Generation of Insulin-Producing Cells from Canine Bone Marrow-Derived Mesenchymal Stem Cells: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Retrieval and Expansion of cBM-MSCs
2.2. Characterization of cBM-MSCs
2.3. In Vitro Differentiation of cBM-MSCs into IPCs
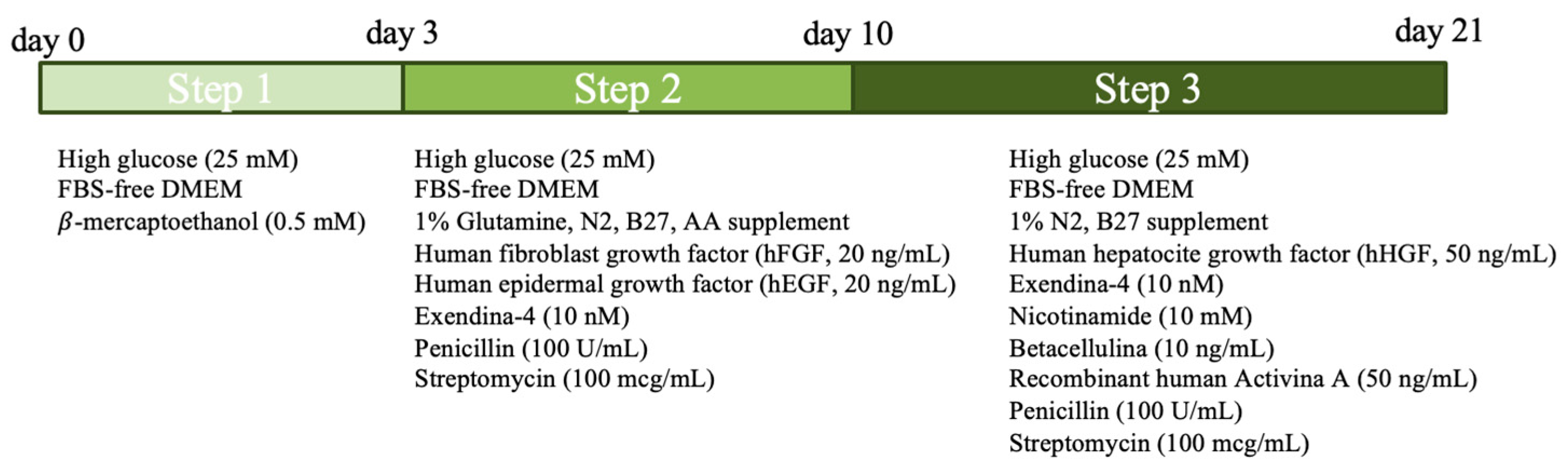
2.3.1. Two-Step Protocol
2.3.2. Three-Step Protocol
2.4. Morphological Analysis
2.5. Gene Expression Analysis by RT-qPCR
2.6. Analysis of Insulin Protein Expression by Immunofluorescence
2.7. Glucose-Stimulated Insulin Secretion Assay
2.8. Determination of Insulin Protein Content
3. Results
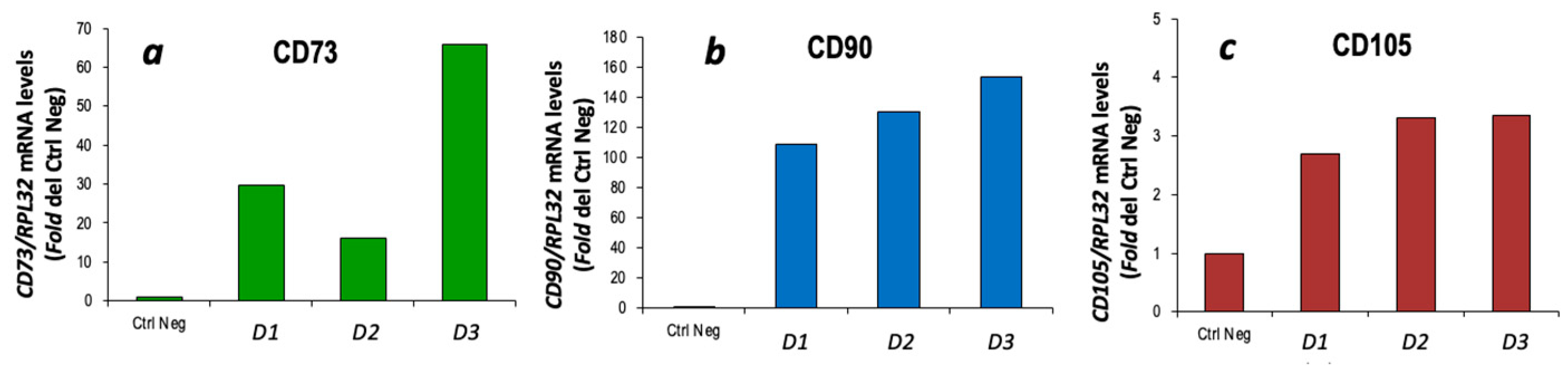
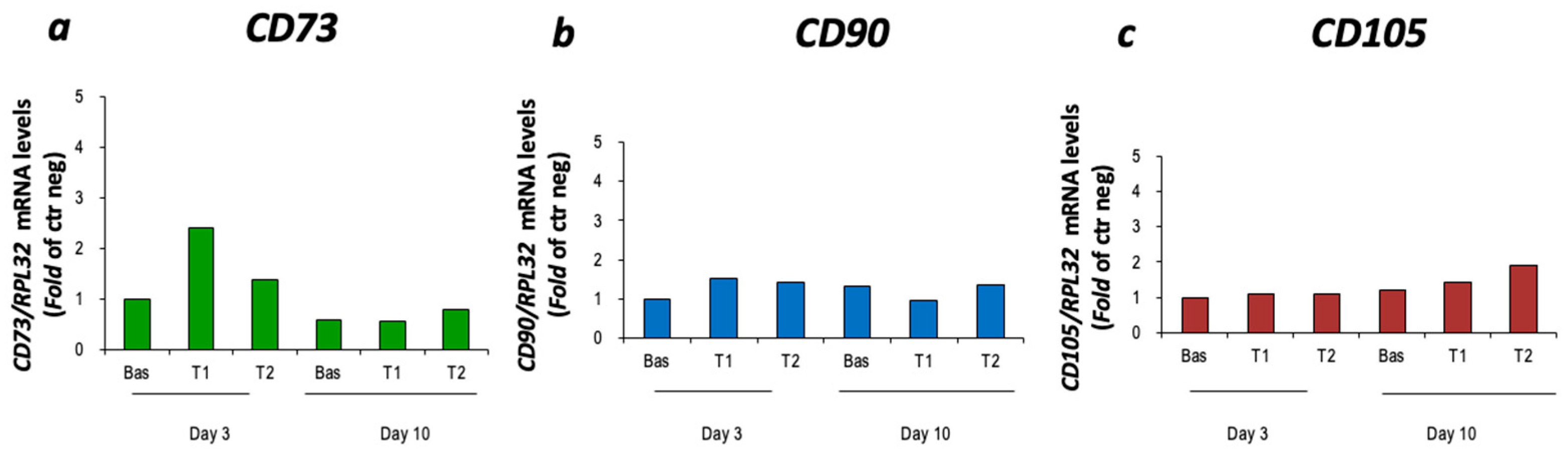
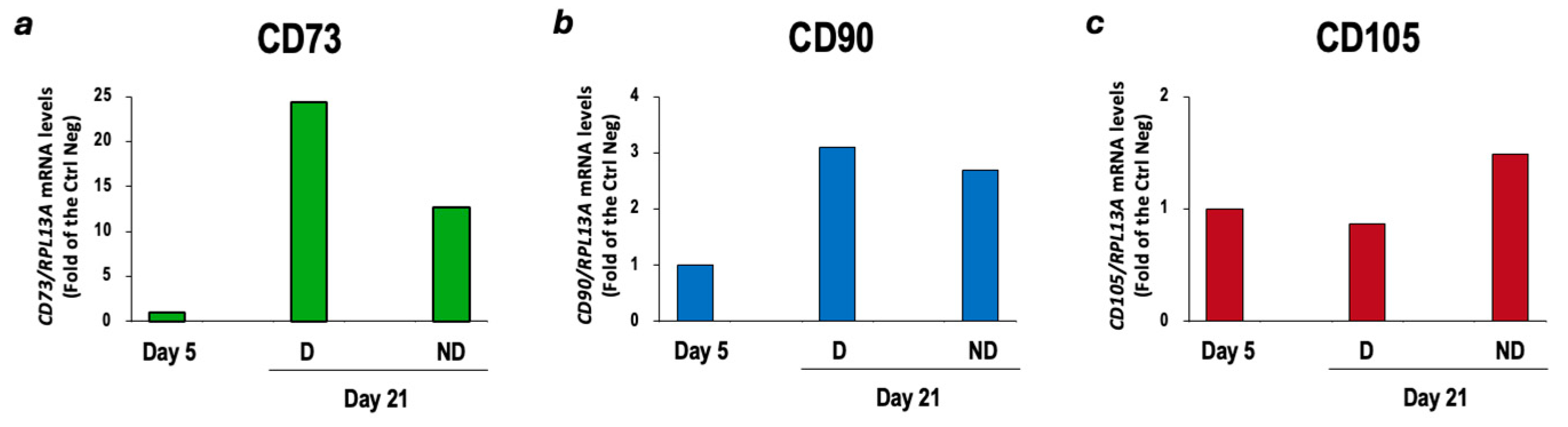
3.1. Characterization of cBM-MSCs
3.2. In Vitro Differentiation of cBM-MSCs into IPCs
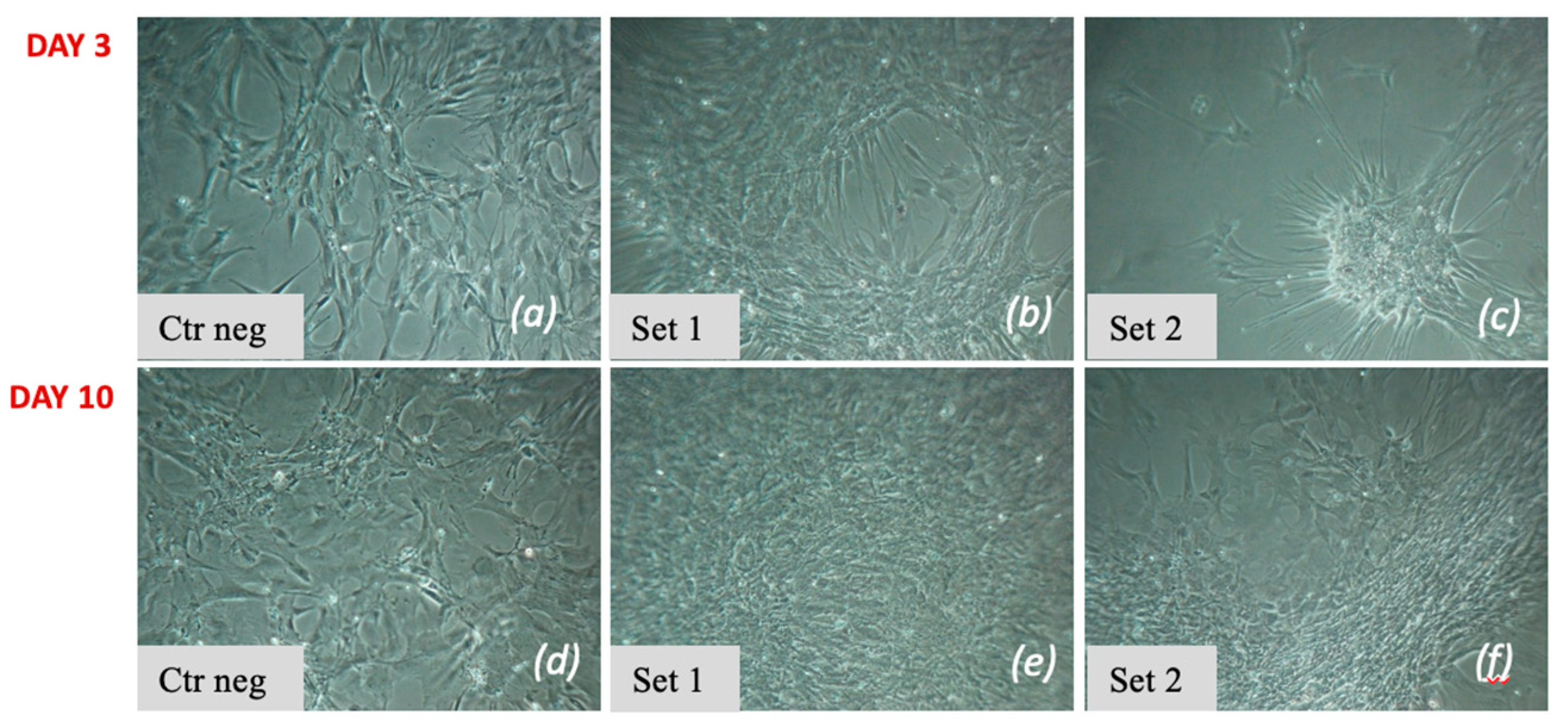
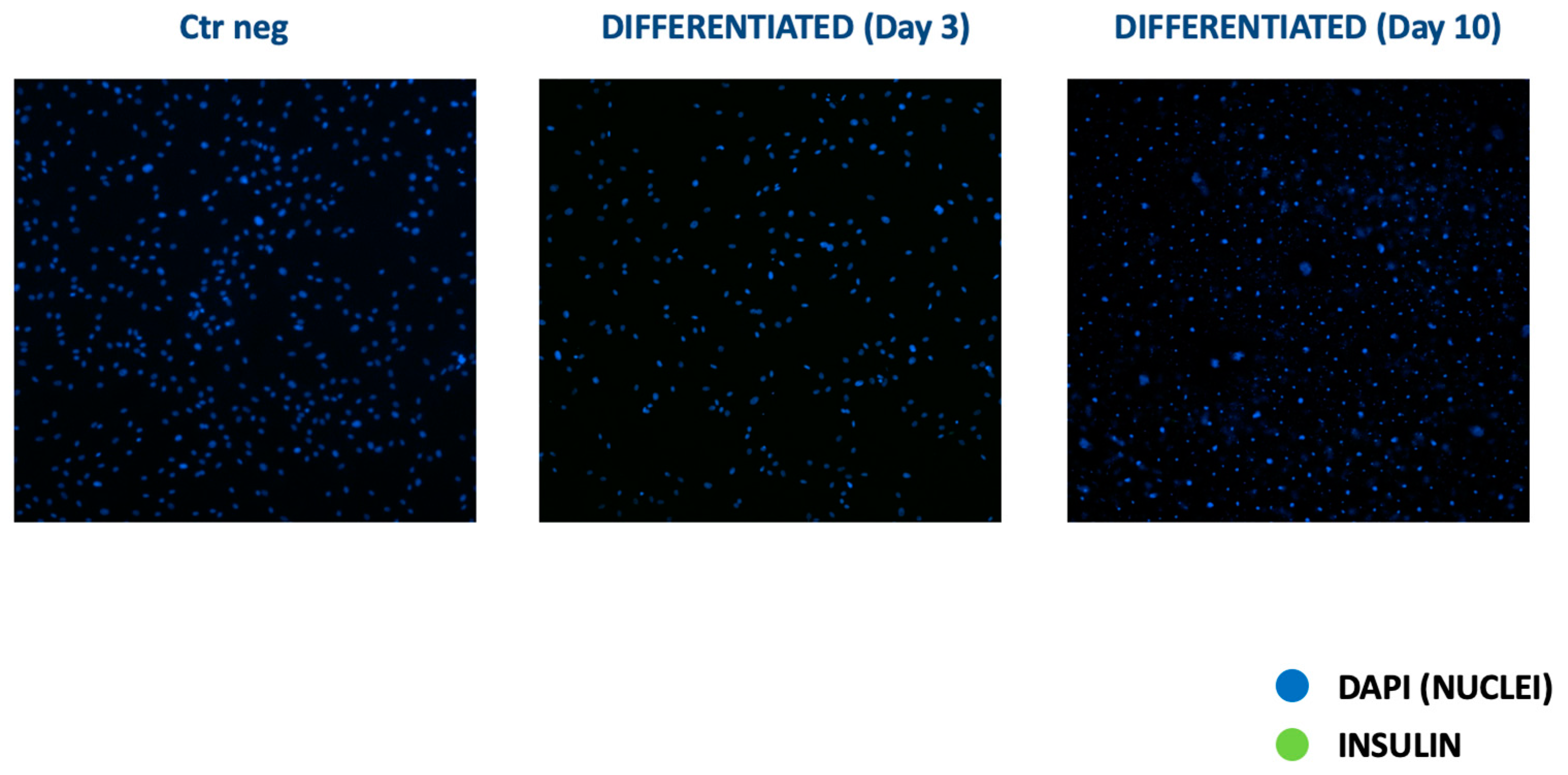
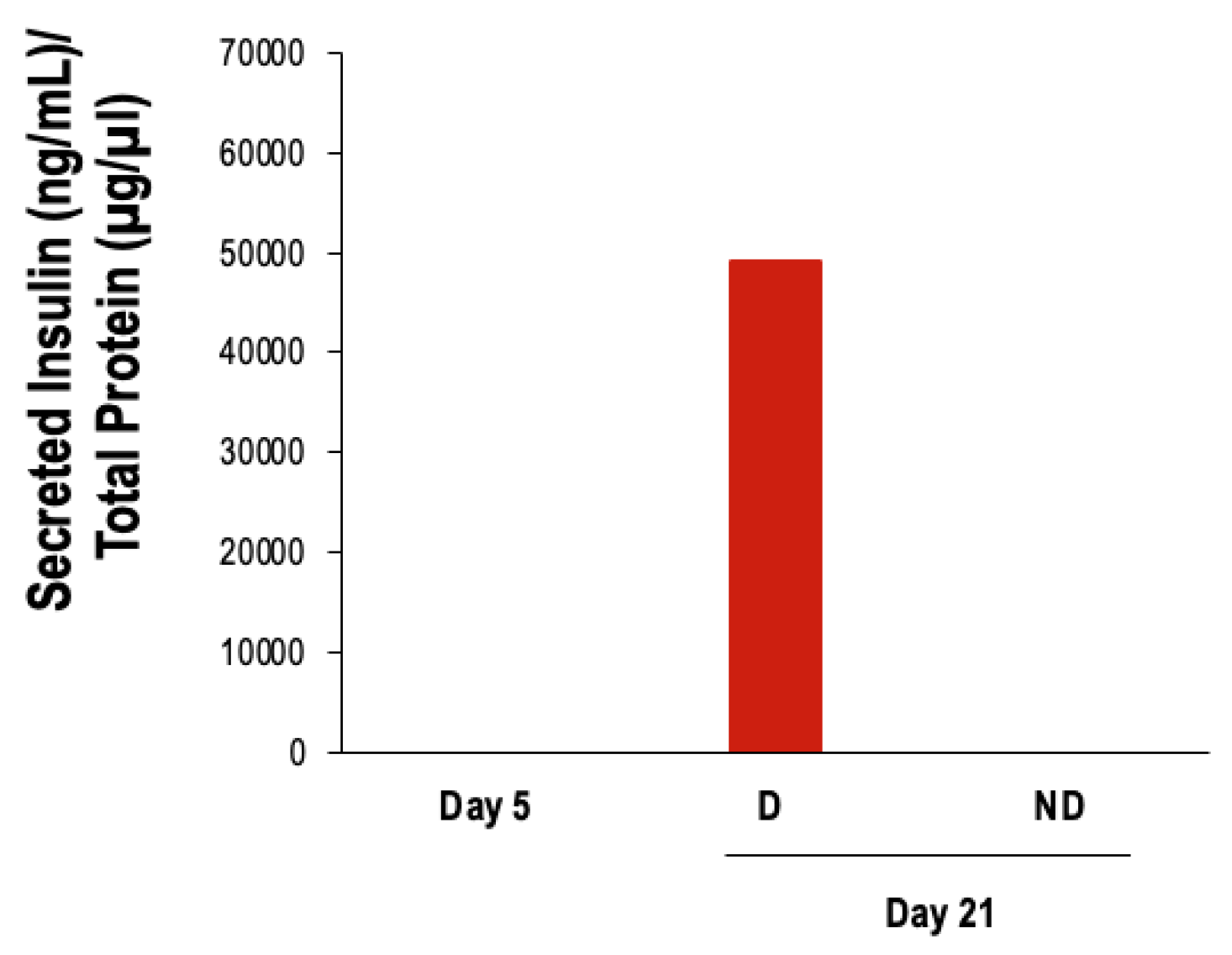
3.2.1. Two-Step Protocol
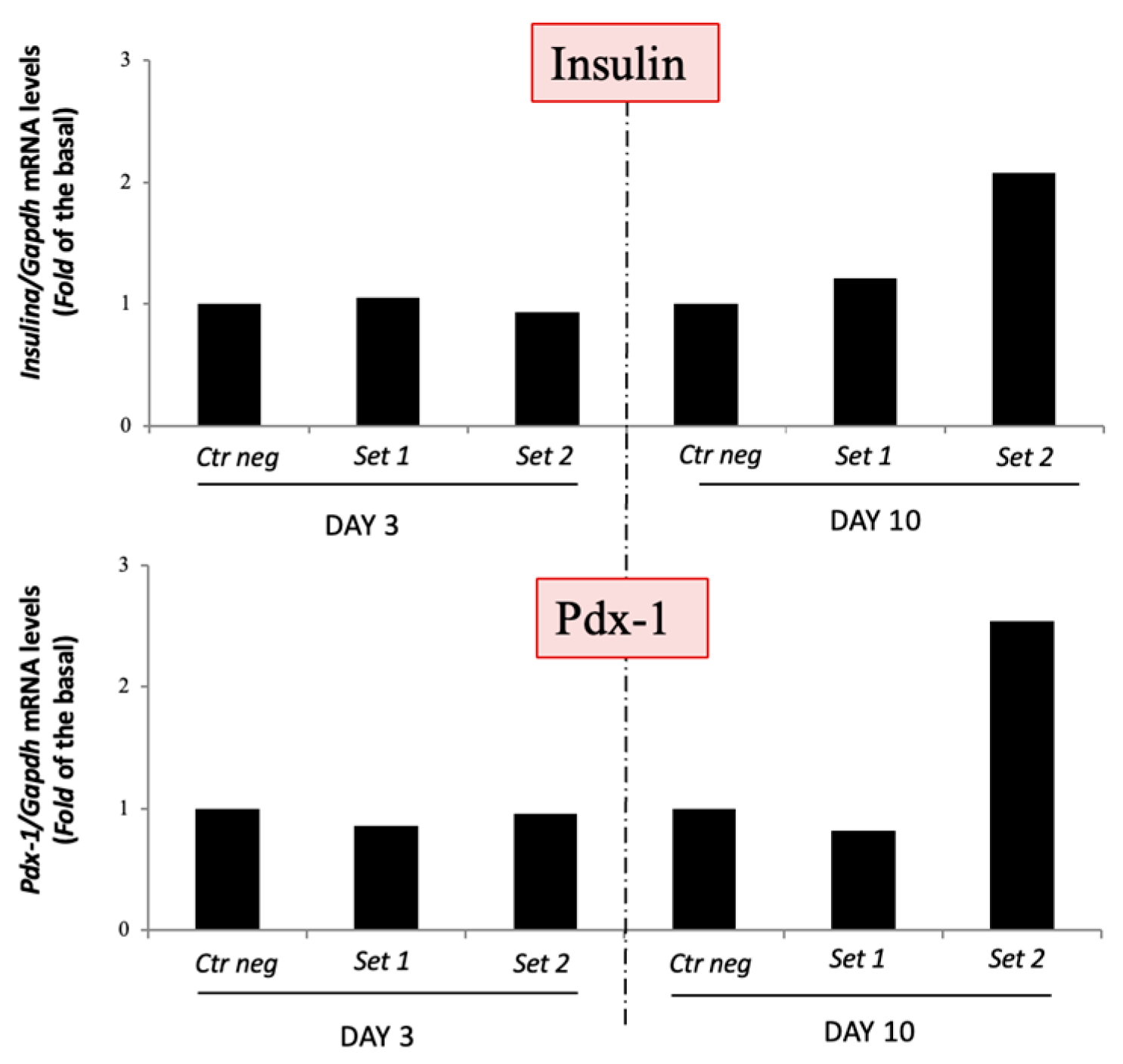
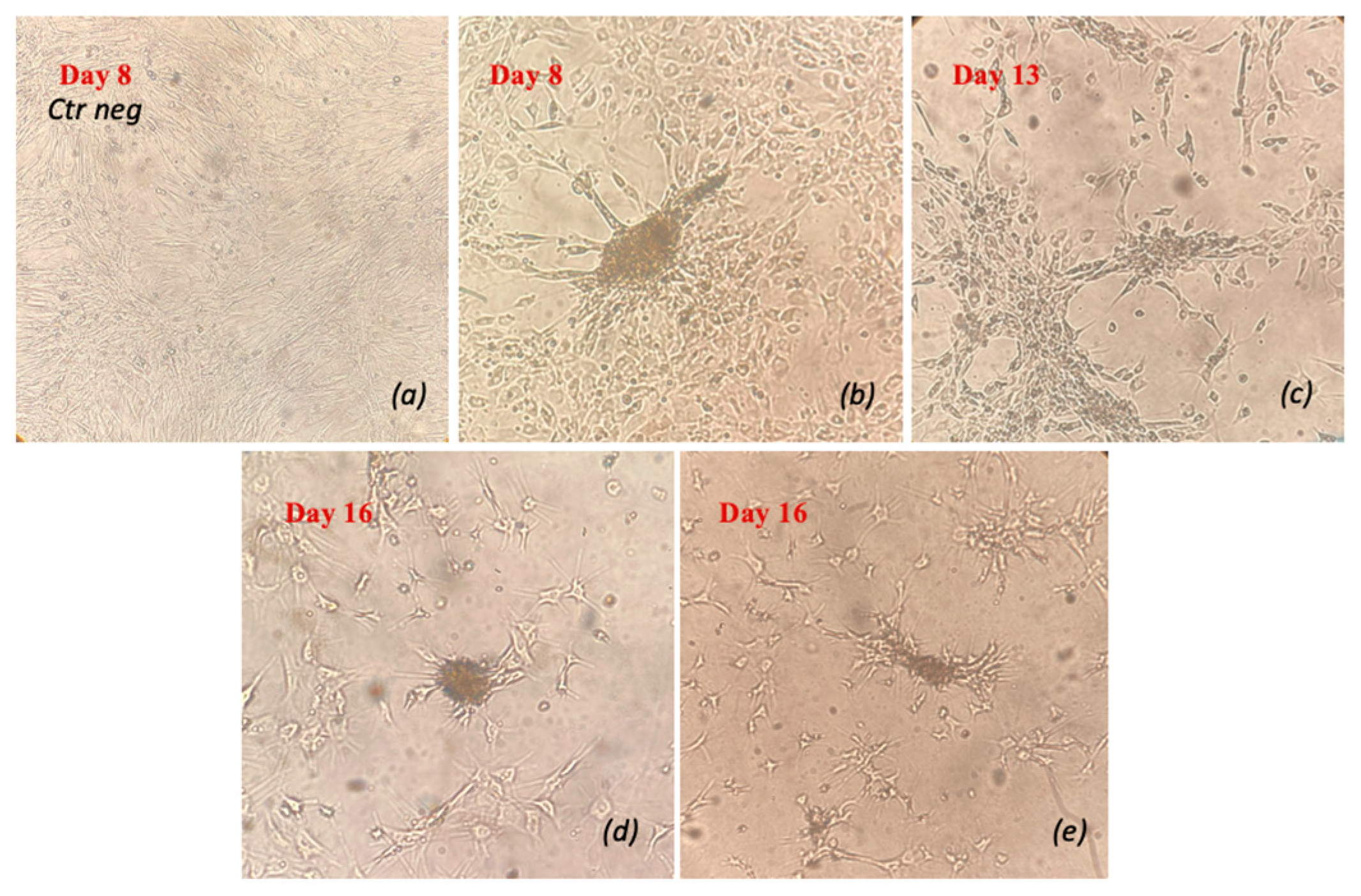
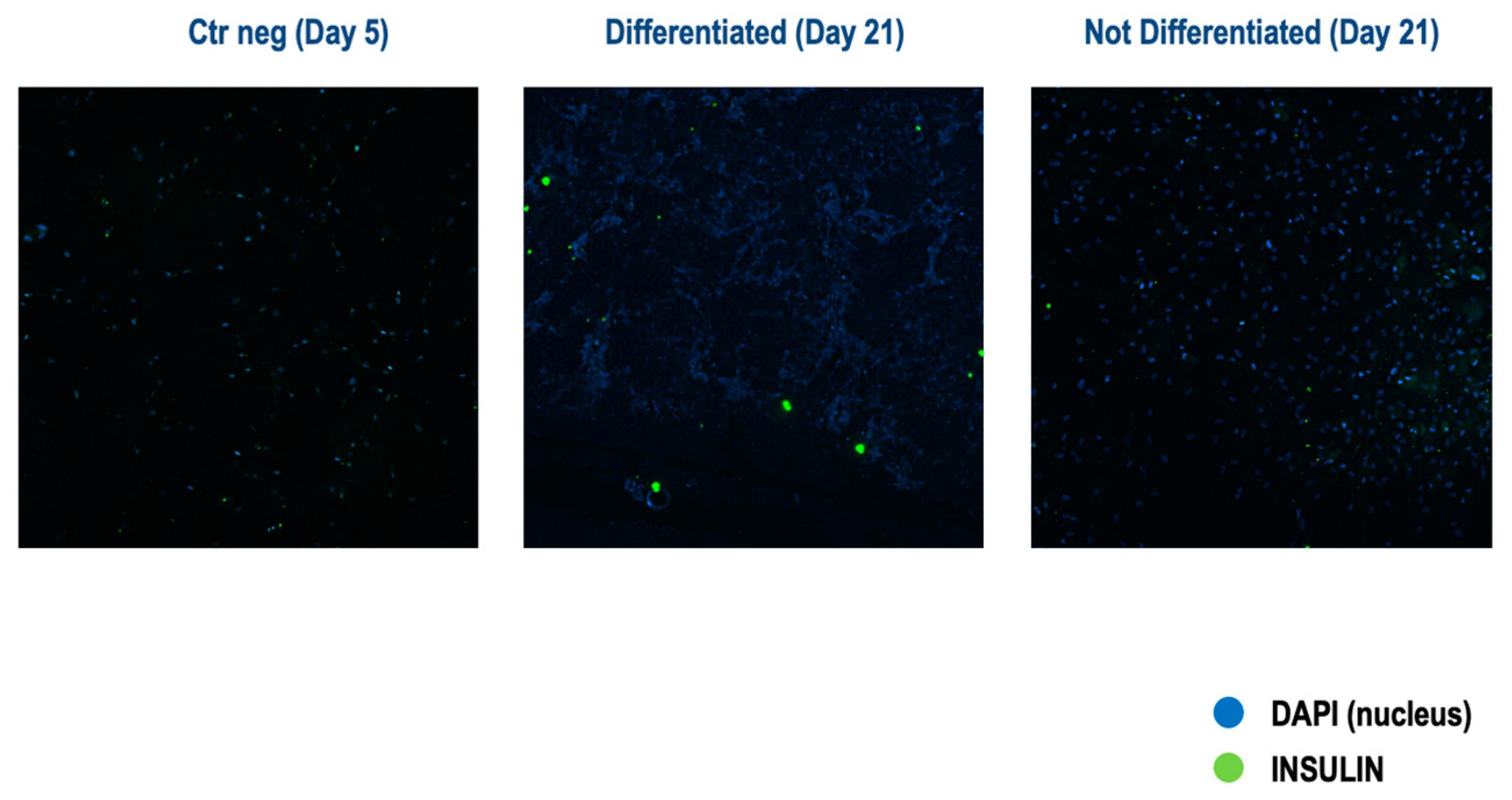
3.2.2. Three-Step Protocol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldman, E.C.; Nelson, R.W.; Reusch, C.; Scott-Moncrieff, J.C.R. Canine & Feline Endocrinology, 4th ed.; Elsevier Saunders: St. Louis, MO, USA, 2015; ISBN 978-1-4557-4456-5. [Google Scholar]
