Wildlife–Livestock Host Community Maintains Simultaneous Epidemiologic Cycles of Mycoplasma conjunctivae in a Mountain Ecosystem
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
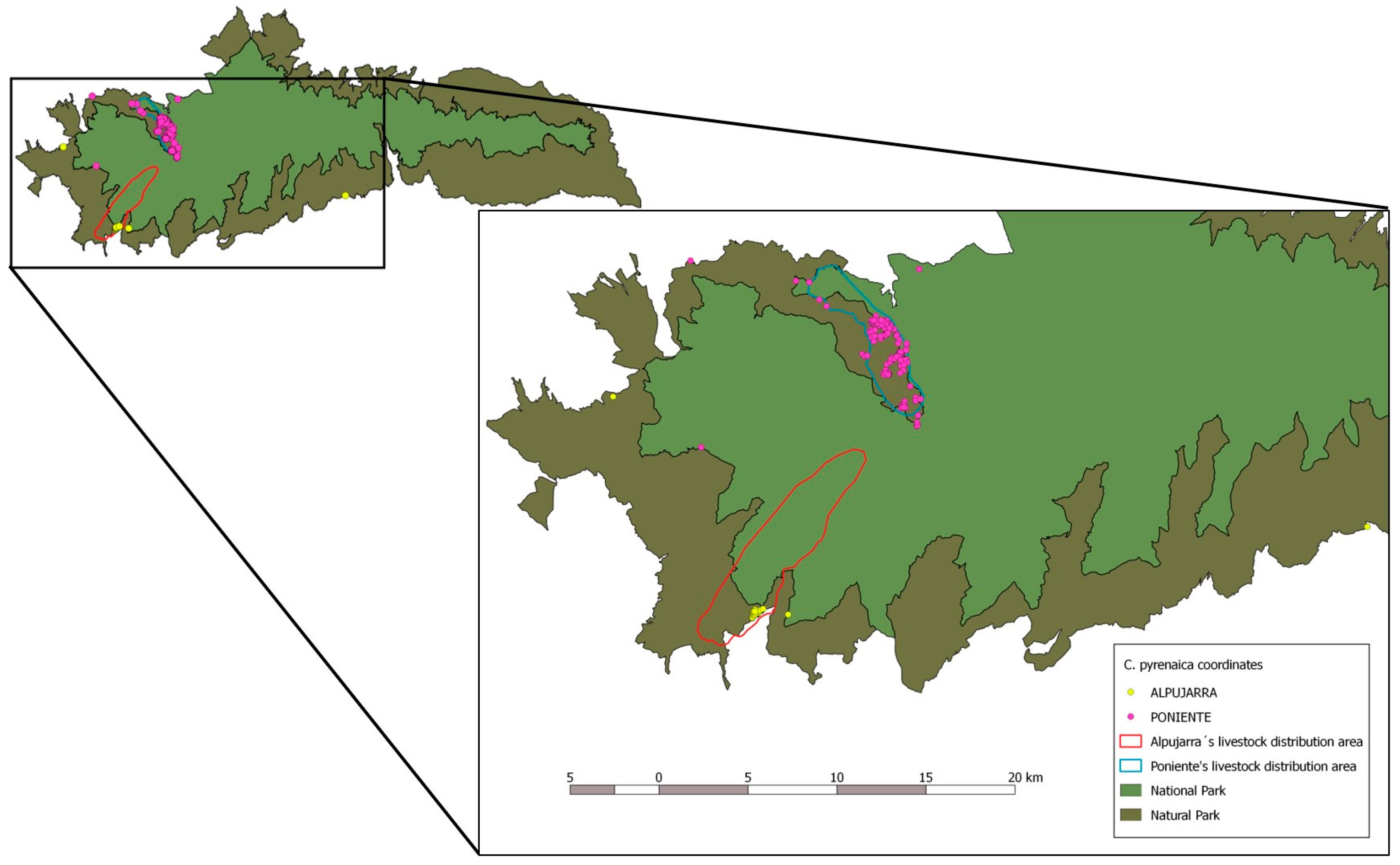
2.1. Study Area
2.2. Sampling
2.3. Molecular Detection of M. conjunctivae DNA
2.4. Statistical Analyses
2.5. Mycoplasma Conjunctivae Subtyping and Cluster Analyses
3. Results
3.1. M. conjunctivae Infection in Iberian ibex
3.2. M. conjunctivae Infection in Domestic Small Ruminants
3.3. Clustering of M. conjunctivae Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacometti, M.; Janovsky, M.; Belloy, L.; Frey, J. Infectious keratoconjunctivitis of ibex, chamois and other Caprinae. Rev. Sci. Tech. OIE 2002, 21, 335–345. [Google Scholar] [CrossRef]
- Dagnall, G.J. The role of Branhamella ovis, Mycoplasma conjunctivae and Chlamydia psittaci in conjunctivitis of sheep. Br. Vet. J. 1994, 150, 65–71. [Google Scholar] [CrossRef]
- Dubay, S.A.; Williams, E.S.; Mills, K.; Boerger-Fields, A.M. Association of Moraxella ovis with keratoconjunctivitis in mule deer and moose in Wyoming. J. Wildl. Dis. 2000, 36, 241–247. [Google Scholar] [CrossRef][Green Version]
- Jansen, B.D.; Heffelfinger, J.R.; Noon, T.H.; Krausman, P.R.; Devos, J.C., Jr. Infectious keratoconjunctivitis in bighorn sheep, Silver Bell Mountains, Arizona, USA. J. Wildl. Dis. 2006, 42, 407–411. [Google Scholar] [CrossRef]
- Holzwarth, N.; Pospischil, A.; Marreros, N.; Ryser-Degiorgis, M.P.; Mavrot, F.; Frey, J.; Thoma, R.; Borel, N. Alpine ibex (Capra i. ibex) is not a reservoir for chlamydial infections of domestic ruminants and humans. Eur. J. Wildl. Res. 2011, 57, 233–240. [Google Scholar] [CrossRef]
- Holzwarth, N.; Pospischil, A.; Mavrot, F.; Vilei, E.M.; Hilbe, M.; Zlinszky, K.; Regenscheit, N.; Pewsner, M.; Thoma, R.; Borel, N. Occurrence of Chlamydiaceae, Mycoplasma conjunctivae, and pestiviruses in Alpine chamois (Rupicapra r. rupicapra) of Grisons, Switzerland. J. Vet. Diagn. Investig. 2011, 23, 333–337. [Google Scholar] [CrossRef]
- Arnal, M.; Herrero, J.; de la Fe, C.; Revilla, M.; Prada, C.; Martínez-Durán, D.; Gómez-Martín, Á.; Fernández-Arberas, O.; Amores, J.; Contreras, A.; et al. Dynamics of an infectious keratoconjunctivitis outbreak by Mycoplasma conjunctivae on Pyrenean chamois Rupicapra p. pyrenaica. PLoS ONE 2013, 8, e61887. [Google Scholar] [CrossRef]
- Osman, K.M.; Ali, H.A.; ElJakee, J.A.; Galal, H.M. Prevalence of Chlamydophila psittaci infections in the eyes of cattle, buffaloes, sheep and goats in contact with a human population. Transbound. Emerg. Dis. 2013, 60, 245–251. [Google Scholar] [CrossRef]
- Gupta, S.; Chahota, R.; Bhardwaj, B.; Priyanka, P.; Verma, S.; Sharma, M. Identification of chlamydiae and mycoplasma species in ruminants with ocular infections. Lett. Appl. Microbiol. 2015, 60, 135–139. [Google Scholar] [CrossRef]
- Handeland, K.; Madslien, K.; Bretten, T.; Røtvei, I.; Våge, J.; Tengs, T. Mycoplasma conjunctivae-associated keratoconjunctivitis in Norwegian muskox (Ovibos moschatus). J. Wildl. Dis. 2020, 56, 489–491. [Google Scholar] [CrossRef]
