Awake 160-Slice Computed Tomography for Upper Airway Evaluation in 17 Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
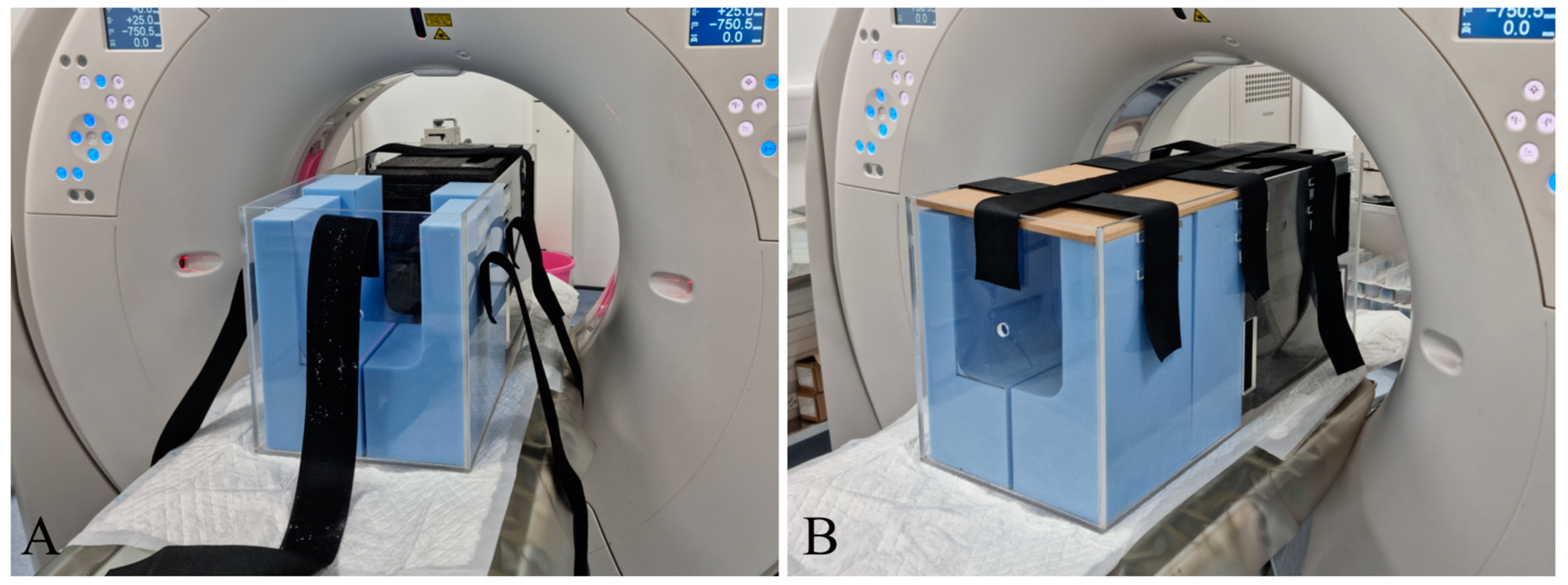
2.3. Computed Tomography
2.4. Upper Airway Examination
2.5. Statistical Analysis
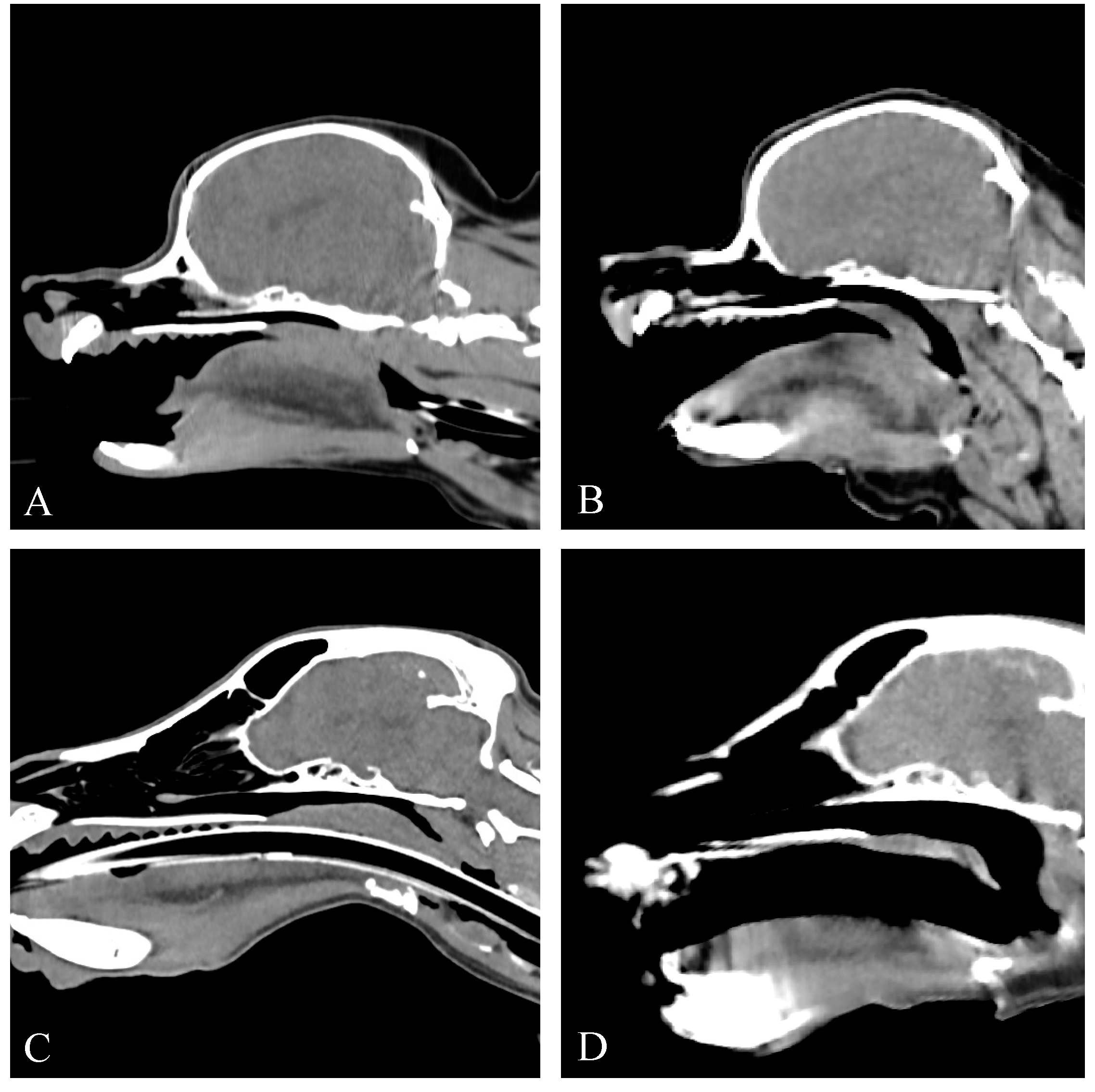
3. Results
3.1. Signalment
3.2. History and Clinical Signs
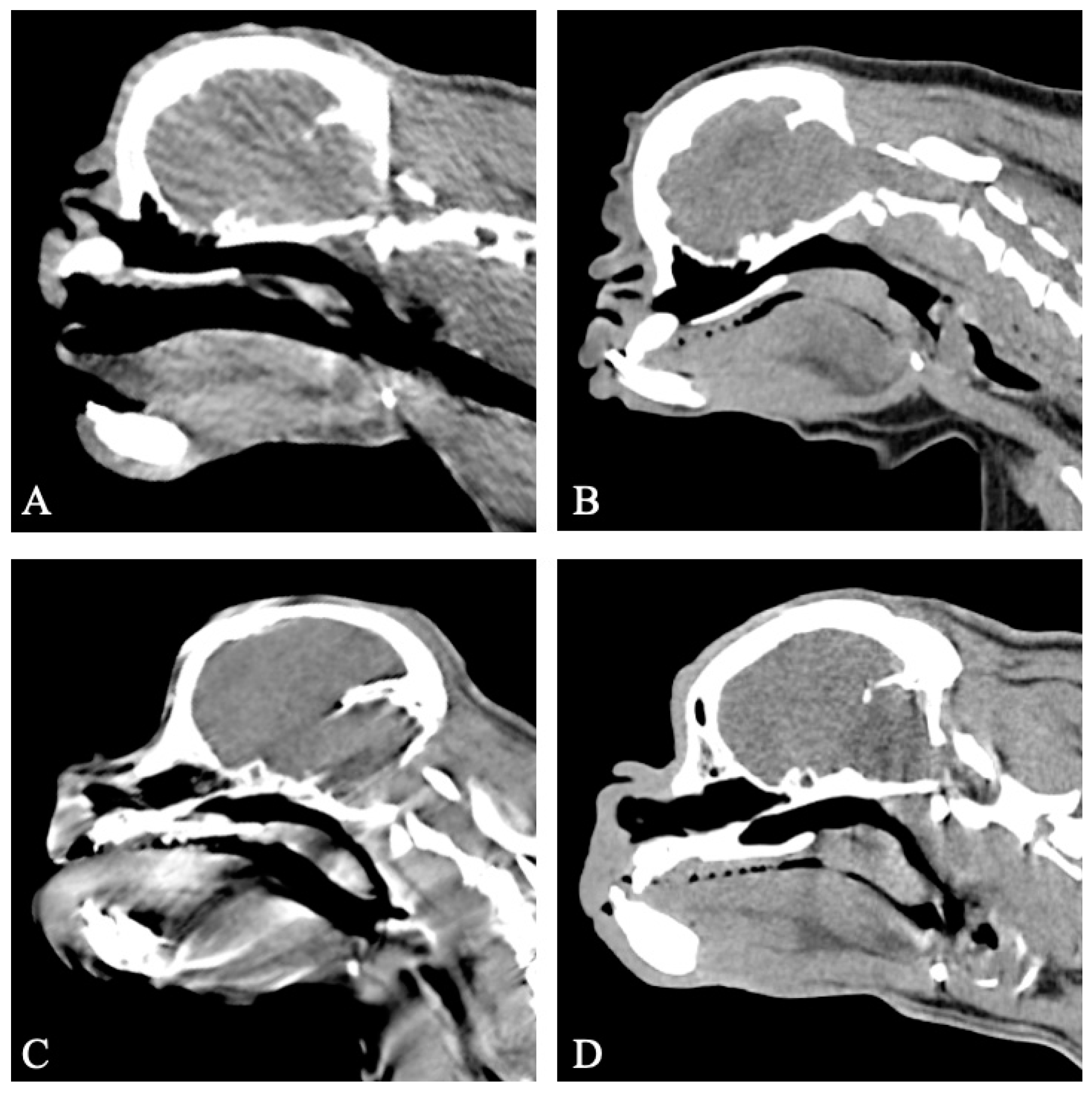
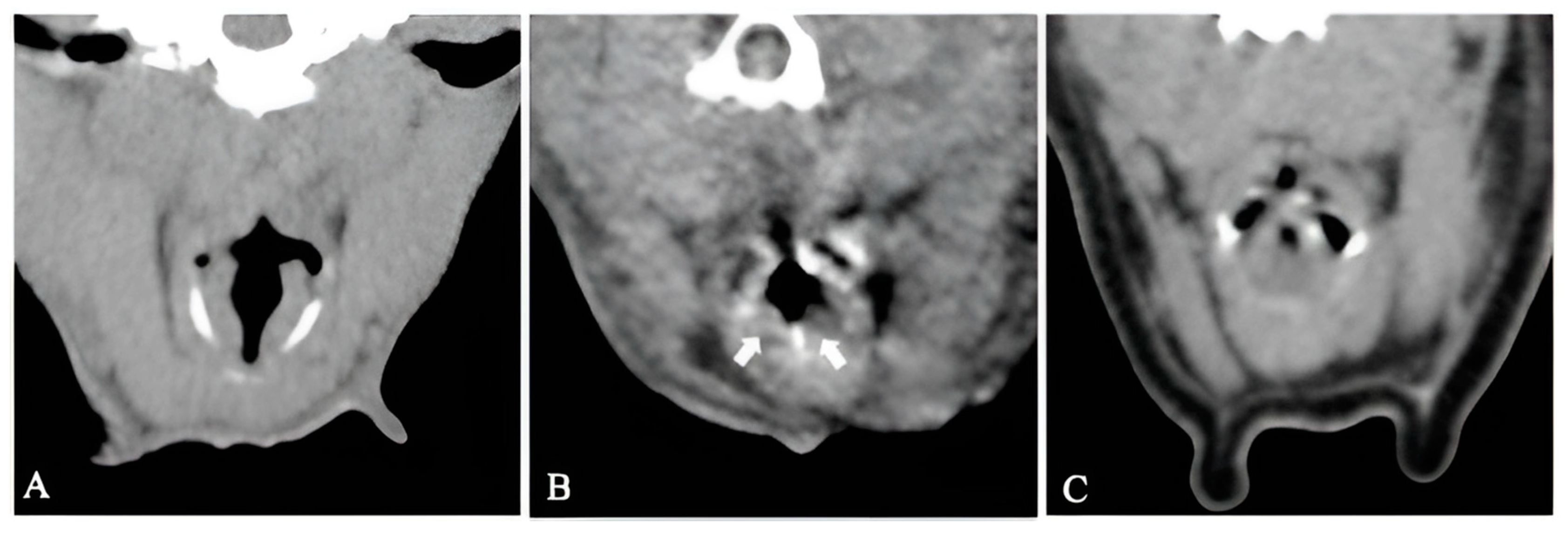
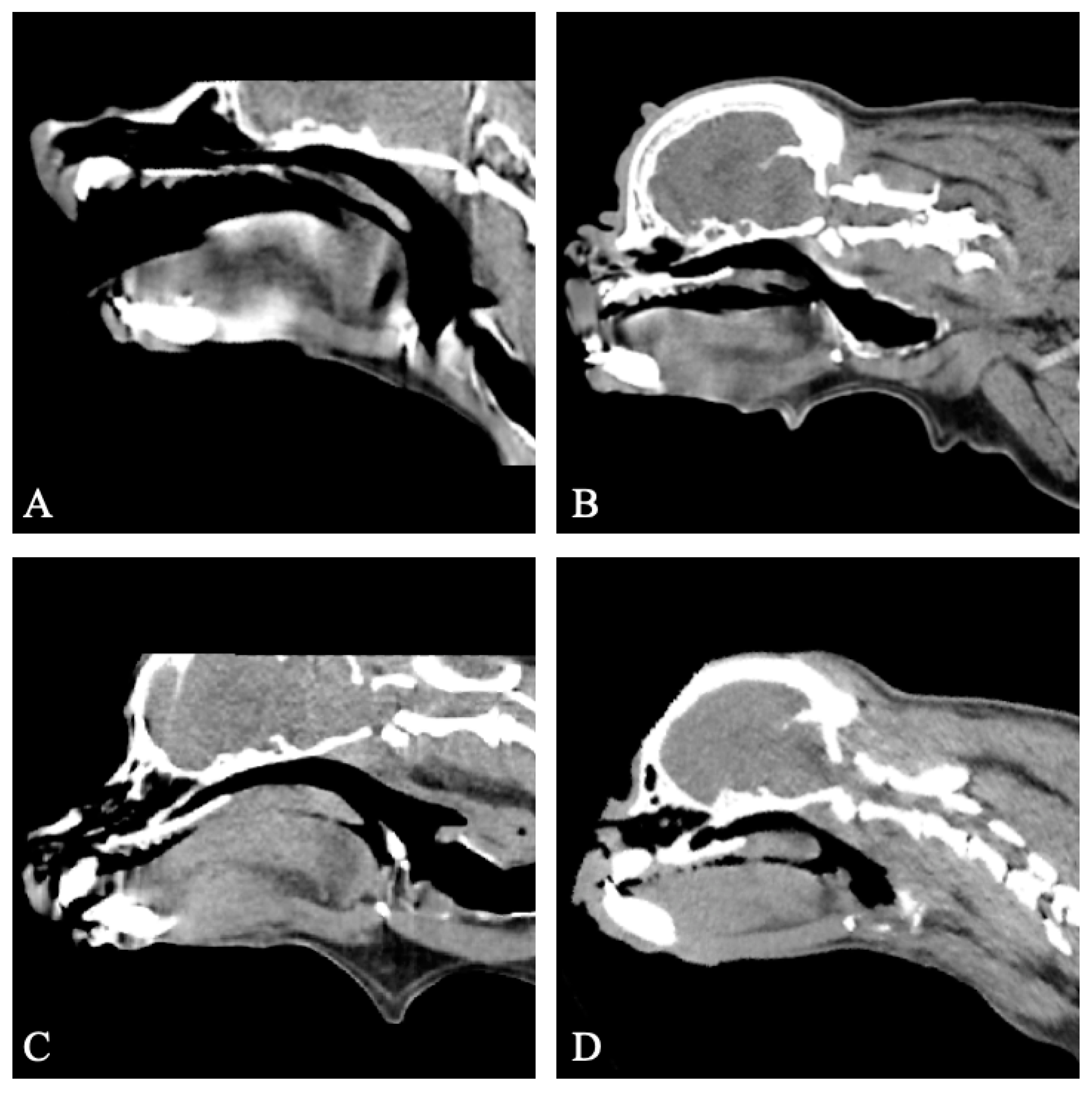
3.3. Computed Tomography Findings
3.3.1. Group I
3.3.2. Group II
3.3.3. Group III
3.4. Upper Airway Examination
3.4.1. Group I
3.4.2. Group II
3.4.3. Group III
3.5. Endoscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ewart, S.L. Overview of Respiratory Function: Ventilation of the Lungs. In Cunningham’s Textbook of Veterinary Physiology, 6th ed.; Klein, B.G., Ed.; Elsevier: Missouri, United States of America, 2020; pp. 518–531. [Google Scholar]
- Auger, M.; Alexander, K.; Beauchamp, G.; Dunn, M. Use of CT to evaluate and compare intranasal features in brachycephalic and normocephalic dogs. J. Small Anim. Pract. 2016, 57, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, D.; Gradner, G.; Kneissl, S.; Dupre, G. Nasopharyngeal Dimensions From Computed Tomography of Pugs and French Bulldogs With Brachycephalic Airway Syndrome. Vet. Surg. 2016, 45, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Deprez, P.; Irubetagoyena, I.; Grand, J.G.; Harran, N. Intraobserver and interobserver reliability of computed tomography measurements of the soft palate in French bulldogs. Vet. Rec. Open 2019, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Grand, J.G.; Bureau, S. Structural characteristics of the soft palate and meatus nasopharyngeus in brachycephalic and non-brachycephalic dogs analysed by CT. J. Small Anim. Pract. 