Case Series: Computed Tomography Features of Extraskeletal Osteosarcoma in Six Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Study Design
2.3. Data Collection, CT Examinations, and Image Analysis
3. Results
3.1. Patient Description
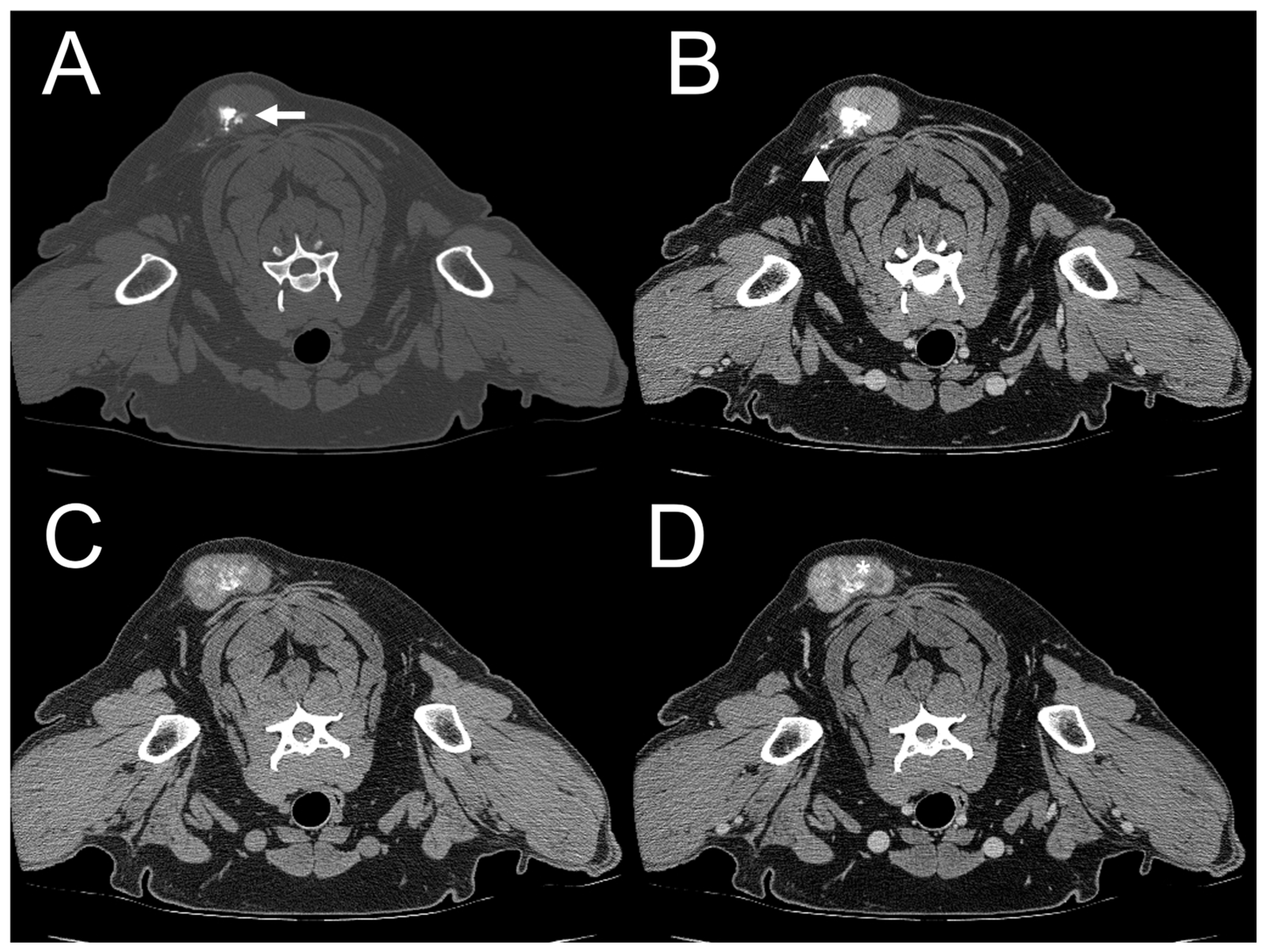
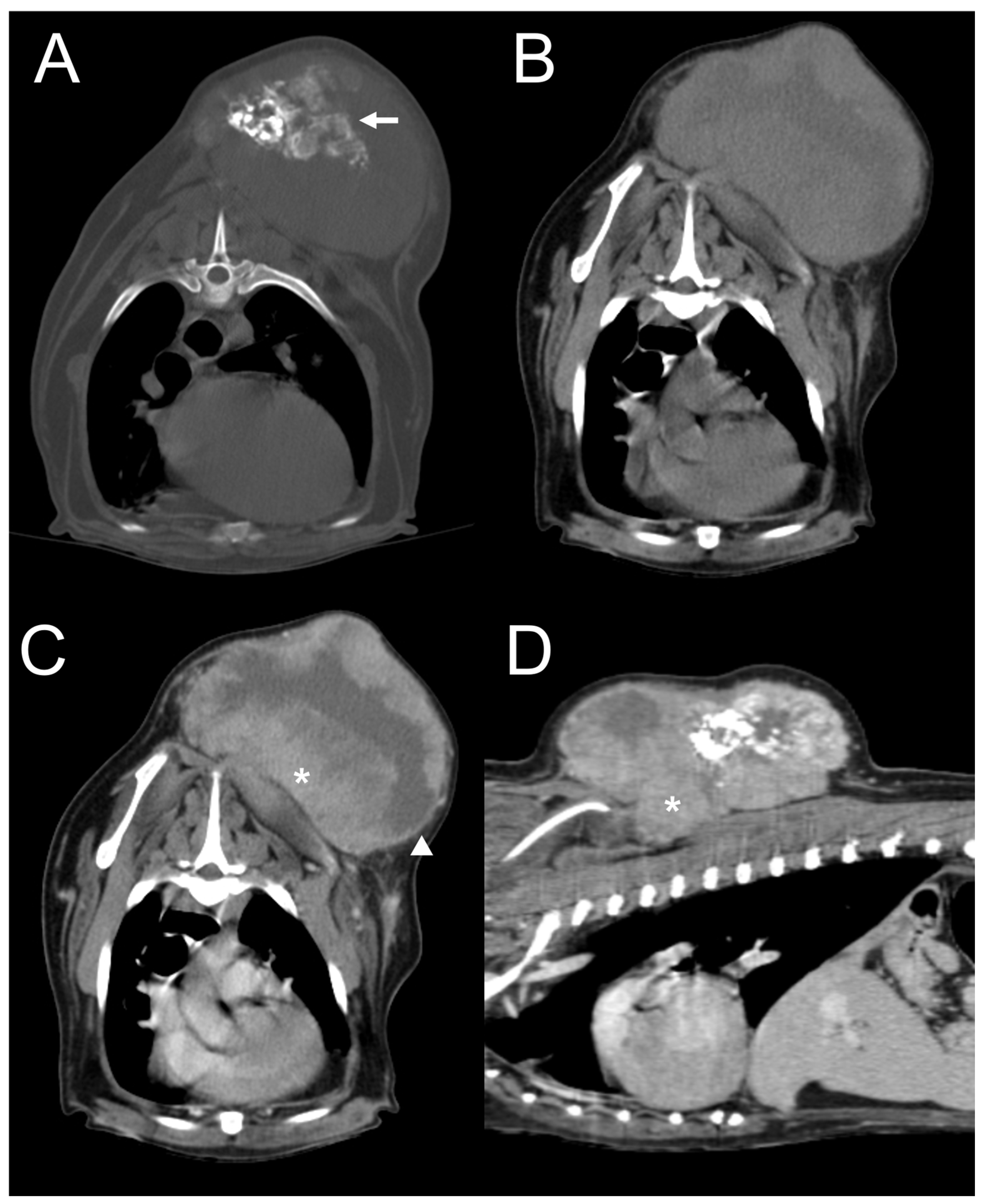
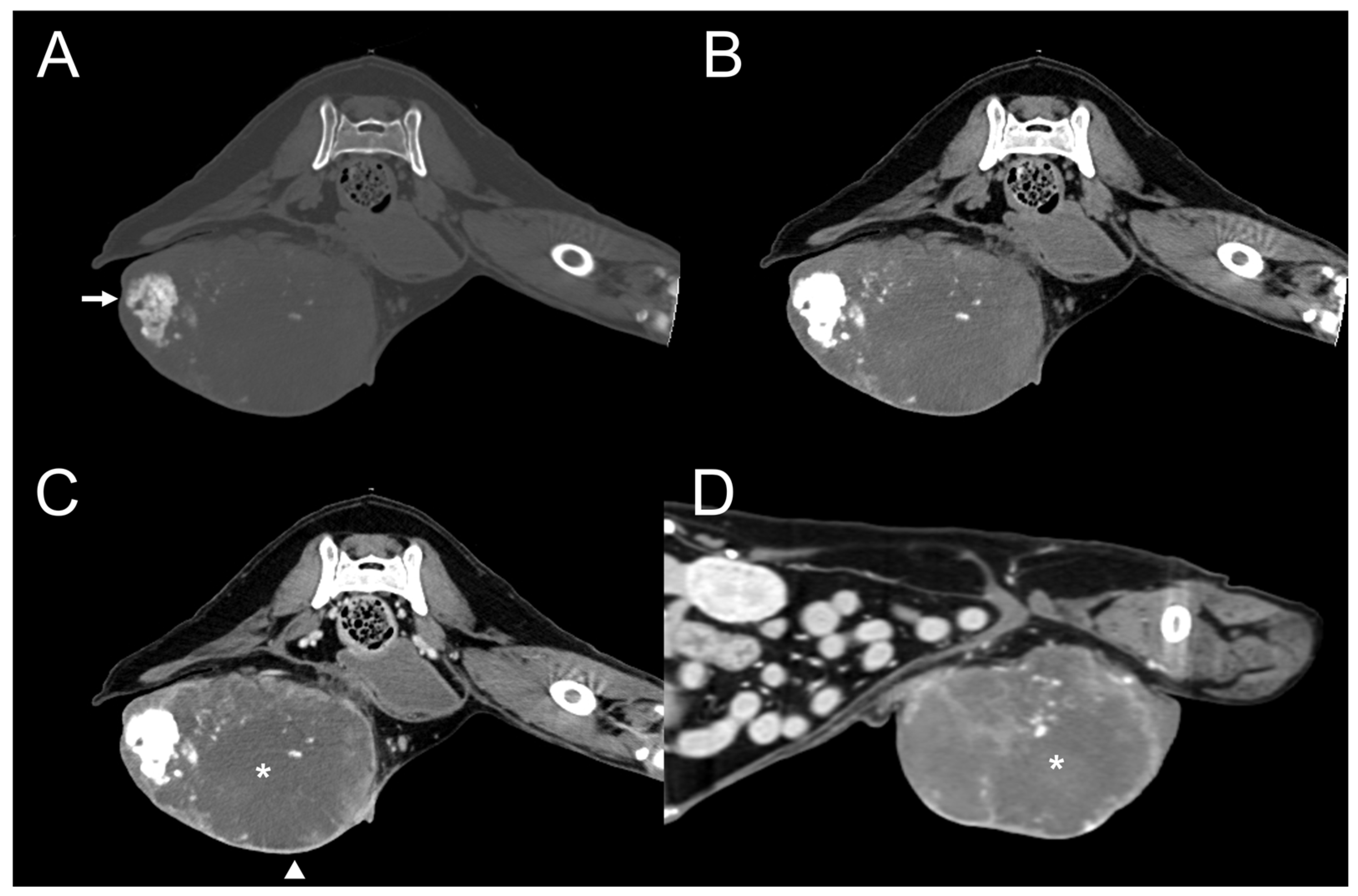
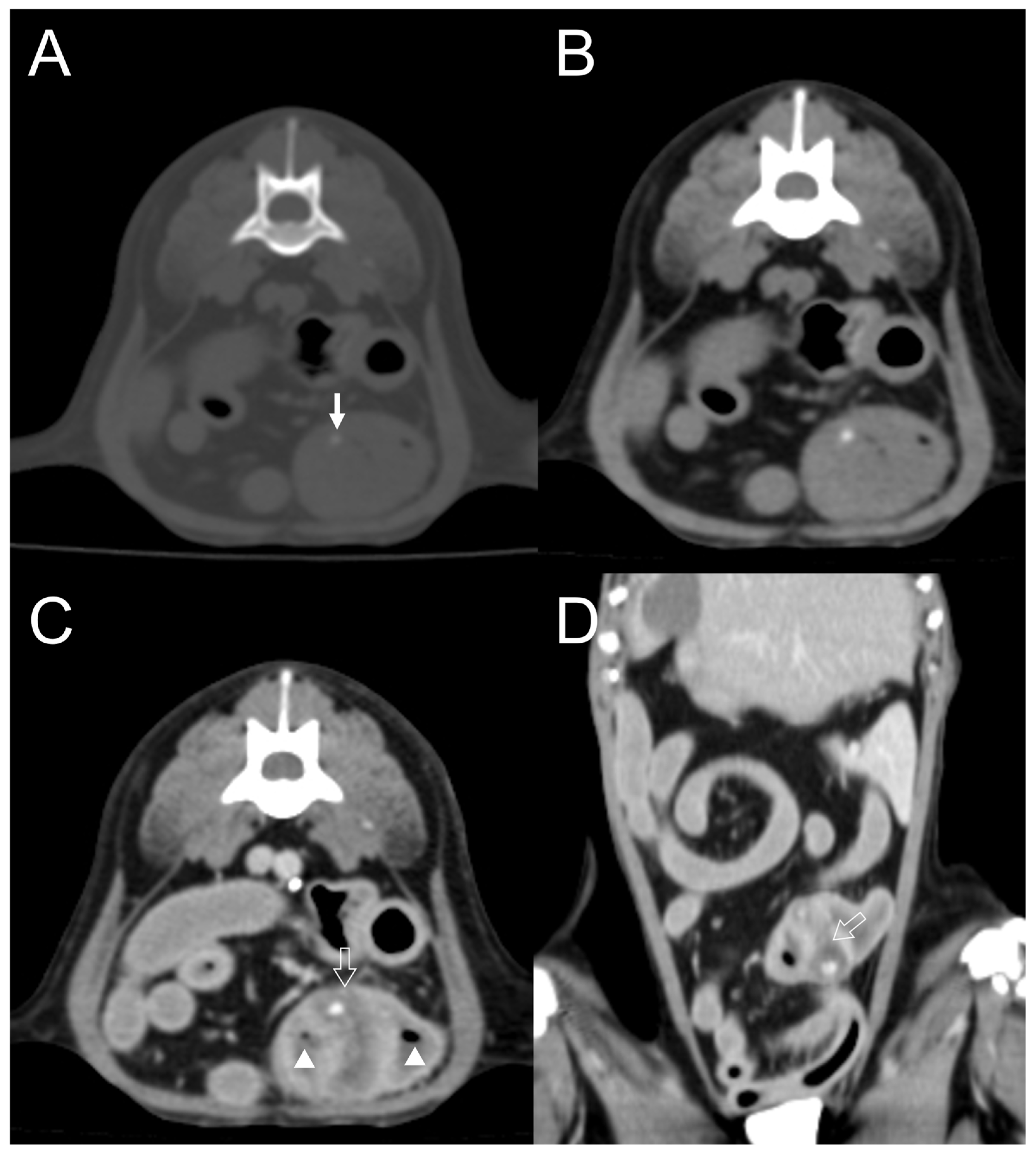
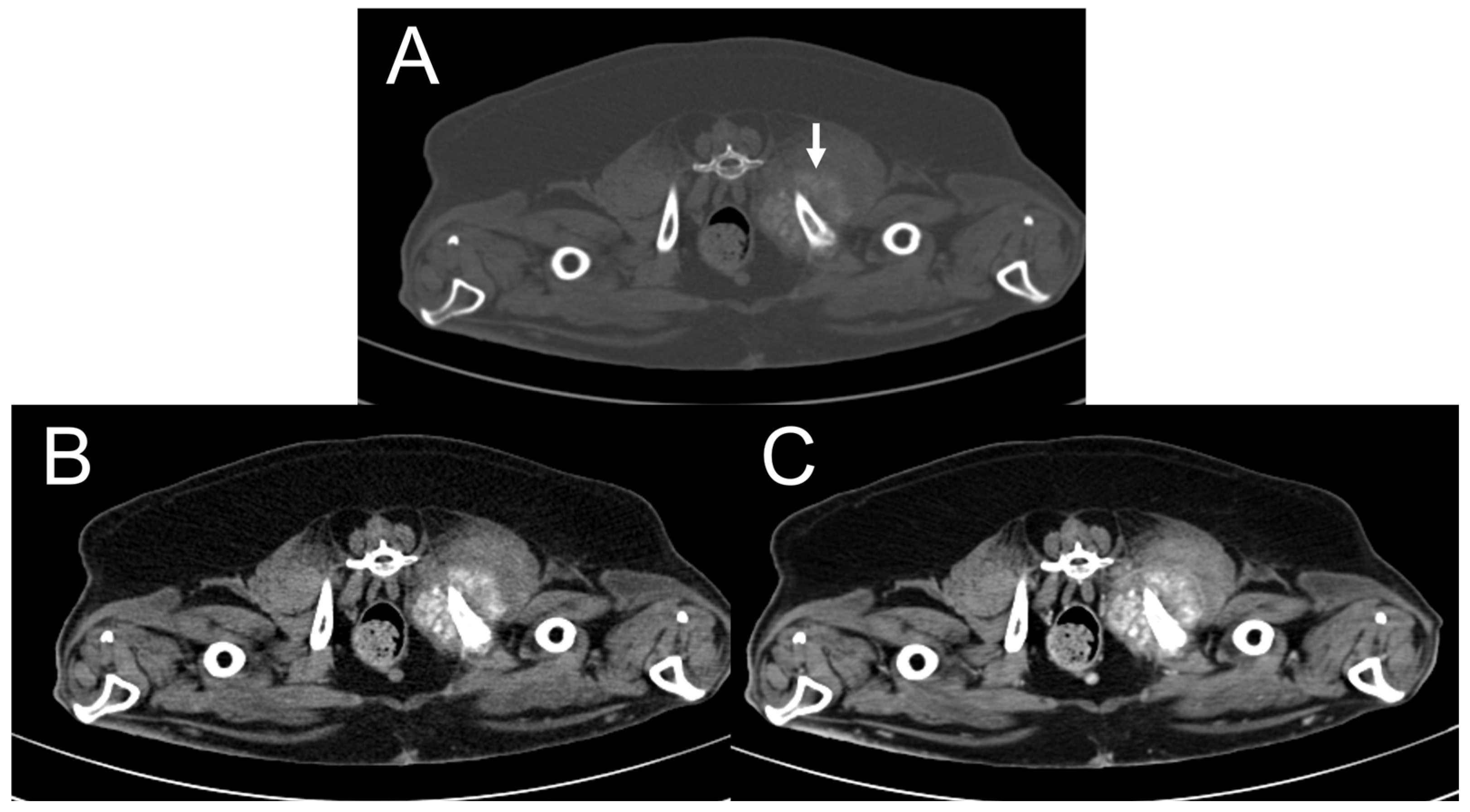
3.2. CT Features
3.3. Follow-up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brodey, R.S.; Riser, W.H. Canine osteosarcoma: A clinicopathologic study of 194 cases. Clin. Orthop. Relat. Res. 1969, 62, 54–64. [Google Scholar] [CrossRef]
- Spodnick, G.J.; Berg, J.; Rand, W.M.; Schelling, S.H.; Couto, G.; Harvey, H.J.; Henderson, R.A.; MacEwen, G.; Mauldin, N.; McCaw, D.L. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). J. Am. Vet. Med. Assoc. 1992, 200, 995–999. [Google Scholar] [CrossRef]
- Wolke, R.E.; Nielsen, S.W. Site incidence of canine osteosarcoma. J. Small Anim. Pract. 1966, 7, 489–492. [Google Scholar] [CrossRef]
- Thompson, K.G.; Dittmer, K.E. Tumors of bone. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 356–424. [Google Scholar]