- Dias-Alves, A.; Cabezón, O.; Borel, N.; López-Olvera, J.R.; Mentaberre, G.; Lavín, S.; Fernández Aguilar, X. Molecular detection and identification of Chlamydiaceae in the eyes of wild and domestic ruminant hosts from Northern Spain. Pathogens 2021, 10, 383. [Google Scholar] [CrossRef]
- Baas, E.J.; Trotter, S.L.; Franklin, R.M.; Barile, M.F. Epidemic caprine keratoconjunctivitis: Recovery of Mycoplasma conjunctivae and its possible role in pathogenesis. Infect. Immun. 1977, 18, 806–815. [Google Scholar] [CrossRef]
- Mayer, D.; Nicolet, J.; Giacometti, M.; Schmitt, M.; Wahli, T.; Meier, W. Isolation of Mycoplasma conjunctivae from conjunctival swabs of Alpine ibex (Capra ibex ibex) affected with infectious keratoconjunctivitis. J. Vet. Med. B 1996, 43, 155–161. [Google Scholar] [CrossRef]
- Grattarola, C.; Frey, J.; Abdo, E.M.; Orusa, R.; Nicolet, J.; Giacometti, M. Mycoplasma conjunctivae infections in chamois and ibexes affected with infectious keratoconjunctivitis in the Italian Alps. Vet. Rec. 1999, 145, 588–589. [Google Scholar] [CrossRef]
- Baker, S.E.; Bashiruddin, J.B.; Ayling, R.D.; Nicholas, R.A. Molecular detection of Mycoplasma conjunctivae in English sheep affected by infectious keratoconjunctivitis. Vet. Rec. 2001, 148, 240–241. [Google Scholar] [CrossRef]
- Motha, M.X.; Frey, J.; Hansen, M.F.; Jamaludin, R.; Tham, K.M. Detection of Mycoplasma conjunctivae in sheep affected with conjunctivitis and infectious keratoconjunctivitis. N. Z. Vet. J. 2003, 51, 186–190. [Google Scholar] [CrossRef]
- Ryser-Degiorgis, M.-P.; Bischof, D.F.; Marreros, N.; Willisch, C.; Signer, C.; Filli, F.; Brosi, G.; Frey, J.; Vilei, E.M. Detection of Mycoplasma conjunctivae in the eyes of healthy, free-ranging Alpine ibex: Possible involvement of Alpine ibex as carriers for the main causing agent of infectious keratoconjunctivitis in wild Caprinae. Vet. Microbiol. 2009, 134, 368–374. [Google Scholar] [CrossRef]
- Fernández-Aguilar, X.; Cabezón, Ó.; Marco, I.; Mentaberre, G.; Frey, J.; Lavín, S.; López-Olvera, J.R. Mycoplasma conjunctivae in domestic small ruminants from high mountain habitats in Northern Spain. BMC Vet. Res. 2013, 9, 253. [Google Scholar] [CrossRef]
- Fernández-Aguilar, X.; Cabezón, Ó.; Frey, J.; Velarde, R.; Serrano, E.; Colom-Cadena, A.; Gelormini, G.; Marco, I.; Mentaberre, G.; Lavín, S.; et al. Long-term dynamics of Mycoplasma conjunctivae at the wildlife-livestock interface in the Pyrenees. PLoS ONE 2017, 12, e0186069. [Google Scholar] [CrossRef]
- Fernández-Aguilar, X.; Cabezón, Ó.; Granados, J.E.; Frey, J.; Serrano, E.; Velarde, R.; Cano-Manuel, F.J.; Mentaberre, G.; Ráez-Bravo, A.; Fandos, P.; et al. Postepizootic persistence of asymptomatic Mycoplasma conjunctivae infection in Iberian ibex. Appl. Environ. Microb. 2017, 83, e00690-17. [Google Scholar] [CrossRef]
- Fernández-Aguilar, X.; Rossi, L.; Cabezón, Ó.; Giorgino, A.; Llopis, I.V.; Frey, J.; López-Olvera, J.R. Infectious keratoconjunctivitis and occurrence of Mycoplasma conjunctivae and Chlamydiaceae in small domestic ruminants from Central Karakoram, Pakistan. Vet. Rec. 2017, 181, 237. [Google Scholar] [CrossRef]
- Giangaspero, M.; Orusa, R.; Nicholas, R.A.; Harasawa, R.; Ayling, R.D.; Churchward, C.P.; Whatmore, A.; Bradley, D.; Robetto, S.; Sacchi, L.; et al. Characterization of Mycoplasma isolated from an ibex (Capra ibex) suffering from keratoconjunctivitis in northern Italy. J. Wildl. Dis. 2010, 46, 1070–1078. [Google Scholar] [CrossRef]
