Bilateral Global Nephrocalcinosis in a Uremic Puppy
Abstract
Simple Summary
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical Findings
2.2. Laboratory Findings
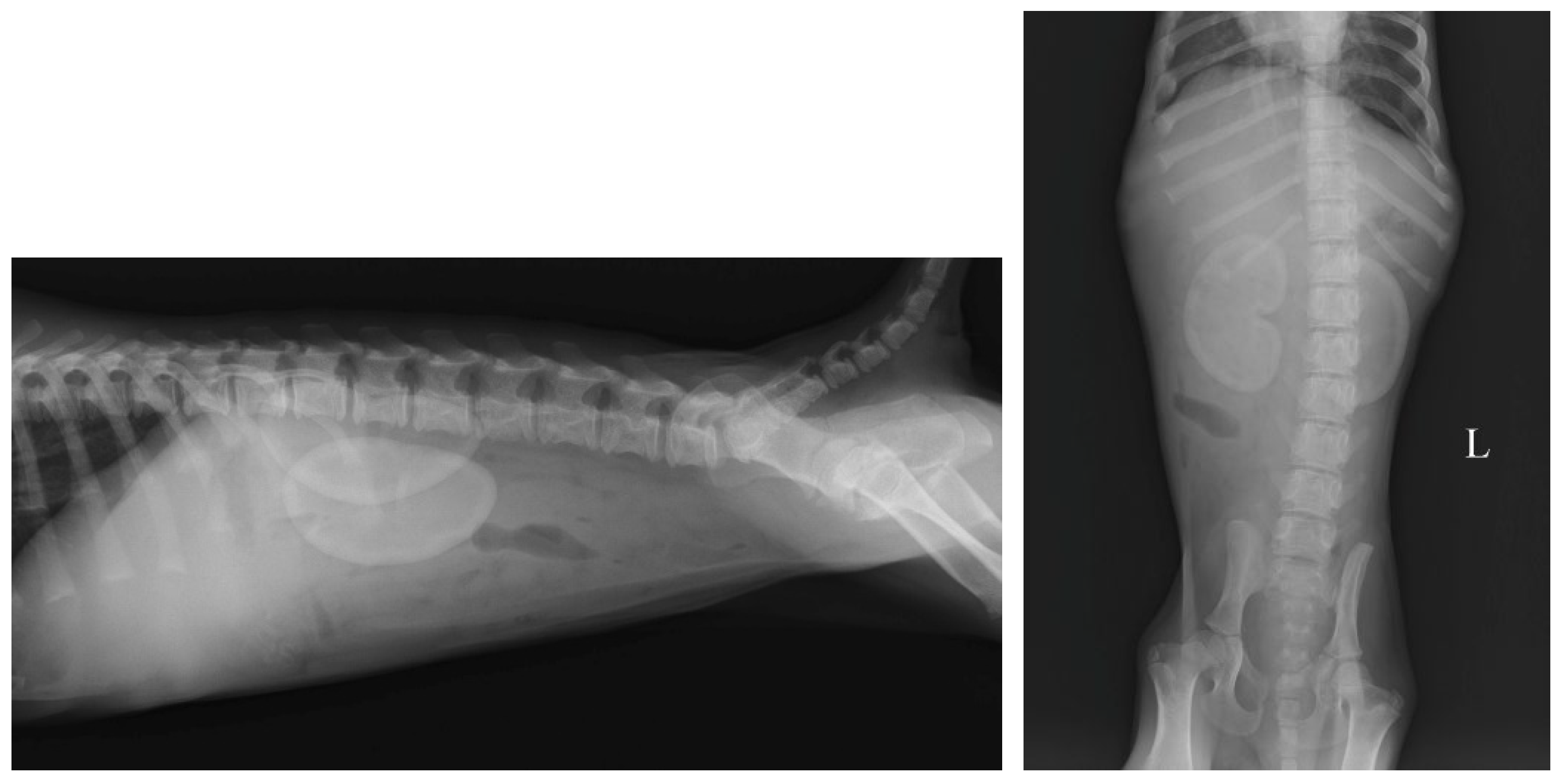
2.3. Imaging Findings
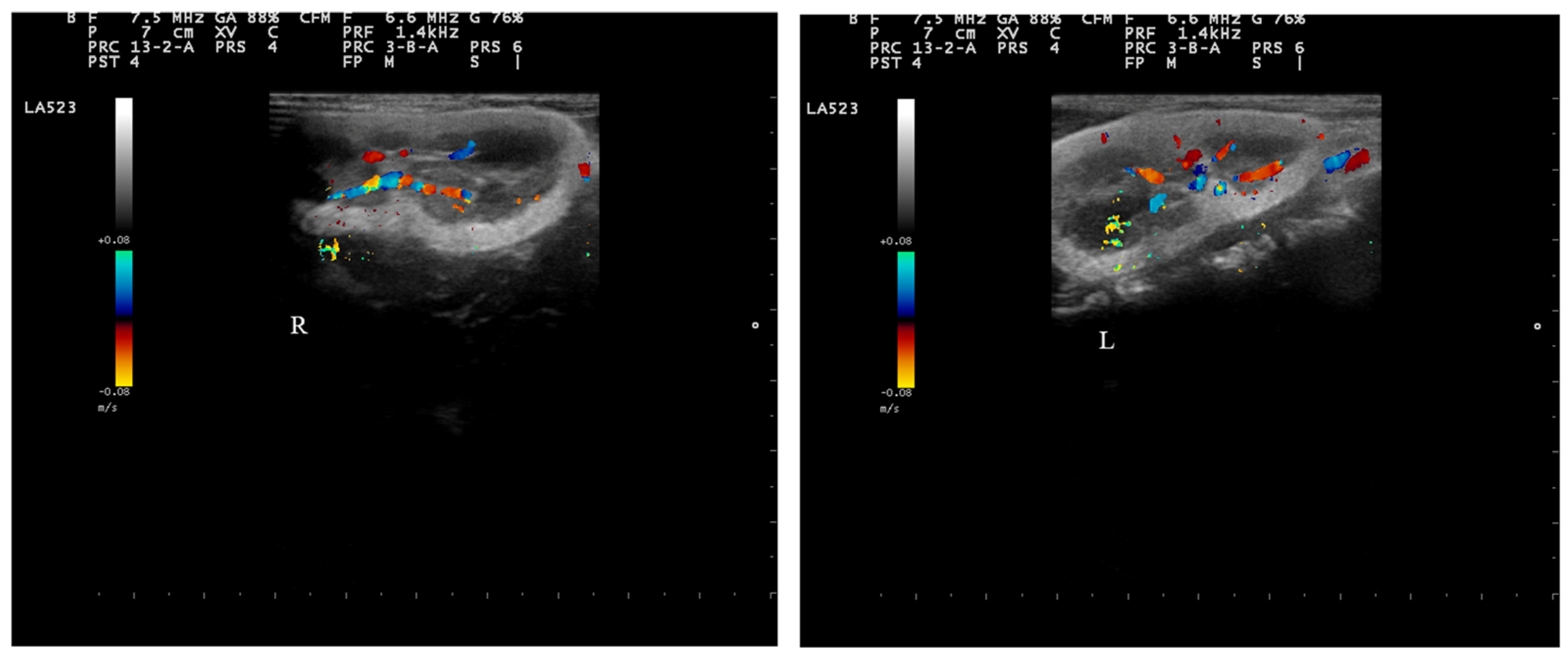
2.4. Doppler Examination
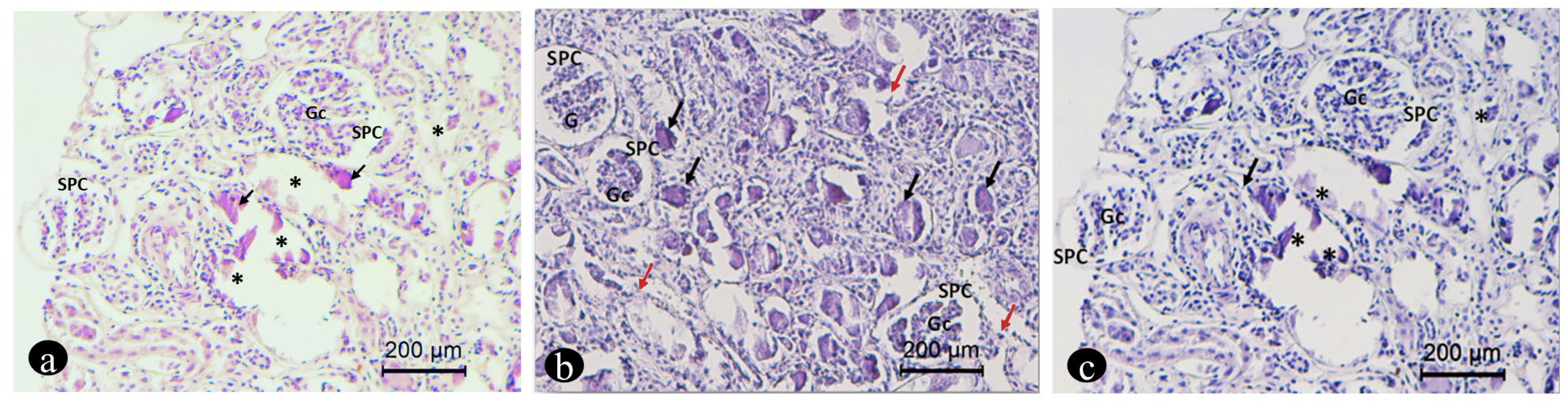
2.5. Renal Biopsy and Histology
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rørtveit, R.; Vigdís Eggertsdóttir, A.; Thomassen, R.; Lingaas, F.; Høgset Jansen, J. A clinical study of canine collagen type III glomerulopathy. BMC Vet. Res. 2013, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Segev, G.; Cortellini, S.; Foster, J.D.; Francey, T.; Langston, C.; Londoño, L.; Schweighauser, A.; Jepson, R.E. International Renal Interest Society best practice consensus guidelines for the diagnosis and management of acute kidney injury in cats and dogs. Vet. J. 2024, 305, 106068. [Google Scholar] [CrossRef] [PubMed]