- Banks, W.C. Parosteal osteosarcoma in a dog and a cat. J. Am. Vet. Med. Assoc. 1971, 158, 1412–1415. [Google Scholar]
- Patnaik, A.K. Canine extraskeletal osteosarcoma and chondrosarcoma: A clinicopathologic study of 14 cases. Vet. Pathol. 1990, 27, 46–55. [Google Scholar] [CrossRef]
- Langenbach, A.; Anderson, M.A.; Dambach, D.M.; Sorenmo, K.U.; Shofer, F.D. Extraskeletal osteosarcomas in dogs: A retrospective study of 169 cases (1986–1996). J. Am. Anim. Hosp. Assoc. 1998, 34, 113–120. [Google Scholar] [CrossRef]
- Kuntz, C.A.; Dernell, W.S.; Powers, B.E.; Withrow, S. Extraskeletal osteosarcomas in dogs: 14 cases. J. Am. Anim. Hosp. Assoc. 1998, 34, 26–30. [Google Scholar] [CrossRef]
- Rezende Souza, F.; de Morais Avelar, N.; Moreira Lopes, T.C.; Dantas Cassali, G.; Yumi Ribeiro Nakagaki, K. Extraskeletal osteosarcoma in the duodenum of a dog. Acta Sci. Vet. 2023, 51, 874. [Google Scholar]
- Alberti, T.D.; Zamboni, R.; Venancio, F.D.; Brunner, C.B.; Raffi, M.B.; Schild, A.L.; Sallis, E.S. Mediastinal extraskeletal osteosarcoma in a canine with pulmonary and cerebral metastasis. Acta Sci. Vet. 2021, 49, 626. [Google Scholar] [CrossRef]