- Degiorgis, M.P.; Obrecht, E.; Ryser, A.; Giacometti, M. The possible role of eye-frequenting flies in the transmission of Mycoplasma conjunctivae. Mitt. Schweiz. Entomol. Ges. 1999, 72, 189–194. [Google Scholar]
- Fernández Aguilar, X.; López-Olvera, J.R.; Puig Ribas, M.; Begovoeva, M.; Velarde, R.; Cardells, J.; Cabezón, Ó. Mycoplasma conjunctivae in insect vectors and anatomic locations related to transmission and persistence. Vet. Microbiol. 2019, 228, 7–11. [Google Scholar] [CrossRef]
- Bassano, B.; Bollo, E.; Peracino, V.; Guarda, F. Brain lesions associated with infectious keratoconjunctivitis in chamois and alpine ibex. Ibex Suppl. J. Mount. Ecol. 1994, 2, 17–22. [Google Scholar]
- Giacometti, M.; Nicolet, J.; Frey, J.; Krawinkler, M.; Meier, W.; Welle, M.; Johansson, K.E.; Degiorgis, M.-P. Susceptibility of Alpine ibex to conjunctivitis caused by inoculation of a sheep-strain of Mycoplasma conjunctivae. Vet. Microbiol. 1998, 61, 279–288. [Google Scholar] [CrossRef]
- Giacometti, M.; Janovsky, M.; Jenny, H.; Nicolet, J.; Belloy, L.; Goldschmidt-Clermont, E.; Frey, J. Mycoplasma conjunctivae infection is not maintained in alpine chamois in eastern Switzerland. J. Wildlife Dis. 2002, 38, 297–304. [Google Scholar] [CrossRef]
- Tschopp, R.; Frey, J.; Zimmermann, L.; Giacometti, M. Outbreaks of infectious keratoconjunctivitis in alpine chamois and ibex in Switzerland between 2001 and 2003. Vet. Rec. 2005, 157, 13–18. [Google Scholar] [CrossRef]
- González-Candela, M.; Cubero, M.; Martín-Atance, P.; León, L. Potential pathogens carried by Spanish ibex (Capra pyrenaica hispanica) in southern Spain. J. Wildlife Dis. 2006, 42, 325–334. [Google Scholar] [CrossRef][Green Version]
- González-Candela, M.; Verbisck-Bucker, G.; Martín-Atance, P.; Cubero-Pablo, M.J.; León-Vizcaíno, L. Mycoplasmas isolated from Spanish ibex (Capra pyrenaica hispanica): Frequency and risk factors. Vet. Rec. 2007, 161, 167–168. [Google Scholar] [CrossRef]
- Marco, I.; Mentaberre, G.; Ballesteros, C.; Bischof, D.F.; Lavín, S.; Vilei, E.M. First report of Mycoplasma conjunctivae from wild Caprinae with infectious keratoconjunctivitis in the Pyrenees (NE Spain). J. Wildlife Dis. 2009, 45, 238–241. [Google Scholar] [CrossRef]
- Gelormini, G.; Gauthier, D.; Vilei, E.M.; Crampe, J.-P.; Frey, J.; Ryser-Degiorgis, M.-P. Infectious keratoconjunctivitis in wild Caprinae: Merging field observations and molecular analyses sheds light on factors shaping outbreak dynamics. BMC Vet. Res. 2017, 13, 67. [Google Scholar] [CrossRef]
- Valldeperes, M.; Prieto Yerro, P.; López-Olvera, J.R.; Fandos, P.; Lavín, S.; Soriguer Escofet, R.C.; Mentaberre, G.; Cano-Manuel León, F.J.; Espinosa, J.; Ráez-Bravo, A.; et al. Diseases of Iberian ibex (Capra pyrenaica). Eur. J. Wildlife Res. 2023, 69, 63. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Tizzani, P.; Rambozzi, L.; Moroni, B.; Meneguz, P.G. Sanitary emergencies at the wild/domestic caprines interface in Europe. Animals 2019, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Degiorgis, M.P.; Frey, J.; Nicolet, J.; Abdo, E.-M.; Fatzer, R.; Schlatter, Y.; Reist, S.; Janovsky, M.; Giacometti, M. An outbreak of infectious keratoconjunctivitis in Alpine chamois (Rupicapra r. rupicapra) in Simmental-Gruyères, Switzerland. Schweiz. Arch Tierh. 2000, 142, 520–527. [Google Scholar]
