Localization of β-Nerve Growth Factor in the Stallion Reproductive Tract
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
2.2. Immunostaining
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, T. Male accessory organs of reproduction, and their secretory product: The seminal plasma. In The Biochemistry of Semen and of the Male Reproductive Tract, 2nd ed.; Methuan and Co., Ltd.: London, UK, 1964; pp. 37–78. [Google Scholar]
- Amann, R.P. Functional anatomy of the adult male. In Equine Reproduction, 2nd ed.; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Ames, IA, USA, 2011; pp. 867–880. [Google Scholar]
- Adams, G.; Ratto, M.; Silva, M.; Carrasco, R. Ovulation-inducing factor (OIF/NGF) in seminal plasma: A review and update. Reprod. Domest. Anim. 2016, 51, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Druart, X.; Rickard, J.P.; Mactier, S.; Kohnke, P.L.; Kershaw-Young, C.M.; Bathgate, R.; Gibb, Z.; Crossett, B.; Tsikis, G.; Labas, V.; et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteom. 2013, 91, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yuen, Z.; Pan, G. Semen-induced ovulation in the bactrian camel (Camelus bactrianus). Reproduction 1985, 74, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, H.; Zeng, G.; Jiang, G.; Gao, Y. Hormone concentrations before and after semen-induced ovulation in the Bactrian camel (Camelus bactrianus). J. Reprod. Fertil. 1985, 74, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhao, X.; Chen, S.; Jiang, S.; Huang, Y.; Zu, Y.; Wang, H. The ovulation inducing effect of seminal plasma in the Bactrian camel. In Proceedings of the First International Camel Conference, Dubai, The United Arab Emirates, 2–6 February 1992; pp. 159–161. [Google Scholar]
- Adams, G.P.; Ratto, M.H.; Huanca, W.; Singh, J. Ovulation-inducing factor in the seminal plasma of alpacas and llamas. Biol. Reprod. 2005, 73, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Ratto, M.H.; Leduc, Y.A.; Valderrama, X.P.; Van Straaten, K.E.; Delbaere, L.T.J.; Pierson, R.A.; Adams, G.P. The nerve of ovulation-inducing factor in semen. Proc. Nat. Acad. Sci. USA 2012, 109, 15042–15047. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.P.; Thoenen, H. The distribution of nerve growth factor in the male sex organs of mammals. J. Neurochem. 1980, 34, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H.; Barde, Y.A. Physiology of nerve growth factor. Physiol. Rev. 1980, 60, 1284–1335. [Google Scholar] [CrossRef] [PubMed]
