Polymicrobial Septic Peritonitis Caused by Enterococcus faecium and Enterococcus casseliflavus following Uterine Rupture in a Goat
Abstract
Simple Summary
Abstract
1. Introduction
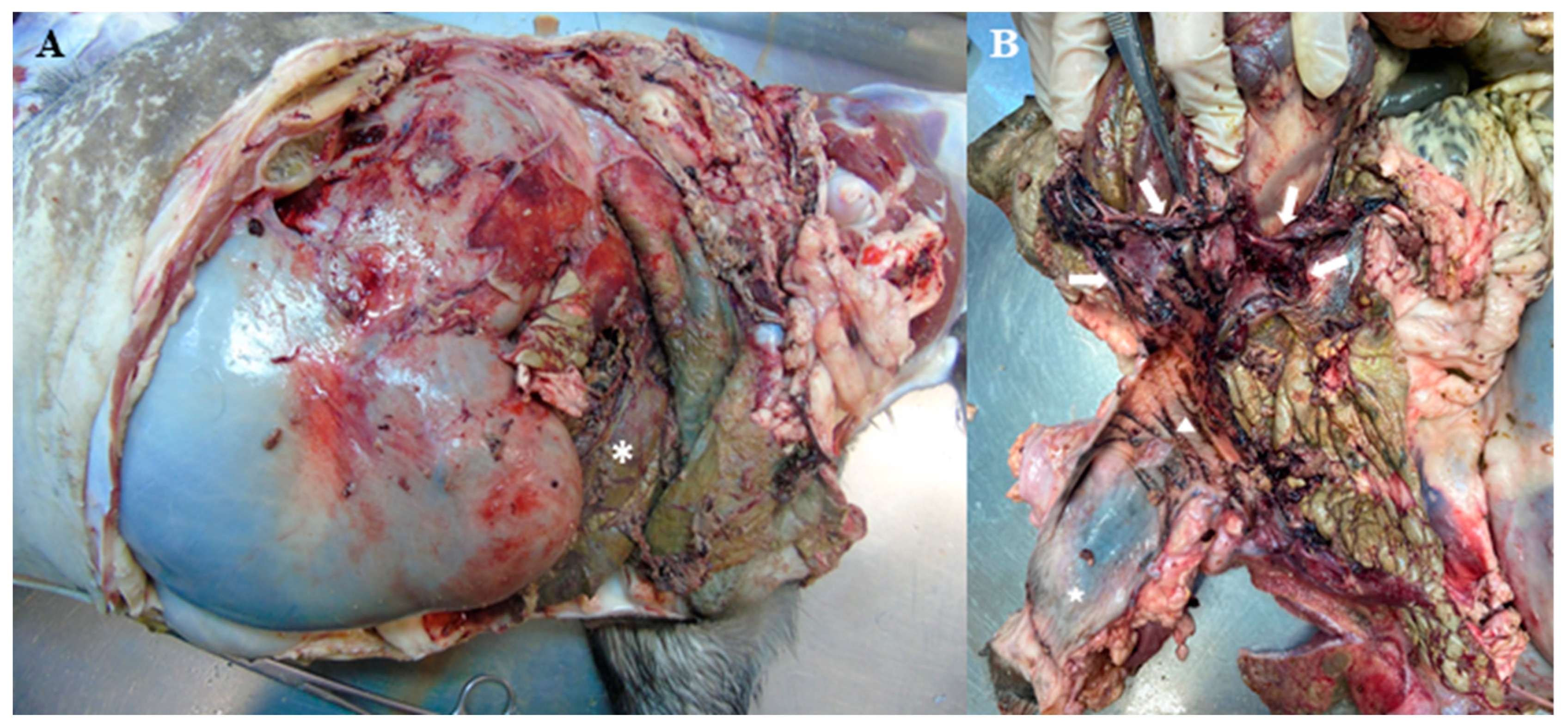
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coll-Roman, L.M.; Cabrera, C.; Vander Broek, A.R.; Bauck, A.G.; Kelleman, A.A.; Pozor, M.A.; Stockler, J.W.; Wiley, C.; Scully, C.; Mackay, E.E.; et al. Multicenter study of uterine tears and other reproductive complications in periparturient goats presented to veterinary teaching hospitals. J. Vet. Intern. Med. 2023, 37, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Brounts, S.H.; Hawkins, J.F.; Baird, A.N.; Glickman, L.T. Outcome and subsequent fertility of sheep and goats undergoing cesarean section because of dystocia: 110 cases (1981–2001). J. Am. Vet. Med. Assoc. 2004, 224, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ametaj, B.N.; Ambrose, D.J.; Gänzle, M.G. Characterization of the bacterial microbiota of the vagina of dairy cows and isolation of pediocin-producing Pediococcus acidilactici. BMC Microbiol. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; Van Schyndel, S.J.; Spricigo, J.F.W.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Dynamics of uterine microbiota in postpartum dairy cows with clinical or subclinical endometritis. Sci. Rep. 2020, 10, 12353. [Google Scholar] [CrossRef] [PubMed]
- Javsicas, L.H.; Giguère, S.; Freeman, D.E.; Rodgerson, D.H.; Slovis, N.M. Comparison of Surgical and Medical Treatment of 49 Postpartum Mares with Presumptive or Confirmed Uterine Tears. Vet. Surg. 2010, 39, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Rozanski, E.; Tseng, F.; Jennings, S.; Paul, A. Traumatic uterine rupture in three felids. J. Vet. Emerg. Crit. Care 2016, 26, 782–786. [Google Scholar] [CrossRef] [PubMed]
- M100-S33; Performance Standards for Antimicrobial Susceptibility. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023.