- Hars, J.; Gauthier, D. Suivi de l’évolution de la kératoconjonctivite sue le peuplement d’ongulés sauvages du Parc National de la Vanoise en 1983. Trav. Sci. Parc. Nat. Vanoise 1984, 14, 157–210. [Google Scholar]
- Marco, I.; Lavín., S.; Gonzalo, J.; Viñas, L. Estudio de un brote de querato-conjuntivitis infecciosa en los rebecos (Rupicapra pyrenaica) del Pirineo leridano. Vet. Prax. 1992, 6, 57–62. [Google Scholar]
- Loison, A.; Gaillard, J.M.; Jullien, J.M. Demographic patterns after an epizootic of keratoconjunctivitis in a chamois population. J. Wildl. Manag. 1996, 60, 517–527. [Google Scholar] [CrossRef]
- Gortázar, C.; Fernández-de-Luco, D.; Frölich, K. Keratoconjunctivitis in a free-ranging red deer (Cervus elaphus) population in Spain. Z. Jagdwiss. 1998, 44, 257–261. [Google Scholar] [CrossRef]
- Evans, A.L.; Bey, R.F.; Schoster, J.V.; Gaarder, J.E.; Finstad, G.L. Preliminary studies on the etiology of keratoconjunctivitis in reindeer (Rangifer tarandus tarandus) calves in Alaska. J. Wildl. Dis. 2008, 44, 1051–1055. [Google Scholar] [CrossRef]
- Muñoz Gutiérrez, J.F.; Sondgeroth, K.S.; Williams, E.S.; Montgomery, D.L.; Creekmore, T.E.; Miller, M.M. Infectious keratoconjunctivitis in free-ranging mule deer in Wyoming: A retrospective study and identification of a novel alphaherpesvirus. J. Vet. Diagn. Investig. 2018, 30, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Houszka, M.; Bazanow, B.; Wesolowska, I.; Szczepanik, J. Infectious keratoconjunctivitis in red deer (Cervus elaphus) in Poland—A case report. Vet. Med. 2021, 66, 117–120. [Google Scholar] [CrossRef]
- Ruffin, D.C. Mycoplasma infections in small ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Hadani, Y.; Lysnyansky, I.; Bareli, A.; Bernstein, M.; Elad, D.; Ozeri, D.; Rotenberg, D.; Bumbarov, V.; Perl, S.; Brenner, J. An unusual widespread outbreak of blindness caused by Mycoplasma conjunctivae on a large dairy goat farm. Isr. J. Vet. Med. 2013, 68, 175–179. [Google Scholar]
- Shahzad, W.; Munir, R.; Rana, M.Y.; Ahmad, R.; Khan, M.S.; Akbar, G.; Ijaz, M.; Mehmood, F. Prevalence, molecular diagnosis and treatment of Mycoplasma conjunctivae isolated from infectious keratoconjunctivitis affected Lohi sheep maintained at Livestock Experiment Station, Bahadurnagar, Okara, Pakistan. Trop. Anim. Health Prod. 2013, 45, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Razavizadeh, A.R.T.; Razymar, J. Molecular diagnosis of Mycoplasma conjunctivae in an outbreak of infectious keratoconjunctivitis in sheep. Iran. J. Vet. Res. 2014, 15, 72–74. [Google Scholar]
- Prats-van der Ham, M.; de la Fe, C.; Amores, J.; Paterna, A.; Tatay-Dualde, J.; Gómez-Martín, Á. Contagious caprine pleuropneumonia (CCPP) and other emergent mycoplasmal diseases affecting small ruminants in arid lands. J. Arid Environ. 2015, 119, 9–15. [Google Scholar] [CrossRef]