- Johnson, C.; Kim, Y. Hepatic extraskeletal osteosarcoma with systemic metastasis in a dog. Korean J. Vet. Res. 2013, 53, 61–64. [Google Scholar] [CrossRef][Green Version]
- Miller, M.A.; Aper, R.L.; Fauber, A.; Blevins, W.E.; Ramos-Vara, J.A. Extraskeletal osteosarcoma associated with retained surgical sponge in a dog. J. Vet. Diagn. Investig. 2006, 18, 224–228. [Google Scholar] [CrossRef]
- Soares, C.T.; Medeiros, F.P.; Martins, R. Primary omentum extraskeletal osteosarcoma in a dog: Case report. Braz. J. Vet. Med. 2023, 45, e000423. [Google Scholar] [CrossRef]
- Sato, T.; Koie, H.; Shibuya, H.; Suzuki, K. Extraskeletal osteosarcoma in the pericardium of a dog. Vet. Rec. 2004, 155, 780–781. [Google Scholar]
- Riggers, D.S.; Rosati, M.; Köhler, C.; Matiasek, K.; Loderstedt, S. A case of extraosseous intradural osteosarcoma of the spine in a dog. Vet. Rec. 2022, 10, e740. [Google Scholar] [CrossRef]
- Kistler, K.R. Canine osteosarcoma: 1,462 cases reviewed to uncover patterns of height, weight, breed, sex, age and site of involvement. In Phi Zeta Awards; School of Veterinary Medicine, University of Pennsylvania: Philadelphia, PA, USA, 1981. [Google Scholar]
- Heyman, S.J.; Diefenderfer, D.L.; Goldschmidt, M.H.; Newton, C.D. Canine axial skeletal osteosarcoma. A retrospective study of 116 cases (1986 to 1989). Vet. Surg. 1992, 21, 304–310. [Google Scholar] [CrossRef]