- Nocera, F.P.; Papulino, C.; Del Prete, C.; Palumbo, V.; Pasolini, M.P.; De Martino, L. Endometritis associated with Enterococcus casseliflavus in a mare: A case report. Asian Pac. J. Trop. Biomed. 2017, 7, 760–762. [Google Scholar] [CrossRef]
- Friedrichs, K.R.; Jensen, A.L.; Kjelgaard-Hansen, M. Reference Intervals and Decision Limits. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M.B., Harr, K.E., Seelig, D.M., Wardrop, K.J., Weiss, D.J., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 1273–1284. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Latimer, K.S. Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 5th ed.; Latimer, K.S., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Rosenberg, L.M.; Marinoff, J.; Crouch, E.E.; Valenzano, D.M.; Peters-Kennedy, J.; Cheong, S.H.; de Amorim, M.D. Uterine perforation secondary to metritis and placenta percreta in a postpartum bitch. Can. Vet. J. 2020, 61, 584–588. [Google Scholar] [PubMed]
- Alimi, O.A.; Abdulwahab, W.F.; Amid, S.A.; Abdulkadir, S.Z.; Lawal, F.M.; Aliyu, A.; Adediran, S.O.; Ajadi, A.A.; Bolaji, M.; Uthman, H.O.; et al. Hematological prediction study of peritonitis following laparotomy in goats. J. Vet. Med. Sci. 2020, 82, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Beukers, A.G.; Zaheer, R.; Goji, N.; Amoako, K.K.; Chaves, A.V.; Ward, M.P.; McAllister, T.A. Comparative genomics of Enterococcus spp. isolated from bovine feces. BMC Microbiol. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Nooij, P.P. Laparohysterectomy as a treatment for uterine rupture in the cow. Can. Vet. J. 1982, 23, 37–38. [Google Scholar] [PubMed]
- Gilbert, R.O.; Cable, C.; Fubini, S.L.; Steiner, A. Surgery of the Bovine Reproductive System and Urinary Tract. In Farm Animal Surgery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 439–503. [Google Scholar]
| Parameters | Day 1 | Day 2 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | Day 14 | Reference Interval [9,10,11] |
|---|---|---|---|---|---|---|---|---|---|
| RBC cell × 1012/L (cell × 106/µL) | 20.7 (20.7) | 20.5 (20.5) | 12.5 (12.5) | 9.55 (9.55) | 7.8 (7.8) | 5.2 (5.2) | - | - | 8–18 (8–18) |
| Hemoglobin g/L (g/dL) | 102 (10.2) | 103 (10.3) | 78 (7.8) | 62 (6.2) | 46 (4.6) | 31 (3.1) | - | - | 80–120 (8–12) |
| HCT L/L (%) | 0.3 (30) | 0.3 (30) | 0.24 (24) | 0.18 (18) | 0.14 (14) | 0.09 (9) | 0.11 (11) | 0.21 (21) | 0.22–0.38 (22–38) |
| MCV fL (μm3) | 14 (14) | 15 (15) | 19 (19) | 19 (19) | 18 (18) | 17 (17) | - | - | 16–25 (16–25) |
| MCH pg/cell | 4.9 | 5 | 6.3 | 6 | 6 | 6 | - | - | 5.2–8.0 |
| MCHC g/L (g/dL) | 340 (34) | 340 (34) | 320 (32) | 340 (34) | 330 (33) | 340 (34) | - | - | 300–360 (30–36) |
| Reticulocytes cell × 109/µL (cell × 103/µL) | - | - | - | - | - | 30.3 (30.3) | - | - | - |
| WBC cell × 109/L (cell/µL) | 28 (28,000) | 10.2 (10,200) | 11.2 (11,200) | 14.7 (14,700) | 31 (31,000) | 33 (33,000) | - | - | 4–13 (4000–13,000) |
| Band neutrophils cell × 109/L (cell/µL) | 0 | 0.306 (306) | 0 | 0.147 (147) | 0.31 (310) | 0 | - | - | Rare |
| Segmented neutrophils cell × 109/L (cell/µL) | 23.24 (23,240) | 3.162 (3162) | 6.16 (6160) | 7.056 (7056) | 22.94 (22,940) | 24.09 (24,090) | - | - | 1.2–7.2 (1200–7200) |
| Lymphocytes cell × 109/L (cell/µL) | 3.38 (3380) | 5.508 (5508) | 4.368 (4368) | 5.145 (5145) | 6.2 (6200) | 7.92 (7920) | - | - | 2–9 (2000–9000) |