2011, 52, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Kaye, B.M.; Boroffka, S.A.; Haagsman, A.N.; Ter Haar, G. Computed Tomographic, Radiographic, and Endoscopic Tracheal Dimensions in English Bulldogs with Grade 1 Clinical Signs of Brachycephalic Airway Syndrome. Vet. Radiol. Ultrasound 2015, 56, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Oechtering, T.H.; Oechtering, G.U.; Nöller, C. Strukturelle Besonderheiten der Nase brachyzephaler Hunderassen in der Computertomographie. Tierärztliche Prax. Kleintiere/Heimtiere 2007, 35, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, L.; Beever, L.; Bruce, M.; Ter Haar, G. Assessment of computed tomography derived cricoid cartilage and tracheal dimensions to evaluate degree of cricoid narrowing in brachycephalic dogs. Vet. Radiol. Ultrasound 2017, 58, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Siedenburg, J.S.; Dupre, G. Tongue and Upper Airway Dimensions: A Comparative Study between Three Popular Brachycephalic Breeds. Animals 2021, 11, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Wijsman, S.; Ter Haar, G.; Hertog, E.D.; Boroffka, S. Rostral nasopharyngeal CT measurements in Chihuahuas and Pomeranians are smaller than those measured in Dachshunds. Vet. Radiol. Ultrasound 2023, 64, 201–210. [Google Scholar] [CrossRef]
- Reimegård, E.; Lee, H.T.N.; Westgren, F. Prevalence of lung atelectasis in sedated dogs examined with computed tomography. Acta Vet. Scand. 2022, 64, 25–33. [Google Scholar] [CrossRef]