- Schultz, R.M.; Wisner, E.R. Long bones. In Veterinary Computed Tomography, 1st ed.; Tobias, S., Jimmy, S., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2011; p. 385. [Google Scholar]
- Mc Auley, G.; Jagannathan, J.; O’Regan, K.; Krajewski, K.M.; Hornick, J.L.; Butrynski, J.; Ramaiya, N. Extraskeletal osteosarcoma: Spectrum of imaging findings. AJR Am. J. Roentgenol. 2012, 198, 31–37. [Google Scholar] [CrossRef]
- MacKenzie, S.; Hecht, S.; Sura, P.A.; Craig, L.E. What is your diagnosis? Extraskeletal osteosarcoma. J. Am. Vet. Med. Assoc. 2012, 240, 817–818. [Google Scholar] [CrossRef]
- Selmic, L.E.; Griffin, L.R.; Rector, M.H.; Lafferty, M.; Pool, R.; Ehrhart, N.P. Treatment of extraskeletal osteosarcoma at a previous injection site resulting in prolonged survival in 1 dog. Can. Vet. J. 2016, 57, 950–954. [Google Scholar]
- Umeda, N.; Yamazoe, H.; Wada, A.; Nagata, K. A dog with extraskeletal osteosarcoma of the salivary glands survived long-term, following surgical resection and adjuvant therapy. J. Vet. Med. Sci. 2023, 85, 358–362. [Google Scholar] [CrossRef]