- Moshref, M.; Tangey, B.; Gilor, C.; Papas, K.K.; Williamson, P.; Loomba-Albrecht, L.; Sheehy, P.; Kol, A. Concise Review: Canine Diabetes Mellitus as a Translational Model for Innovative Regenerative Medicine Approaches. Stem Cells Transl. Med. 2019, 8, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Dang Le, Q.; Rodprasert, W.; Kuncorojakti, S.; Pavasant, P.; Osathanon, T.; Sawangmake, C. In Vitro Generation of Transplantable Insulin-Producing Cells from Canine Adipose-Derived Mesenchymal Stem Cells. Sci. Rep. 2022, 12, 9127. [Google Scholar] [CrossRef] [PubMed]
- Rodprasert, W.; Nantavisai, S.; Pathanachai, K.; Pavasant, P.; Osathanon, T.; Sawangmake, C. Tailored Generation of Insulin Producing Cells from Canine Mesenchymal Stem Cells Derived from Bone Marrow and Adipose Tissue. Sci. Rep. 2021, 11, 12409. [Google Scholar] [CrossRef] [PubMed]
- Teshima, T.; Okamoto, K.; Dairaku, K.; Nagashima, T.; Michishita, M.; Suzuki, R.; Matsumoto, H.; Koyama, H. Generation of Insulin-Producing Cells from Canine Adipose Tissue-Derived Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 8841865. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, P.; Zou, T.; Lv, Y.; Zhao, W.; Zhang, X.; Zhang, Y. Transcriptome Analysis of the Transdifferentiation of Canine BMSCs into Insulin Producing Cells. BMC Genom. 2021, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- Tayaramma, T.; Ma, B.; Rohde, M.; Mayer, H. Chromatin-Remodeling Factors Allow Differentiation of Bone Marrow Cells into Insulin-Producing Cells. Stem Cells 2006, 24, 2858–2867. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Ismail, A.M.; Khater, S.M.; Ashamallah, S.A.; Azzam, M.M.; Ghoneim, M.A. Insulin-Producing Cells from Adult Human Bone Marrow Mesenchymal Stromal Cells Could Control Chemically Induced Diabetes in Dogs: A Preliminary Study. Cell Transplant. 2018, 27, 937–947. [Google Scholar] [CrossRef]
- Takemitsu, H.; Zhao, D.; Yamamoto, I.; Harada, Y.; Michishita, M.; Arai, T. Comparison of Bone Marrow and Adipose Tissue-Derived Canine Mesenchymal Stem Cells. BMC Vet. Res. 2012, 8, 150. [Google Scholar] [CrossRef]
- Takemitsu, H.; Zhao, D.; Ishikawa, S.; Michishita, M.; Arai, T.; Yamamoto, I. Mechanism of Insulin Production in Canine Bone Marrow Derived Mesenchymal Stem Cells. Gen. Comp. Endocrinol. 2013, 189, 1–6. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Kuncorojakti, S.; Srisuwatanasagul, S.; Kradangnga, K.; Sawangmake, C. Insulin-Producing Cell Transplantation Platform for Veterinary Practice. Front. Vet. Sci. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Khater, S.M.; Ashamallah, S.A.; Ismail, A.M.; El-Badri, N.; Ghoneim, M.A. Generation of Insulin-Producing Cells from Human Bone Marrow-Derived Mesenchymal Stem Cells: Comparison of Three Differentiation Protocols. BioMed Res. Int. 2014, 2014, 832736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marappagounder, D.; Somasundaram, I.; Dorairaj, S.; Sankaran, R. Differentiation of Mesenchymal Stem Cells Derived from Human