- Holopainen, S.; Rautala, E.; Lilja-Maula, L.; Lohi, H.; Rajamaki, M.M.; Lappalainen, A.K. Thoracic high resolution CT using the modified VetMousetrap device is a feasible method for diagnosing canine idiopathic pulmonary fibrosis in awake West Highland White Terriers. Vet. Radiol. Ultrasound 2019, 60, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.; Hussein, K.H.; Woo, H.; Park, K. Emphysematous pyelonephritis associated with calcium oxalate uroliths detected on computed tomography in an awake dog. Korean J. Vet. Res. 2020, 60, 93–96. [Google Scholar] [CrossRef]
- Lee, K.; Heng, H.G.; Jeong, J.; Naughton, J.F.; Rohleder, J.J. Feasibility of computed tomography in awake dogs with traumatic pelvic fracture. Vet. Radiol. Ultrasound 2012, 53, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jeong, J.; Heng, H.G.; Sung, S.; Choi, Y.; Oh, H.; Kim, K.; Cho, Y.; Jung, Y.; Lee, K. Computed tomographic features of tracheal shapes and dimensions in awake dogs. Veterinární Medicína 2018, 63, 131–136. [Google Scholar] [CrossRef]
- Ngwenyama, T.R.; Herring, J.M.; O’Brien, M.; Hartman, S.K.; Galloway, K.A.; O’Brien, R.T. Contrast-enhanced multidetector computed tomography to diagnose pulmonary thromboembolism in an awake dog with pyothorax. J. Vet. Emerg. Crit. Care 2014, 24, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Ranallo, F.N.; Pijanowski, G.J.; Mitchell, M.A.; O’Brien, M.A.; McMichael, M.; Hartman, S.K.; Matheson, J.S.; O’Brien, R.T. The Vetmousetrap™: A Device for Computed Tomographic Imaging of the Thorax of Awake Cats. Vet. Radiol. Ultrasound 2010, 52, 41–52. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Mitchell, M.A.; O’Brien, R.T. Thoracic computed tomography in feline patients without use of chemical restraint. Vet. Radiol. Ultrasound 2011, 52, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Shanaman, M.M.; Hartman, S.K.; O’Brien, R.T. Feasibility for using dual-phase contrast-enhanced multi-detector helical computed tomography to evaluate awake and sedated dogs with acute abdominal signs. Vet. Radiol. Ultrasound 2012, 53, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Hartman, S.; Matheson, J.; O’Brien, R. Computed tomographic imaging of dogs with primary laryngeal or tracheal airway obstruction. Vet. Radiol. Ultrasound 2011, 52, 377–384. [Google Scholar] [CrossRef]
- Stadler, K.; O’Brien, R. Computed tomography of nonanesthetized cats with upper airway obstruction. Vet. Radiol. Ultrasound 2013, 54, 231–236. [Google Scholar] [CrossRef]
- Tomo, Y.; Edamura, K.; Yamazaki, A.; Tanegashima, K.; Seki, M.; Asano, K.; Tinga, S.; Hayashi, K. Evaluation of Hindlimb Deformity and Posture in Dogs with Grade 2 Medial Patellar Luxation during Awake Computed Tomography Imaging while Standing. Vet. Comp. Orthop. Traumatol. 2022, 35, 143–151. [Google Scholar] [CrossRef]
- Poncet, C.M.; Dupre, G.P.; Freiche, V.G.; Bouvy, B.M. Long-term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. J. Small Anim. Pract. 2006, 47, 137–142. [Google Scholar] [CrossRef]
- Harvey, C.E.; Fink, E.A. Tracheal diameter: Analysis of radiographic measurements in brachycephalic and nonbrachycephalic dogs. J Am Anim Hosp Assoc. 1982, 18, 570–576. [Google Scholar]
- Leonard, H.C. Collapse of the larynx and adjacent structures in the dog. J. Am. Vet. Med. Assoc. 1960, 137, 360–363. [Google Scholar]
- Oshita, R.; Katayose, S.; Kanai, E.; Takagi, S. Assessment of Nasal Structure Using CT Imaging of Brachycephalic Dog Breeds. Animals 2022, 12, 1636–1644. [Google Scholar] [CrossRef]