- Garcia, M.R.; Gomes, B.; Irvine, K.; Rosa, C. Metastatic renal extraskeletal osteosarcoma in a dog: Clinical presentation, CT features and the role of ALP cytochemical staining for diagnosis. Vet. Rec. 2023, 11, e679. [Google Scholar] [CrossRef]
- Tremolada, G.; Griffin, L.; Manchester, A.C.; Aboellail, T.; Lapsley, J.M.; Selmic, L.E. Primary extraskeletal osteosarcoma of the post-hepatic caudal vena cava in a dog-Case report. Front. Vet. Sci. 2023, 10, 1197236. [Google Scholar] [CrossRef]
- Crombé, A.; Spinnato, P.; Righi, A.; Leopardi, M.P.; Carpenzano, M.; Izzo, F.; Parmeggiani, A.; Linck, P.A.; Perret, R.; Cesari, M.; et al. Imaging presentation of extraskeletal osteosarcomas on CT and MRI and correlation with patients outcome: A two-center retrospective of 54 patients. Diagn. Interv. Imaging 2023, 104, 297–306. [Google Scholar] [CrossRef]
- Fuerst, J.A.; Reichle, J.K.; Szabo, D.; Cohen, E.B.; Biller, D.S.; Goggin, J.M.; Griffin, J.F., IV; Aarsvold, S.; Emerson, S.E. Computed tomographic findings in 24 dogs with liposarcoma. Vet. Radiol. Ultrasound 2017, 58, 23–28. [Google Scholar] [CrossRef]
- Choi, H.; Kwon, Y.; Chang, J.; Jeong, S.; Lee, H.; Kim, J.; Jung, J.; Lee, Y. Undifferentiated pleomorphic sarcoma (malignant fibrous histiocytoma) of the head in a dog. J. Vet. Med. Sci. 2011, 73, 235–239. [Google Scholar] [CrossRef]