Bone Marrow and Subcutaneous Adipose Tissue into Pancreatic Islet-like Clusters In Vitro. Cell. Mol. Biol. Lett. 2013, 18, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Horibe, T.; Hinotsu, S.; Tanaka, T.; Inoue, T.; Urushihara, H.; Kitagawa, A.; Kawakami, K. Chromosomal Variability of Human Mesenchymal Stem Cells Cultured under Hypoxic Conditions. J. Cell Mol. Med. 2012, 16, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, P.; Gao, D.; Zhang, X.; Ruan, C.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y. Genome-Wide Analysis Reveals Changes in Long Noncoding RNAs in the Differentiation of Canine BMSCs into Insulin-Producing Cells. Int. J. Mol. Sci. 2020, 21, 5549. [Google Scholar] [CrossRef] [PubMed]
- Soria, B. In-Vitro Differentiation of Pancreatic β-Cells. Differentiation 2001, 68, 205–219. [Google Scholar] [CrossRef]
- Haumaitre, C.; Lenoir, O.; Scharfmann, R. Histone Deacetylase Inhibitors Modify Pancreatic Cell Fate Determination and Amplify Endocrine Progenitors. Mol. Cell. Biol. 2008, 28, 6373–6383. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Hou, X.; Hou, W.; Dong, J.; Sun, L.; Tang, K.; Wang, B.; Song, J.; Li, H.; et al. Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells from Diabetic Patients into Insulin-Producing Cells In Vitro. Chin. Med. J. 2007, 120, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Bonner-Weir, S.; Deery, D.; Leahy, J.L.; Weir, G.C. Compensatory Growth of Pancreatic