- Eastwood, P.R.; Platt, P.R.; Shepherd, K.; Maddison, K.; Hillman, D.R. Collapsibility of the Upper Airway at Different Concentrations of Propofol Anesthesia. Anesthesiology 2005, 103, 470–477. [Google Scholar] [CrossRef]
- Drummond, G.B. Influence of thiopentone on upper airway muscles. Br. J. Anaesth. 1989, 63, 12–21. [Google Scholar] [CrossRef]
- Eastwood, P.R.; Szollosi, I.; Platt, P.R.; Hillman, D.R. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology 2002, 97, 786–793. [Google Scholar] [CrossRef]
- Crawford, M.W.; Rohan, D.; Macgowan, C.K.; Yoo, S.J.; Macpherson, B.A. Effect of propofol anesthesia and continuous positive airway pressure on upper airway size and configuration in infants. Anesthesiology 2006, 105, 45–50. [Google Scholar] [CrossRef]
- Nishino, T.; Shirahata, M.; Yonezawa, T.; Honda, Y. Comparison of changes in the hypoglossal and the phrenic nerve activity in response to increasing depth of anesthesia in cats. Anesthesiology 1984, 60, 19–24. [Google Scholar] [CrossRef]
- Ochiai, R.; Guthrie, R.D.; Motoyama, E.K. Effects of varying concentrations of halothane on the activity of the genioglossus, intercostals, and diaphragm in cats: An electromyographic study. Anesthesiology 1989, 70, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.S. Thoracic CT technique in children. J. Thorac. Imaging 2001, 16, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Frush, D.P.; Donnelly, L.F. Helical CT in children: Technical considerations and body applications. Radiology 1998, 209, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Pappas, J.N.; Donnelly, L.F.; Frush, D.P. Reduced frequency of sedation of young children with multisection helical CT. Radiology 2000, 215, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Z.; Ma, J.; Hong, Y.; Pi, Z.; Qu, X.; Xu, M.; Li, J.; Zhou, H. Imaging the Infant Chest without Sedation: Feasibility of Using Single Axial Rotation with 16-cm Wide-Detector CT. Radiology 2018, 286, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, A.; Carraccio, C.; Giardino, A.; Harris, R.H. Sedation for pediatric CT scanning: Is radiology becoming a drug-free zone? Pediatr. Emerg. Care 2005, 21, 295–297. [Google Scholar] [CrossRef]
- Gottumukkala, R.V.; Kalra, M.K.; Tabari, A.; Otrakji, A.; Gee, M.S. Advanced CT Techniques for Decreasing Radiation Dose, Reducing Sedation Requirements, and Optimizing Image Quality in Children. Radiographics 2019, 39, 709–726. [Google Scholar] [CrossRef]
- Miller, N.A.; Gregory, J.S.; Semple, S.I.; Aspden, R.M.; Stollery, P.J.; Gilbert, F.J. The effects of humming and pitch on craniofacial and craniocervical morphology measured using MRI. J. Voice 2012, 26, 90–101. [Google Scholar] [CrossRef]
- Mitchinson, A.G.; Yoffey, J.M. Changes in the vocal folds in humming low and high notes. A radiographic study. J. Anat. 1948, 82, 88–92. [Google Scholar]
- Ha, Y.; Kim, J.; Chung, K.; Yoon, H.; Eom, K. Fluoroscopic evaluation of laryngopharyngeal anatomic variations attributable to head posture in dogs. Am. J. Vet. Res. 2021, 82, 55–62. [Google Scholar] [CrossRef]
| Case | Sex | Age (Months) | Breed | Condition | CT Findings | Direct Laryngoscopy Findings | Endoscopy Findings |
|---|---|---|---|---|---|---|---|
| 1 † | MN | 19 | French Bulldog | BOAS | Normal SP length. Moderate SP thickening. Open larynx. | ||
| 2 ‡ | MN | 17 | Chihuahua | Persistent coughing | Normal SP length. Normal SP thickness. Open larynx. | ||