- Farmer, R.J.M.; Poirier, V.J.; Nykamp, S.; Jensen, M.; Foster, R.A.; Oblak, M.; Appleby, R. CT features of subcutaneous, intermuscular, and intramuscular mast cell tumors in dogs. Vet. Radiol. Ultrasound 2023, 64, 53–60. [Google Scholar] [CrossRef]
- Fukuda, S.; Kobayashi, T.; Robertson, I.D.; Oshima, F.; Fukazawa, E.; Nakano, Y.; Ono, S.; Thrall, D.E. Computed tomographic features of canine nonparenchymal hemangiosarcoma. Vet. Radiol. Ultrasound 2014, 55, 374–379. [Google Scholar] [CrossRef]
- Cordella, A.; Stock, E.; Bertolini, G.; Strohmayer, C.; Serra, G.D.; Saunders, J. CT features of primary bone neoplasia of the thoracic wall in dogs. Vet. Radiol. Ultrasound 2023, 64, 605–614. [Google Scholar] [CrossRef]
- Duffy, D.; Selmic, L.E.; Kendall, A.R.; Powers, B.E. Outcome following treatment of soft tissue and visceral extraskeletal osteosarcoma in 33 dogs: 2008–2013. Vet. Comp. Oncol. 2017, 15, 46–54. [Google Scholar] [CrossRef]




| Manufacturer | Model | CT Channel | Slice Thickness | Helical Pitch | Matrix Dimension | kVp | mAs | Number of Scans Performed | |
|---|---|---|---|---|---|---|---|---|---|