- Shikata, H.; Utsumi, N.; Hiramatsu, M.; Minami, N.; Nemoto, N.; Shikata, T. Immunohistochemical localization of nerve growth factor and epidermal growth factor in guinea pig prostate gland. Histochemistry 1984, 80, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Bogle, O.A.; Carrasco, R.A.; Ratto, M.H.; Singh, J.; Adams, G.P. Source and localization of ovulation-inducing factor/nerve growth factor in male reproductive tissues among mammalian species. Biol. Reprod. 2018, 99, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Bogle, O.A.; Ambati, D.; Davis, R.P.; Adams, G.P. Evidence for the presence of ovulation-inducing factor in porcine and equine seminal plasma. Reprod. Fertil. Dev. 2009, 21, 101. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Y.; Chen, W.; Hei, J.; Dong, C. Expression and localization of nerve growth factor (NGF) in the testis of alpaca (Llama pacos). Folia. Histochem. Cytobiol. 2011, 49, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, A.; Arias-Alvarez, M.; Timón, P.; Bautista, J.M.; Rebollar, P.G.; Lorenzo, P.L.; Garcia-Garcia, R.M. Characterization of β-Nerve Growth Factor-TrkA system in male reproductive tract of rabbit and the relationship between β-NGF and testosterone levels with seminal quality during sexual maturation. Theriogenology 2019, 126, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Chen, Z.; Liu, X.; Li, D.; Xie, Q.; Ling, F.; Fang, L. Isolation and purification of the ovulation-inducing factor from seminal plasma in the bactrian camel (Camelus bactrianus). Theriogenology 2001, 55, 1863–1879. [Google Scholar] [CrossRef] [PubMed]
- Meriem, F.; José Alvaro, C.P.; Imed, S.; Rosaura, P.P.; Mouldi, S.M.; Adriana, C.; Touhami, K.; Teresa, M.B.; Mohamed, H. Identification of β-nerve growth factor in dromedary camel seminal plasma and its role in induction of ovulation in females. Emir. J. Food Agric. 2017, 29, 293–299. [Google Scholar]
- Tribulo, P.; Bogle, O.; Mapletoft, R.J.; Adams, G.P. Bioactivity of ovulation inducing factor (or nerve growth factor) in bovine seminal plasma and its effects on ovarian function in cattle. Theriogenology 2015, 83, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
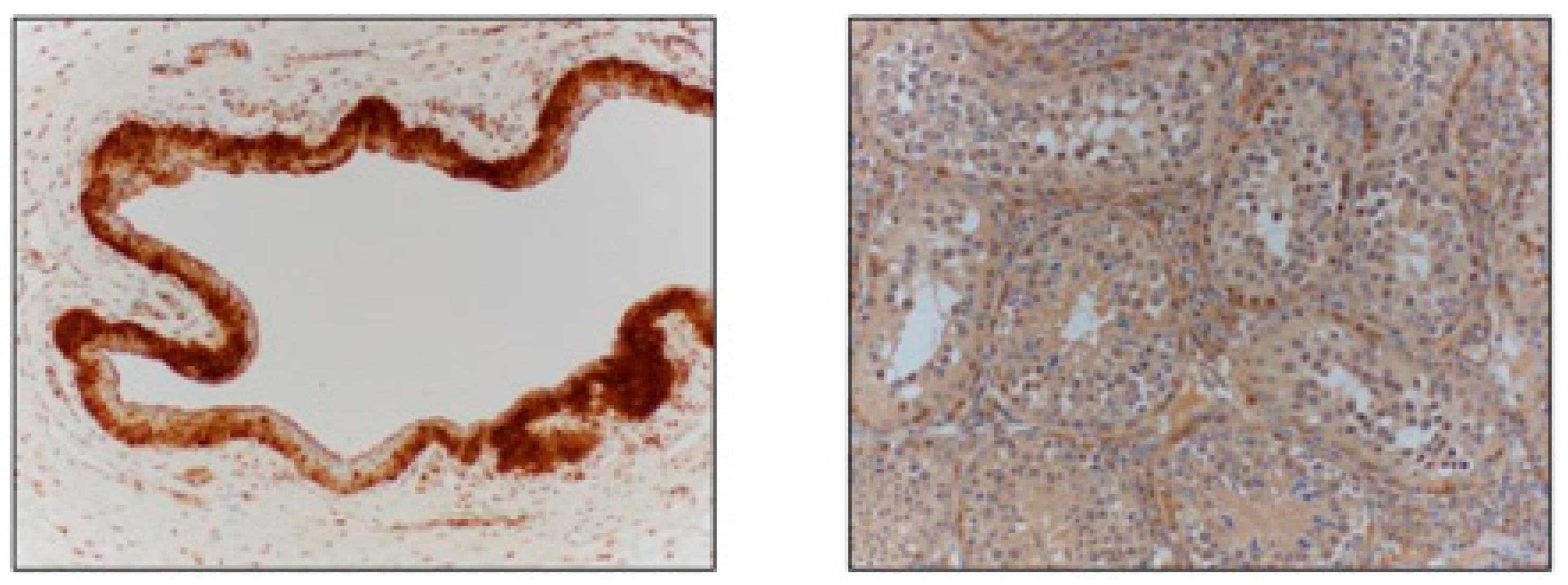
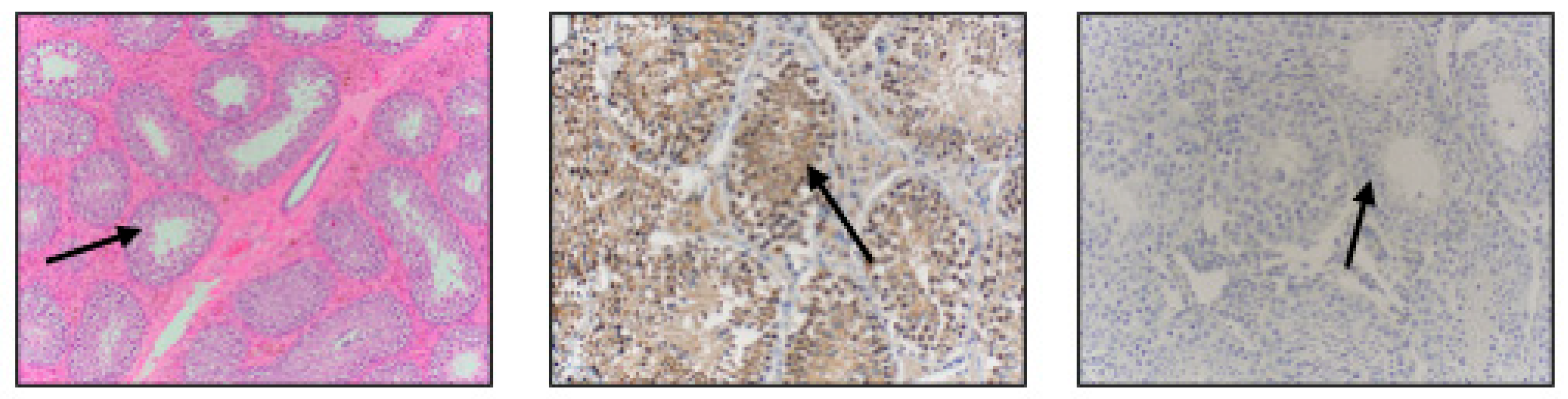

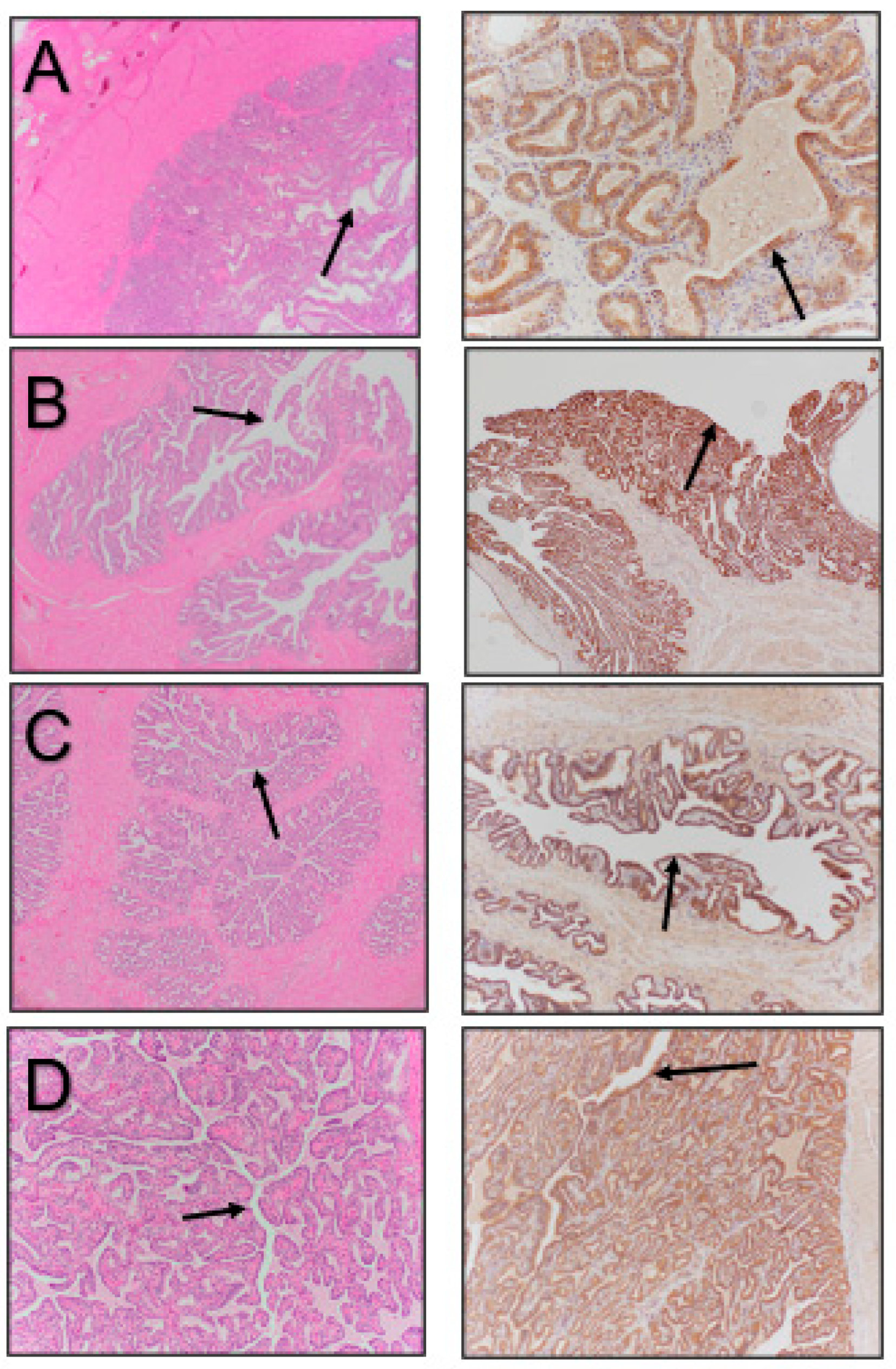
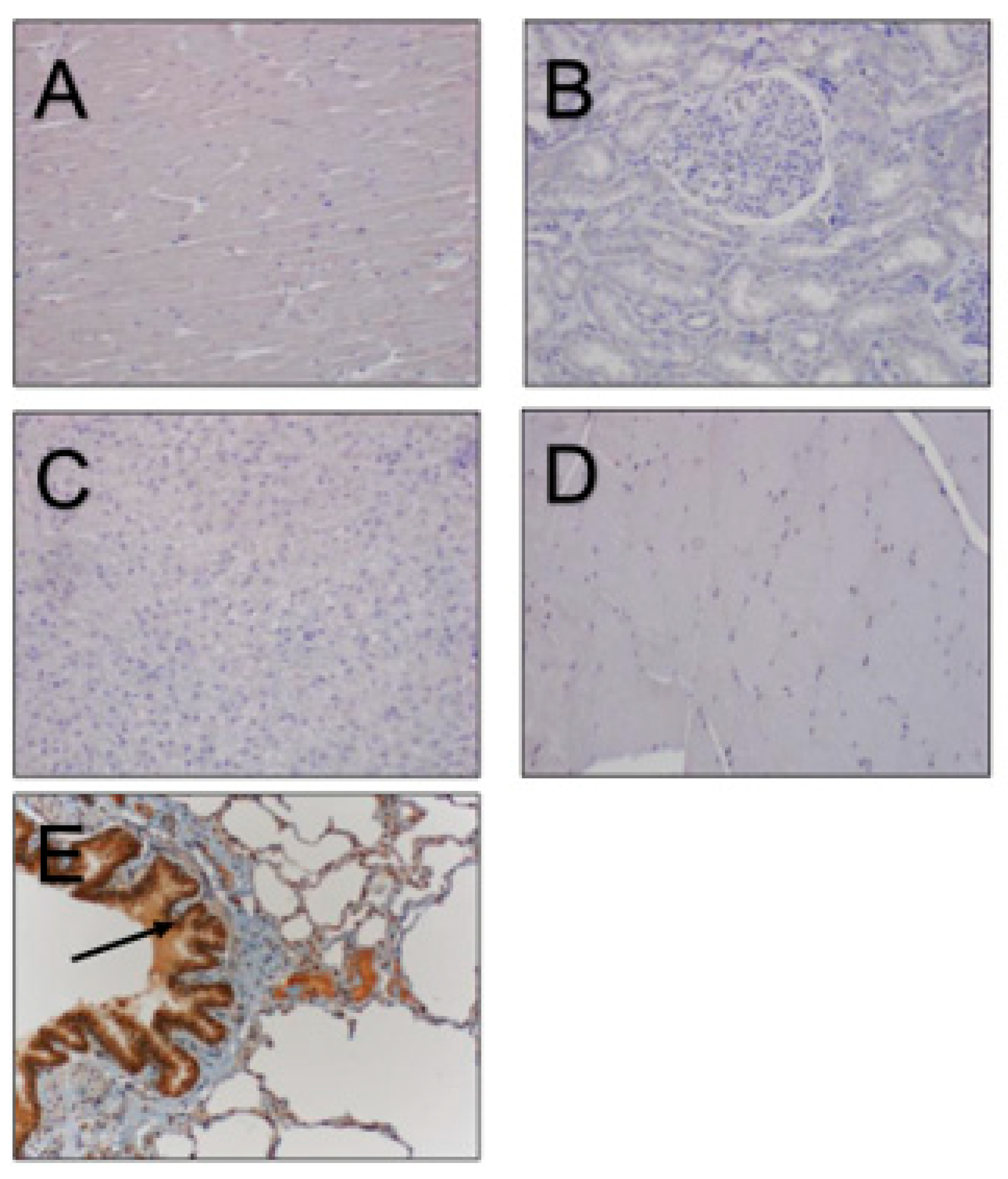
| Reproductive Tissue | NGF-β Staining Intensity |