| Monocytes cell × 109/L (cell/µL) | 0.280 (280) | 1.122 (1122) | 0.672 (672) | 2.352 (2352) | 1.550 (1550) | 0.990 (990) | - | - | 0–0.55 (0–550) |
| Eosinophils cell × 109/L (cell/µL) | 0 | 0.102 (102) | 0 | 0 | 0 | 0 | - | - | 0.050–0.650 (50–650) |
| Basophils cell × 109/L (cell/µL) | 0 | 0 | 0 | 0 | 0 | 0 | - | - | 0–120 (0–120) |
| Thrombocytes cell × 109/L (cell × 103/µL) | 0.641 (0.641) | 0.202 (0.202) | 0.066 (0.066 | 0.227 (0.227) | 0.464 (0.464) | - | - | - | 0.3–0.6 (0.3–0.6) |
| Total protein g/L (g/dL) | 57.9 (5.79) | 48.2 (4.82) | 44 (4.4) | - | 50.9 (5.09) | 57.8 (5.78) | 62(6.2) | 62 (6.2) | 64–70 (6.4–7.0) |
| Albumin g/L (g/dL) | 25.9 (2.59) | 20.3 (2.03) | 15.6 (1.56) | - | 15.4 (1.54) | 17.2 (1.72) | - | - | 27–39 (2.7–3.9) |
| AST µkat/L (units/L) | 0.77 (46) | 2.01 (120.1) | 6.97 (417.5) | - | 7.27 (435.1) | 5.45 (326.5) | - | - | 2.79–8.57 (167–513) |
| GGT µkat/L (units/L) | 0.25 (14.9) | 0.18 (10.6) | 0.08 (4.9) | - | 0.39 (23.2) | 0.51 (30.4) | - | - | 0.33–0.94 (20–56) |
| Total bilirubin μmol/L (mg/dL) | 7.011 (0.41) | 2.736 (0.16) | 1.026 (0.06) | - | 4.788 (0.28) | 8.208 (0.48) | - | - | 0–1.71 (0–0.1) |
| BUN mmol/L (mg/dL) | 29.881 (83.7) | 39.091 (109.5) | 24.704 (69.2) | - | 37.164 (104.1) | 20.599 (57.7) | - | - | 3.57–7.14 (10–20) |
| Creatinine μmol/L (mg/dL) | 167.076 (1.89) | 174.148 (1.97) | 125.528 (1.42) | - | 115.804 (1.31) | 116.688 (1.32) | - | - | 88.4–159.12 (1.0–1.8) |
| Susceptibility Profile [7] | ||
|---|---|---|
| Antimicrobial Tested | Enterococcus faecium | Enterococcus casseliflavus |
| Ampicillin | Resistant | Sensible |
| Penicillin | Resistant | Sensible |
| Chloramphenicol | Sensible | Intermediate |
| Ciprofloxacin | Resistant | Intermediate |
| Erythromycin | Intermediate | Intermediate |
| Tetracycline | Resistant | Sensible |
| Vancomycin | Sensible | Intermediate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, G.S.; Francischetti, G.S.; Garritano, N.F.; Hagen, S.C.F.; Cagnim, A.F.; Catão-Dias, J.L.; Ferreira Neto, J.S.; Sucupira, M.C.A.; Heinemann, M.B. Polymicrobial Septic Peritonitis Caused by Enterococcus faecium and Enterococcus casseliflavus following Uterine Rupture in a Goat. Vet. Sci. 2024, 11, 268. https://doi.org/10.3390/vetsci11060268
dos Santos GS, Francischetti GS, Garritano NF, Hagen SCF, Cagnim AF, Catão-Dias JL, Ferreira Neto JS, Sucupira MCA, Heinemann MB. Polymicrobial Septic Peritonitis Caused by Enterococcus faecium and Enterococcus casseliflavus following Uterine Rupture in a Goat. Veterinary Sciences. 2024; 11(6):268. https://doi.org/10.3390/vetsci11060268
Chicago/Turabian Styledos Santos, Gabriel S., Giovanna S. Francischetti, Natália F. Garritano, Stefano C. F. Hagen, Artur F. Cagnim, José Luiz Catão-Dias, José S. Ferreira Neto, Maria Claudia A. Sucupira, and Marcos B. Heinemann. 2024. "Polymicrobial Septic Peritonitis Caused by Enterococcus faecium and Enterococcus casseliflavus following Uterine Rupture in a Goat" Veterinary Sciences 11, no. 6: 268. https://doi.org/10.3390/vetsci11060268
APA Styledos Santos, G. S., Francischetti, G. S., Garritano, N. F., Hagen, S. C. F., Cagnim, A. F., Catão-Dias, J. L., Ferreira Neto, J. S., Sucupira, M. C. A., & Heinemann, M. B. (2024). Polymicrobial Septic Peritonitis Caused by Enterococcus faecium and Enterococcus casseliflavus following Uterine Rupture in a Goat. Veterinary Sciences, 11(6), 268. https://doi.org/10.3390/vetsci11060268