- Shamsaddini Bafti, M.; Pourbakhsh, S.A.; Ezatkhah, M.; Ashtari, A. Detection of Mycoplasma agalactiae in small ruminants of Southeast Iran. Arch Razi Inst. 2017, 72, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Meekins, J.M.; Apley, M.D.; Lubbers, B.; Peddireddi, L.; Rankin, A.J. Evaluation of conjunctival bacterial flora in a herd of goats in the Midwestern United States. Vet. Ophthalmol. 2017, 20, 40–45. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Parray, O.R.; Mir, M.S.; Qureshi, S.; Kashoo, Z.A.; Nadeem, M.; Fazili, M.U.R.; Tufani, N.A.; Kanwar, M.S.; Chakraborty, S.; et al. Mycoplasmosis in small ruminants in India. J. Exp. Biol. Agric. Sci. 2018, 6, 264–281. [Google Scholar] [CrossRef]
- Ryser-Degiorgis, M.-P.; Ingold, P.; Tenhu, H.; Less, A.M.T.; Ryser, A.; Giacometti, M. Encounters between Alpine ibex, Alpine chamois and domestic sheep in the Swiss Alps. Hystrix 2002, 13, 1–11. [Google Scholar] [CrossRef]
- Belloy, L.; Janovsky, M.; Vilei, E.M.; Pilo, P.; Giacometti, M.; Frey, J. Molecular epidemiology of Mycoplasma conjunctivae in Caprinae: Transmission across species in natural outbreaks. Appl. Environ. Microb. 2003, 69, 1913–1919. [Google Scholar] [CrossRef]
- Abbona, F.; Venturino, E. An eco-epidemic model for infectious keratoconjunctivitis caused by Mycoplasma conjunctivae in domestic and wild herbivores, with possible vaccination strategies. Math. Method. Appl. Sci. 2018, 41, 2269–2280. [Google Scholar] [CrossRef]
- Mavrot, F.; Vilei, E.M.; Marreros, N.; Signer, C.; Frey, J.; Ryser-Degiorgis, M.-P. Occurrence, quantification, and genotyping of Mycoplasma conjunctivae in wild Caprinae with and without infectious keratoconjunctivitis. J. Wildlife Dis. 2012, 48, 619–631. [Google Scholar] [CrossRef]
- Mayer, D.; Degiorgis, M.-P.; Meier, W.; Nicolet, J.; Giacometti, M. Lesions associated with infectious keratoconjunctivitis in Alpine ibex. J. Wildlife Dis. 1997, 33, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Blanchard, A. Mycoplasmas and their host: Emerging and re-emerging minimal pathogens. Trends Microbiol. 2013, 21, 196–203. [Google Scholar] [CrossRef]
- Mavrot, F.; Zimmermann, F.; Vilei, E.M.; Ryser-Degiorgis, M.-P. Is the development of infectious keratoconjunctivitis in Alpine ibex and Alpine chamois influenced by topographic features? Eur. J. Wildlife Res. 2012, 58, 869–874. [Google Scholar] [CrossRef][Green Version]
- Manceau, V.; Crampe, J.P.; Boursot, P.; Taberlet, P. Identification of evolutionary significant units in the Spanish wild goat, Capra pyrenaica (Mammalia, Artiodactyla). Anim. Conserv. 1999, 2, 33–39. [Google Scholar] [CrossRef]
- Amills, M.; Jiménez, N.; Jordana, J.; Riccardi, A.; Fernández-Arias, A.; Guiral, J.; Bouzat, J.L.; Folch, J.; Sànchez, A. Low diversity in the major histocompatibility complex class II DRB1 gene of the Spanish ibex, Capra pyrenaica. Heredity 2004, 93, 266–272. [Google Scholar] [CrossRef][Green Version]
- Acevedo, P.; Cassinello, J. Biology, ecology and status of Iberian ibex Capra pyrenaica: A critical review and research prospectus. Mammal Rev. 2009, 39, 17–32. [Google Scholar] [CrossRef]
- Alados, C.L.; Escós, J. Cabra montés—Capra pyrenaica. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Cassinello, J., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2017; Available online: http://www.vertebradosibericos.org (accessed on 17 December 2022).