| Dog 1 | Toshiba | Alexion | 16 | 3 | 0.9 | 512 | 100 | 120 | 1 |
| Dog 2 | Siemens | Emotion 16 | 16 | 0.75 | 0.8 | 512 | 130 | 134 | 2 * |
| Dog 3 | Toshiba | Aquilion 64 | 64 | 1 | N/A | 512 | 120 | 150 | 1 |
| Dog 4 | GE | LightSpeed | 4 | 2.5 | 1.5 | 512 | 120 | 200 | 1 |
| Dog 5 | Toshiba | Alexion | 16 | 3 | 0.9 | 512 | 120 | 200 | 1 |
| Dog 6 | GE | Revolution ACT | 32 | 2.5 | 0.5 | 512 | 120 | 80 | 1 |
| Location | Margins | Enhancement | Mineralization | |||||
|---|---|---|---|---|---|---|---|---|
| Homogeneity | Peripheral Rim Enhancement | Presence | Degree | Shape | Location | |||
| Dog 1 | Subcutaneous (right parotid) | Ill-defined | Heterogenous | Absent | Present | Marked | Amorphous | Mixed |
| Dog 2 | Subcutaneous (right forelimb) | Well-defined | Absent | Present | Present | Moderate | Spindle | Mixed |
| Dog 3 | Subcutaneous (dorsum at level of the 4th–5th cervical vertebrae (C4–5)) | Ill-defined | Heterogenous | Absent | Present | Moderate | Amorphous | Mixed |
| Dog 4 | Subcutaneous (dorsum at the level of the 1st–8th thoracic vertebrae (T1–8)) | Well-defined | Heterogeneous | Present | Present | Moderate | Amorphous | Mixed |
| Dog 5 | Mammary (rt. caudal) | Well-defined | Heterogenous | Present | Present | Moderate | Amorphous | Eccentric |