|---|---|
| Sertoli Cells of Testes | + |
| Leydig Cells of Testes (interstitial) | + |
| Efferent Duct of Testes | +++ |
| Head of Epididymis | + |
| Body of Epididymis | + |
| Tail of Epididymis | ++ |
| Vas Deferens | ++ |
| Ampulla | +++ |
| Seminal Vesicle | +++ |
| Prostate Gland | +++ |
| Bulbourethral Gland | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickelson, A.; Magunda, F.; Graham, J.; McCue, P. Localization of β-Nerve Growth Factor in the Stallion Reproductive Tract. Vet. Sci. 2024, 11, 367. https://doi.org/10.3390/vetsci11080367
Mickelson A, Magunda F, Graham J, McCue P. Localization of β-Nerve Growth Factor in the Stallion Reproductive Tract. Veterinary Sciences. 2024; 11(8):367. https://doi.org/10.3390/vetsci11080367
Chicago/Turabian StyleMickelson, Alison, Forgivemore Magunda, James Graham, and Patrick McCue. 2024. "Localization of β-Nerve Growth Factor in the Stallion Reproductive Tract" Veterinary Sciences 11, no. 8: 367. https://doi.org/10.3390/vetsci11080367
APA StyleMickelson, A., Magunda, F., Graham, J., & McCue, P. (2024). Localization of β-Nerve Growth Factor in the Stallion Reproductive Tract. Veterinary Sciences, 11(8), 367. https://doi.org/10.3390/vetsci11080367