- Garnier, A. Conservation of Ibex Species (Capra ibex et Capra pyrenaica) in France: Contribution of Monitoring Data from Reintroduced and Settled Population. Biodiversity and Ecology. Ph.D. Thesis, Université Paul Sabatier—Toulouse III, Toulouse, France, 2021. Available online: https://theses.hal.science/tel-03560035/ (accessed on 1 March 2024).
- Junta de Andalucía; UNESCO. Memoria de Actividades y Resultados 2015. Sierra Nevada, Parque Nacional, Parque Natural, Memoria 20; Junta de Andalucía: Granada, Spain, 2015; pp. 140–160. [Google Scholar]
- Casas-Díaz, E.; Marco, I.; López-Olvera, J.R.; Mentaberre, G.; Lavín, S. Comparison of xylazine–ketamine and medetomidine–ketamine anaesthesia in the Iberian ibex (Capra pyrenaica). Eur. J. Wildlife Res. 2011, 57, 887–893. [Google Scholar] [CrossRef]
- López-Olvera, J.R.; Marco, I.; Montané, J.; Casas-Díaz, E.; Mentaberre, G.; Lavín, S. Comparative evaluation of effort, capture and handling effects of drive nets to capture roe deer (Capreolus capreolus), Southern chamois (Rupicapra pyrenaica) and Spanish ibex (Capra pyrenaica). Eur. J. Wildlife Res. 2009, 55, 193–202. [Google Scholar] [CrossRef]
- Fandos, P. La Cabra Montés (Capra pyrenaica) en el Parque Natural de Cazorla, Segura y Las Villas; ICONA: Madrid, Spain, 1991; 176p. [Google Scholar]
- Vilei, E.M.; Bonvin-Klotz, L.; Zimmermann, L.; Ryser-Degiorgis, M.-P.; Giacometti, M.; Frey, J. Validation and diagnostic efficacy of a TaqMan real-time PCR for the detection of Mycoplasma conjunctivae in the eyes of infected Caprinae. J. Microbiol. Meth. 2007, 70, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; 490p. [Google Scholar]
- R Development Team. R: A Language and Environment for Statistical Computing; R Development Team: Vienna, Austria, 2022; Available online: http://www.r-project.org (accessed on 1 March 2024).