- International Renal Interest Society. 2016. Available online: http://www.iris-kidney.com/guidelines/grading.html (accessed on 4 July 2024).
- Peeters, D.; Clercx, C.; Michiels, L.; Desmecht, D.; Snaps, F.; Henroteaux, M.; Daya, M.J. Juvenile nephropathy in a Boxer, a Rottweiler, a Collie and an Irish Wolfhound. Aust. Vet. J. 2000, 78, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Maxie, M.G. The urinary system: Anomalies of development. In Pathology of Domestic Animals, 4th ed.; Jubb, K.V.F., Kennedy, P.C., Palmer, N., Eds.; Academic Press: London, UK, 1993; Volume 2, pp. 459–468. [Google Scholar]
- Bartges, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. Small Anim. Pract. 2012, 42, 669–692. [Google Scholar] [CrossRef]
- Geddes, R.F.; Finch, N.C.; Syme, H.M.; Elliott, J. The role of phosphorus in the pathophysiology of chronic kidney disease. J. Vet. Emerg. Crit. Care 2013, 23, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.D. Update on mineral and bone disorders in chronic kidney disease. Vet. Clin. Small Anim. Pract. 2016, 46, 1131–1149. [Google Scholar] [CrossRef] [PubMed]
- Rudinsky, A.J.; Harjes, L.M.; Byron, J.; Chew, D.J.; Toribio, R.E.; Langston, C.; Parker, V.J. Factors associated with survival in dogs with chronic kidney disease. J. Vet. Intern. Med. 2018, 32, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Lippi, I.; Guidi, G.; Marchetti, V.; Tognetti, R.; Meucci, V. Prognostic role of the product of serum calcium and phosphorus concentrations in dogs with chronic kidney disease: 31 cases (2008–2010). J. Am. Vet. Med. Assoc. 2014, 245, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Relford, R.; Robertson, J.; Clements, C. Symmetric Dimethylarginine: Improving the Diagnosis and Staging of Chronic Kidney Disease in Small Animals. Vet. Clin. Small Anim. Pract. 2016, 46, 941–960. [Google Scholar] [CrossRef] [PubMed]
- International Renal Interest Society Guidelines. 2019. Available online: http://www.iriskidney.com/pdf/staging-of-ckd.pdf (accessed on 22 December 2022).