β-Cells in Adult Rats After Short-Term Glucose Infusion. Diabetes 1989, 38, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Nakauchi, H.; Taniguchi, H. Glucagon-like Peptide 1 (1–37) Converts Intestinal Epithelial Cells into Insulin-Producing Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5034–5039. [Google Scholar] [CrossRef]
- Wu, X.-H. Reversal of Hyperglycemia in Diabetic Rats by Portal Vein Transplantation of Islet-like Cells Generated from Bone Marrow Mesenchymal Stem Cells. WJG 2007, 13, 3342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lu, Y.; Zhu, J.; Xu, J.; Huang, H.; Zhu, M.; Chen, Y.; Zhou, Y.; Fan, X.; Wang, Z. Effects of Intrahepatic Bone-Derived Mesenchymal Stem Cells Autotransplantation on the Diabetic Beagle Dogs. J. Surg. Res. 2011, 168, 213–223. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sieradzki, J.; Schatz, H. Epidermal Growth Factor Stimulates (Pro-)Insulin Biosynthesis and 3 H-Thymidine Incorporation in Isolated Pancreatic Rat Islets. Horm. Metab. Res. 1986, 18, 873–874. [Google Scholar] [CrossRef] [PubMed]
- Lumelsky, N.; Blondel, O.; Laeng, P.; Velasco, I.; Ravin, R.; McKay, R. Differentiation of Embryonic Stem Cells to Insulin-Secreting Structures Similar to Pancreatic Islets. Science 2001, 292, 1389–1394. [Google Scholar] [CrossRef]