- Belloy, L.; Vilei, E.M.; Giacometti, M.; Frey, J. Characterization of LppS, an adhesin of Mycoplasma conjunctivae. Microbiology 2003, 149, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy. The Principles and Practice of Numerical Classification; W. H. Freeman & Co.: San Francisco, CA, USA, 1973; 573p. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Valldeperes, M.; Pascual-Rico, R.; Fandos, P.; Soriguer Escofet, R.C.; Pérez, J.M.; Cano-Manuel León, F.J.; Prieto Yerro, P.; López-Olvera, J.R.; Granados, J.E. Home range in genus Capra: From polygons to Brownian bridges of scabietic and healthy Iberian ibexes (Capra pyrenaica). J. Mammal. 2024, 2024, gyae013. [Google Scholar] [CrossRef]
- Gauthier, D. La kerato-Conjonctivite Infectieuse du Chamois: Étude Épidémiologique Dans le Département de la Savoie, 1983–1990. DVM Thesis, Univsersité Claude Bernard, Lyon, France, 1991. [Google Scholar]
- Pfeiffer, D.U. Veterinary Epidemiology—An Introduction; The Royal Veterinary College, University of London: London, UK, 2009; 174p. [Google Scholar] [CrossRef]
- Janovsky, M.; Frey, J.; Nicolet, J.; Belloy, L.; Goldschmidt-Clermont, E.; Giacometti, M. Mycoplasma conjunctivae infection is self-maintained in the Swiss domestic sheep population. Vet. Microbiol. 2001, 83, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Richomme, C.; Gauthier, D.; Fromont, E. Contact rates and exposure to inter-species disease transmission in mountain ungulates. Epidemiol. Infect. 2006, 134, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.D.; Dobson, A.P.; Dhondt, K.V.; Hawley, D.M.; Dhondt, A.A. Evidence of trade-offs shaping virulence evolution in an emerging wildlife pathogen. J. Evolution. Biol. 2014, 27, 1271–1278. [Google Scholar] [CrossRef]
- Barroso, P.; Relimpio, D.; Zearra, J.A.; Cerón, J.J.; Palencia, P.; Cardoso, B.; Ferreras, E.; Escobar, M.; Cáceres, G.; López-Olvera, J.R.; et al. Using integrated wildlife monitoring to prevent future pandemics through one health approach. One Health 2023, 16, 100479. [Google Scholar] [CrossRef]
- Zimmermann, L.; Jambresic, S.; Giacometti, M.; Frey, J. Specificity of Mycoplasma conjunctivae strains for alpine chamois Rupicapra r. rupicapra. Wildlife Biol. 2008, 14, 118–124. [Google Scholar] [CrossRef]
- Kamath, P.L.; Foster, J.T.; Drees, K.P.; Luikart, G.; Quance, C.; Anderson, N.J.; Clarke, P.R.; Cole, E.K.; Drew, M.L.; Edwards, W.H.; et al. Genomics reveals historic and contemporary transmission dynamics of a bacterial disease among wildlife and livestock. Nat. Commun. 2016, 7, 11448. [Google Scholar] [CrossRef] [PubMed]
- Boots, M.; Hudson, P.J.; Sasaki, A. Large shifts in pathogen virulence relate to host population structure. Science 2004, 303, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.; López-Olvera, J.R.; Kiluba wa Kiluba, T.; Gortázar, C. Overcoming the limitations of wildlife disease monitoring. Res. Dir. One Health 2024, 2, e3. [Google Scholar] [CrossRef]


| 2015 | 2016 | 2017 | 2015–2017 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALP | PO | ALP | PO | ALP | PO | ALP | PO | NSSN | |||||||||||||
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | Total ALP | F | M | Total PO | F | M | Total | |
| Iberian ibex | |||||||||||||||||||||
| Subadults | 3 | 2 | 1 | 11 | 1 | 2 | 2 | 18 | 1 | 0 | 3 | 2 | 5 | 4 | 9 | 6 | 31 | 37 | 11 | 35 | 46 |
| Adults | 5 | 3 | 12 | 14 | 2 | 0 | 0 | 30 | 3 | 0 | 0 | 32 | 10 | 3 | 13 | 12 | 76 | 88 | 22 | 79 | 101 |
| Subtotal ibex | 8 | 5 | 13 | 25 | 3 | 2 | 2 | 48 | 4 | 0 | 3 | 34 | 15 | 7 | 22 | 18 | 107 | 125 | 33 | 114 | 147 |
| Domestic sheep | 33 | 0 | 66 | 2 | 33 | 0 | 33 | 66 | 2 | 68 | 99 | 2 | 101 | ||||||||
| Domestic goat | 53 | 15 | 0 | 0 | 53 | 15 | 68 | 0 | 0 | 0 | 53 | 15 | 68 | ||||||||
| Subtotal livestock | 86 | 15 | 66 | 2 | 86 | 15 | 101 | 66 | 2 | 68 | 152 | 17 | 169 | ||||||||