- Shavit, L.; Jaeger, P.; Unwin, R.J. What is nephrocalcinosis? Kidney Int. 2015, 88, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner-Sigl, S.; Haberlandt, E.; Mumm, S.; Scholl-Burgi, S.; Sergi, C.; Ryan, L.; Ericson, K.L.; Whyte, M.P.; Högler, W. Pyridoxine-responsive seizures as the first symptom of infantile hypophosphatasia caused by two novel missense mutations of the tissue non-specific alkaline phosphatase gene. Bone 2007, 40, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Vervaet, B.A.; Verhulst, A.; D’Haese, P.C.; De Broe, M.E. Nephrocalcinosis: New insights into mechanisms and consequences. Nephrol. Dial. Transpl. 2009, 24, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Evan, A.P. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr. Nephrol. 2010, 25, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Nephrocalcinosis in animal models with and without stones. Urol. Res. 2010, 38, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Takahashi, Y.; Morita, N.; Moriyama, M.T.; Kosaka, T.; Nishio, M.; Yoshimoto, T.; Suzuki, K. Cyclooxygenase 2 and prostaglandin E2 regulate the attachment of calcium oxalate crystals to renal epithelial cells. Int. J. Urol. 2012, 19, 936–943. [Google Scholar] [CrossRef] [PubMed]
- La Scola, C.; Mencarelli, F.; Pasini, A.; Visentin, S.; Montini, G. Nefrocalcinosi in età pediatrica. G. Di Tec. Nefrol. E Dial. 2009, 21, 25–31. [Google Scholar] [CrossRef]
- Mulay, S.R.; Shi, C.; Ma, X.; Anders, H.J. Novel Insights into Crystal-Induced Kidney Injury. Kidney Dis. 2018, 4, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Schell-Feith, E.A.; Kistvan Holthe, J.E.; van der Heijden, A.J. Nephrocalcinosis in preterm neonates. Pediatr. Nephrol. 2010, 25, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Mhalhel, K.; Montalbano, G.; Giurdanella, G.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Germanà, A.; Levanti, M. Histological and Immunohistochemical Study of Gilt-head Seabream Tongue from the Early Stage of Development: TRPV4 Potential Roles. Ann. Anat. 2022, 244, 151985. [Google Scholar] [CrossRef] [PubMed]
- Mhalhel, K.; Briglia, M.; Aragona, M.; Porcino, C.; Abbate, F.; Guerrera, M.C.; Laurà, R.; Krichen, Y.; Guerbej, H.; Germanà, A.; et al. Nothobranchius as a Model for Anorexia of Aging Research: An Evolutionary, Anatomical, Histological, Immunohistochemical, and Molecular Study. Ann. Anat. 2023, 250, 152116. [Google Scholar] [CrossRef] [PubMed]
- Ammenti, A.; Pelizzoni, A.; Cecconi, M.; Molinari, P.P.; Montini, G. Nephrocalcinosis in children: A retrospective multicentre study. Acta Paediatr. 2009, 98, 1628–1631. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzi, M.G.; Jacobson-Dickman, E.; DeBoer, M.D.; Drugs and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: A review of current literature. J. Clin. Endocrinol. Metab. 2014, 99, 1132–1141. [Google Scholar] [CrossRef]
