Phosphorous (mg/dL) b | 2.8–5.9 | 3.9 (2.4–4.8) | - | 4.3 (3.6–6.0) |
| Bicarbonate (mmol/L) b | 15–23 | 15 (13–18) | - | 16 (14–19) |
| Anion gap (mmol/L) | 19–29 | 22 (18–29) | - | 22 (18–25) |
| Potassium (mmol/L) | 3.6–5.3 | 4.5 (3.7–5.0) | - | 4.0 (3.7–5.2) |
| UPC | <0.20 | 0.10 (0–0.40) | - | 0.10 (0.04–0.40) |
| BP (mmHg) | 100–160 | 133 (108–157) | - | 129 (104–173) |
| IDS (ng/mL) a,b | - | 1704 (1244–5666) | 1369 (558–4465) | 1426 (631–3874) |
| pCS (ng/mL) b | - | 11,839 (5250–45,684 | 5952 (2451–20,477) | 7055 (2031–20,168) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschall, R.E.; Quimby, J.M.; Lourenço, B.N.; Summers, S.C.; Schmiedt, C.W. The Effect of Renaltec on Serum Uremic Toxins in Cats with Experimentally Induced Chronic Kidney Disease. Vet. Sci. 2024, 11, 379. https://doi.org/10.3390/vetsci11080379
Paschall RE, Quimby JM, Lourenço BN, Summers SC, Schmiedt CW. The Effect of Renaltec on Serum Uremic Toxins in Cats with Experimentally Induced Chronic Kidney Disease. Veterinary Sciences. 2024; 11(8):379. https://doi.org/10.3390/vetsci11080379
Chicago/Turabian StylePaschall, Rene E., Jessica M. Quimby, Bianca N. Lourenço, Stacie C. Summers, and Chad W. Schmiedt. 2024. "The Effect of Renaltec on Serum Uremic Toxins in Cats with Experimentally Induced Chronic Kidney Disease" Veterinary Sciences 11, no. 8: 379. https://doi.org/10.3390/vetsci11080379
APA StylePaschall, R. E., Quimby, J. M., Lourenço, B. N., Summers, S. C., & Schmiedt, C. W. (2024). The Effect of Renaltec on Serum Uremic Toxins in Cats with Experimentally Induced Chronic Kidney Disease. Veterinary Sciences, 11(8), 379. https://doi.org/10.3390/vetsci11080379