| 3 * | FS | 64 | Pug | BOAS | Overlong SP. Moderate SP thickening. Severe LC. Diffuse oesophageal gas dilation. | ||
| 4 ‡ | FS | 148 | Cocker Spaniel | Nasopharyngeal narrowing | Normal SP length. Normal SP thickness. Open larynx. Resolution of nasopharyngeal narrowing. Unilateral otitis media. | Normal SP length. Normal SP thickness. Normal larynx. Everted tonsils. | |
| 5 † | FS | 99 | CKCS | BOAS | Normal SP length. Normal SP thickness. Open larynx. | ||
| 6 * | MN | 13 | Pug | BOAS | Overlong SP. Mild SP thickening Everted LS Aberrant caudal nasal conchae | Overlong SP. Thickened SP. Stage 1 LC. Everted tonsils. | |
| 7 * | MN | 15 | French Bulldog | BOAS | Overlong SP. Marked SP thickening. Open larynx. | Overlong SP. Thickened SP. Normal larynx. Everted tonsils. | |
| 8 ‡ | FS | 30 | CKCS | Sleep apnoea | Overlong SP. Mild SP thickening. Open larynx. | Normal SP length. Normal SP thickness. Normal larynx. Everted tonsils. | |
| 9 * | M | 28 | French Bulldog | BOAS | Overlong SP. Marked SP thickening. Everted LS. Unilateral otitis media. | ||
| 10 * | F | 3 | French Bulldog | BOAS | Overlong SP. Moderate SP thickening. Open larynx. Tracheal hypoplasia. Unilateral otitis media. | Overlong SP. Thickened SP. Stage 1 LC. Everted tonsils. | |
| 11 * | MN | 47 | French Bulldog | BOAS | Overlong SP. Marked SP thickening. Open larynx. | Overlong SP. Thickened SP. Stage 1 LC. Everted tonsils | |
| 12 ‡ | FS | 47 | Maltese | Nasopharyngeal narrowing and tracheal collapse | Overlong SP. Mild SP thickening. Open larynx. Resolution of nasopharyngeal narrowing. Moderate tracheal collapse. | Normal SP length. Normal SP thickness. Normal larynx. | Resolution of nasopharyngeal narrowing. Grade 2 tracheal collapse. |