| Model | K | AIC | Δi | ωi |
|---|---|---|---|---|
| Year x age class + period + sex | 7 | 116.60 | 0.00 | 0.40 |
| Year x age class + area + sex | 6 | 118.04 | 1.44 | 0.20 |
| Year x age class + period + area + sex | 8 | 118.66 | 2.06 | 0.14 |
| Year x age class | 4 | 118.79 | 2.19 | 0.14 |
| Year x age class + period + area | 7 | 119.67 | 3.07 | 0.09 |
| 2015 | 2016 | 2017 | 2015–2017 | ||
|---|---|---|---|---|---|
| Subadults | Positive/sampled | 5/17 | 3/23 | 2/6 | 10/46 |
| Prevalence | 29.4% a | 13.0% a | 33.3% | 21.7% | |
| Confidence interval 95% | 7.8–51.1 | 0.0–26.8 | 0.0–71.1 | 9.8–33.7 | |
| Adults | Positive/sampled | 1/34 | 0/32 | 15/35 | 16/101 |
| Prevalence | 2.9% bx | 0.0% bx | 42.9% y | 15.8% | |
| Confidence interval 95% | 0.0–8.6 | 0.0–0.0 | 26.5–59.3 | 8.7–23.0 | |
| Total | Positive/sampled | 6/51 | 3/55 | 17/41 | 26/147 |
| Prevalence | 11.8% x | 5.5% x | 41.5% y | 17.7% | |
| Confidence interval 95% | 2.9–20.6 | 0.0–11.5 | 26.4–56.5 | 11.5–23.9 |
| PERIOD | Pre | During | Post |
|---|---|---|---|
| Positive/sampled | 6/46 | 15/74 | 5/27 |
| Prevalence | 13.0% a | 20.3% b | 18.5% b |
| Confidence interval 95% | 3.3–22.8 | 11.1–29.4 | 3.9–33.2 |
| SEX | Female | Male | |
| Positive/sampled | 5/33 | 20/114 | |
| Prevalence | 15.2% | 17.5% | |
| Confidence interval 95% | 2.9–27.4 | 10.6–24.5 |
| Pre | Post | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALP | PO | Total Pre | ALP | PO | Total Post | |||||||||
| Female | Male | Subtotal ALP | Female | Male | Subtotal PO | Female | Male | Subtotal ALP | Female | Male | Subtotal PO | |||
| Goat | 10/53 | 1/15 | 11/68 | 0/0 | 0/0 | 0/0 | 11/68 a | 11/53 | 0/15 | 11/68 | 0/0 | 0/0 | 0/0 | 11/68 |
| 18.9% | 6.7% | 16.2% | 16.2% | 20.8% | 0.0% | 16.2% | 16.2% | |||||||
| 8.3–29.4 | 0.0–19.3 | 7.4–24.9 | 7.4–24.9 | 9.8–31.7 | 0.0–0.0 | 7.4–24.9 | 7.4–24.9 | |||||||
| Sheep | 2/33 | 0/0 | 2/33 | 3/65 | 0/2 | 3/67 | 5/100 b | 4/33 | 0/0 | 4/33 | 4/66 | 0/2 | 4/68 | 8/101 |
| 6.1% | 6.1% | 4.6% | 0.0% | 4.5% | 5.0% | 12.1% | 12.1% | 6.1% | 0.0% | 5.9% | 7.9% | |||
| 0.0–14.2 | 0.0–14.2 | 0.0–9.7 | 0.0–0.0 | 0.0–9.4 | 0.7–9.3 | 1.0–23.3 | 1.0–23.3 | 0.3–11.8 | 0.0–0.0 | 0.3–11.5 | 2.7–13.2 | |||
| TOTAL | 12/86 | 1/15 | 13/101 | 3/65 | 0/2 | 3/67 | 16/168 | 15/86 | 0/15 | 15/101 | 4/66 | 0/2 | 4/68 | 19/169 |
| 14.0% | 6.7% | 12.9% | 4.6% | 0.0% | 4.5% | 9.5% | 17.4% | 0.0% | 14.9% | 6.1% | 0.0% | 5.9% | 11.2% | |
| 6.6–21.3 | 0.019.3 | 6.3–19.4 | 0.0–9.7 | 0.0–0.0 | 0.0–9.4 | 5.1–14.0 | 9.4–25.5 | 0.0–0.0 | 7.9–21.8 | 0.3–11.8 | 0.0–0.0 | 0.3–11.5 | 6.5–16.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Olvera, J.R.; Ramírez, E.; Martínez-Carrasco, C.; Granados, J.E. Wildlife–Livestock Host Community Maintains Simultaneous Epidemiologic Cycles of Mycoplasma conjunctivae in a Mountain Ecosystem. Vet. Sci. 2024, 11, 217. https://doi.org/10.3390/vetsci11050217
López-Olvera JR, Ramírez E, Martínez-Carrasco C, Granados JE. Wildlife–Livestock Host Community Maintains Simultaneous Epidemiologic Cycles of Mycoplasma conjunctivae in a Mountain Ecosystem. Veterinary Sciences. 2024; 11(5):217. https://doi.org/10.3390/vetsci11050217
Chicago/Turabian StyleLópez-Olvera, Jorge Ramón, Eva Ramírez, Carlos Martínez-Carrasco, and José Enrique Granados. 2024. "Wildlife–Livestock Host Community Maintains Simultaneous Epidemiologic Cycles of Mycoplasma conjunctivae in a Mountain Ecosystem" Veterinary Sciences 11, no. 5: 217. https://doi.org/10.3390/vetsci11050217
APA StyleLópez-Olvera, J. R., Ramírez, E., Martínez-Carrasco, C., & Granados, J. E. (2024). Wildlife–Livestock Host Community Maintains Simultaneous Epidemiologic Cycles of Mycoplasma conjunctivae in a Mountain Ecosystem. Veterinary Sciences, 11(5), 217. https://doi.org/10.3390/vetsci11050217