- Kassem, D.H.; Kamal, M.M.; El-Kholy, A.E.-L.G.; El-Mesallamy, H.O. Exendin-4 Enhances the Differentiation of Wharton’s Jelly Mesenchymal Stem Cells into Insulin-Producing Cells through Activation of Various β-Cell Markers. Stem Cell Res. Ther. 2016, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Franklin, I.; St-Onge, L.; Biason-Lauber, A.; Schoenle, E.J.; Wollheim, C.B.; Gauthier, B.R. The Diabetes-Linked Transcription Factor PAX4 Promotes β-Cell Proliferation and Survival in Rat and Human Islets. J. Cell Biol. 2004, 167, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Ng, W.M.; Tan, H.S.; Vinitha, D.; Yang, Z.; Fan, J.B.; Zou, Y.; Hui, J.H.; Lee, E.H.; Lim, B. Molecular Basis of Immortalization of Human Mesenchymal Stem Cells by Combination of P53 Knockdown and Human Telomerase Reverse Transcriptase Overexpression. Stem Cells Dev. 2013, 22, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Xue, W.-J.; Duan, Y.-F.; Xie, L.-Y.; Lu, W.-H.; Zheng, J.; Yin, A.-P. Nicotinamide Facilitates Mesenchymal Stem Cell Differentiation Into Insulin-Producing Cells and Homing to Pancreas in Diabetic Mice. Transplant. Proc. 2015, 47, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Burkart, V. Nicotinamide in Type 1 Diabetes. Mechanism of Action Revisited. Diabetes Care 1999, 22 (Suppl. S2), B16–B20. [Google Scholar]
- Wang, R.; Yashpal, N.; Bacchus, F.; Li, J. Hepatocyte Growth Factor Regulates Proliferation and Differentiation of Epithelial Monolayers Derived from Islets of Postnatal Rat Pancreas. J. Endocrinol. 2004, 183, 163–171. [Google Scholar] [CrossRef][Green Version]