| 13 ‡ | MN | 50 | Mixed breed | Reversed sneezing | Overlong SP. Mild SP thickening. Open larynx. | ||
| 14† | FS | 150 | CKCS | BOAS | Normal SP length. Mild SP thickening. Open larynx. | ||
| 15 * | MN | 57 | Pug | BOAS | Overlong SP. Moderate SP thickening. Severe LC Aberrant caudal nasal conchae. | Overlong SP. Thickened SP. Stage 3 LC | |
| 16 ‡ | M | 67 | Pomeranian | Tracheal collapse | Normal SP length. Normal SP thickness. Open larynx. Severe tracheal collapse. | Normal SP length. Normal SP thickness. Normal larynx. | Grade 4 tracheal collapse. |
| 17 † | MN | 104 | French Bulldog | BOAS | Normal SP length. Mild SP thickening. Everted LS. Advanced pulmonary metastatic disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stordalen, M.B.; Bray, S.; Stringer, F.; Stonebrook, C.; Guilherme, S.; Bray, J.P. Awake 160-Slice Computed Tomography for Upper Airway Evaluation in 17 Dogs. Vet. Sci. 2024, 11, 342. https://doi.org/10.3390/vetsci11080342
Stordalen MB, Bray S, Stringer F, Stonebrook C, Guilherme S, Bray JP. Awake 160-Slice Computed Tomography for Upper Airway Evaluation in 17 Dogs. Veterinary Sciences. 2024; 11(8):342. https://doi.org/10.3390/vetsci11080342
Chicago/Turabian StyleStordalen, Marius B., Sharyn Bray, Felicity Stringer, Callum Stonebrook, Sergio Guilherme, and Jonathan P. Bray. 2024. "Awake 160-Slice Computed Tomography for Upper Airway Evaluation in 17 Dogs" Veterinary Sciences 11, no. 8: 342. https://doi.org/10.3390/vetsci11080342
APA StyleStordalen, M. B., Bray, S., Stringer, F., Stonebrook, C., Guilherme, S., & Bray, J. P. (2024). Awake 160-Slice Computed Tomography for Upper Airway Evaluation in 17 Dogs. Veterinary Sciences, 11(8), 342. https://doi.org/10.3390/vetsci11080342