| Dog 6 | Small intestine | Well-defined | Homogenous | Absent | Present | Mild | Dot | Eccentric |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.; Kim, M.; Kim, S.-S.; Hwang, H.; Jung, J.; Park, N.-W.; Kim, J.; Eom, K. Case Series: Computed Tomography Features of Extraskeletal Osteosarcoma in Six Dogs. Vet. Sci. 2024, 11, 282. https://doi.org/10.3390/vetsci11060282
Jeong J, Kim M, Kim S-S, Hwang H, Jung J, Park N-W, Kim J, Eom K. Case Series: Computed Tomography Features of Extraskeletal Osteosarcoma in Six Dogs. Veterinary Sciences. 2024; 11(6):282. https://doi.org/10.3390/vetsci11060282
Chicago/Turabian StyleJeong, Jeongyun, Minjoo Kim, Sung-Soo Kim, Hyunju Hwang, Joohyun Jung, Noh-Won Park, Jaehwan Kim, and Kidong Eom. 2024. "Case Series: Computed Tomography Features of Extraskeletal Osteosarcoma in Six Dogs" Veterinary Sciences 11, no. 6: 282. https://doi.org/10.3390/vetsci11060282
APA StyleJeong, J., Kim, M., Kim, S.-S., Hwang, H., Jung, J., Park, N.-W., Kim, J., & Eom, K. (2024). Case Series: Computed Tomography Features of Extraskeletal Osteosarcoma in Six Dogs. Veterinary Sciences, 11(6), 282. https://doi.org/10.3390/vetsci11060282