- Mashima, H.; Shibata, H.; Mine, T.; Kojima, I. Formation of Insulin-Producing Cells from Pancreatic Acinar AR42J Cells by Hepatocyte Growth Factor. Endocrinology 1996, 137, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.B.; Limaye, L.S.; Kale, V.P. Mimicking the Functional Hematopoietic Stem Cell Niche in Vitro: Recapitulation of Marrow Physiology by Hydrogel-Based Three-Dimensional Cultures of Mesenchymal Stromal Cells. Haematologica 2012, 97, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Abdel-Rahman, E.A.; Reda, A.M.; Ali, S.S.; Khater, S.M.; Ashamallah, S.A.; Ismail, A.M.; Ismail, H.E.-D.A.; et al. From Human Mesenchymal Stem Cells to Insulin-Producing Cells: Comparison between Bone Marrow- and Adipose Tissue-Derived Cells. BioMed Res. Int. 2017, 2017, 3854232. [Google Scholar] [CrossRef]
- Gonez, L.J.; Knight, K.R. Cell Therapy for Diabetes: Stem Cells, Progenitors or Beta-Cell Replication? Mol. Cell. Endocrinol. 2010, 323, 55–61. [Google Scholar] [CrossRef] [PubMed]




| Target | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Insulin | AGGGAGGTGGAGGACCTG | CAGCACTGCTCCACGATG |
| RPL13A | GCCGGAAGGTTGTAGTCGT | GGAGGAAGGCCAGGTAATTC |
| RPL32 | TGGTTACAGGAGCAACAAGAAA | GCACATCAGCAGCACTTCA |
| PDX-1 | GACAACAGGAACTACAAGTCGGAAT | TGTTTCGGGACAGATGAAGGT |
| CD73 | GCCGGAAGGTTGTAGTCGT | GGAGGAAGGCCAGGTAATTC |
| CD90 | TGGTTACAGGAGCAACAAGAAA | GCACATCAGCAGCACTTCA |
| CD105 | GACAACAGGAACTACAAGTCGGAAT | TGTTTCGGGACAGATGAAGGT |