- Rönnefarth, G.; Misselwitz, J.; Members of APN. Nephrocalcinosis in children: A retrospective survey. Pediatr. Nephrol. 2000, 14, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, H.; Hsu, H.H.; Ogawa, M.; Akabane, R.; Miyagawa, Y.; Takemura, N. Association between serum fibroblast growth factor-23 concentration and development of hyperphosphatemia in normophosphatemic dogs with chronic kidney disease. J. Vet. Intern. Med. 2021, 35, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Crowell, W.A.; Barsanti, J.A.; White, J.V.; Finco, D.R. Beneficial effects of dietary mineral restriction in dogs with marked reduction of functional renal mass. J. Am. Soc. Nephrol. 1991, 1, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Pedrinelli, V.; Lima, D.M.; Duarte, C.N.; Teixeira, F.A.; Porsani, M.; Zarif, C.; Amaral, A.R.; Vendramini, T.H.A.; Kogika, M.M.; Brunetto, M.A. Nutritional and laboratory parameters affect the survival of dogs with chronic kidney disease. PLoS ONE 2020, 15, e0234712. [Google Scholar] [CrossRef]
- Reiss, A.B.; Miyawaki, N.; Moon, J.; Kasselman, L.J.; Voloshyna, I.; D’Avino, R., Jr.; De Leon, J. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 2018, 278, 49–59. [Google Scholar] [CrossRef]
- Morrow, C.K.; Volmer, P.A. Hypercalcemia, hyperphosphatemia and soft tissue mineralization. Compend. Contin. Educ. Pract. Vet. 2002, 24, 380–387. [Google Scholar]
- Kiefer-Hecker, B.; Bauer, A.; Dobenecker, B. Effects of low phosphorus intake on serum calcium, phosphorus, alkaline phosphatase activity and parathyroid hormone in growing dogs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- De Brito Galvao, J.F.; Nagode, L.A.; Schenck, P.A.; Chew, D.J. Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor-23 interactions in chronic kidney disease. J. Vet. Emerg. Crit. Care 2013, 23, 134–162. [Google Scholar] [CrossRef] [PubMed]
- Finco, D.R.; Brown, S.A.; Crowell, W.A.; Groves, C.A.; Duncan, J.R.; Barsanti, J.A. Effects of phosphorus/calcium-restricted and phosphorus/calcium-replete 32% protein diets in dogs with chronic renal failure. Am. J. Vet. Res. 1992, 53, 157–163. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, M.; Pennisi, M.; Macrì, F.; Falcone, A.; Di Pietro, S.; Mhalhel, K.; Giudice, E. Bilateral Global Nephrocalcinosis in a Uremic Puppy. Vet. Sci. 2024, 11, 338. https://doi.org/10.3390/vetsci11080338
Rizzo M, Pennisi M, Macrì F, Falcone A, Di Pietro S, Mhalhel K, Giudice E. Bilateral Global Nephrocalcinosis in a Uremic Puppy. Veterinary Sciences. 2024; 11(8):338. https://doi.org/10.3390/vetsci11080338
Chicago/Turabian StyleRizzo, Maria, Melissa Pennisi, Francesco Macrì, Annastella Falcone, Simona Di Pietro, Kamel Mhalhel, and Elisabetta Giudice. 2024. "Bilateral Global Nephrocalcinosis in a Uremic Puppy" Veterinary Sciences 11, no. 8: 338. https://doi.org/10.3390/vetsci11080338
APA StyleRizzo, M., Pennisi, M., Macrì, F., Falcone, A., Di Pietro, S., Mhalhel, K., & Giudice, E. (2024). Bilateral Global Nephrocalcinosis in a Uremic Puppy. Veterinary Sciences, 11(8), 338. https://doi.org/10.3390/vetsci11080338