| GAPDH | CGAGATCCCGCCAACATCAA | CTCCATGGTGGTGAAGACCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colella, A.; Biondi, G.; Marrano, N.; Francioso, E.; Fracassi, L.; Crovace, A.M.; Recchia, A.; Natalicchio, A.; Paradies, P. Generation of Insulin-Producing Cells from Canine Bone Marrow-Derived Mesenchymal Stem Cells: A Preliminary Study. Vet. Sci. 2024, 11, 380. https://doi.org/10.3390/vetsci11080380
Colella A, Biondi G, Marrano N, Francioso E, Fracassi L, Crovace AM, Recchia A, Natalicchio A, Paradies P. Generation of Insulin-Producing Cells from Canine Bone Marrow-Derived Mesenchymal Stem Cells: A Preliminary Study. Veterinary Sciences. 2024; 11(8):380. https://doi.org/10.3390/vetsci11080380
Chicago/Turabian StyleColella, Antonella, Giuseppina Biondi, Nicola Marrano, Edda Francioso, Laura Fracassi, Alberto M. Crovace, Alessandra Recchia, Annalisa Natalicchio, and Paola Paradies. 2024. "Generation of Insulin-Producing Cells from Canine Bone Marrow-Derived Mesenchymal Stem Cells: A Preliminary Study" Veterinary Sciences 11, no. 8: 380. https://doi.org/10.3390/vetsci11080380
APA StyleColella, A., Biondi, G., Marrano, N., Francioso, E., Fracassi, L., Crovace, A. M., Recchia, A., Natalicchio, A., & Paradies, P. (2024). Generation of Insulin-Producing Cells from Canine Bone Marrow-Derived Mesenchymal Stem Cells: A Preliminary Study. Veterinary Sciences, 11(8), 380. https://doi.org/10.3390/vetsci11080380







