miR-329b-5p Affects Sheep Intestinal Epithelial Cells against Escherichia coli F17 Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell culture and Transfection
2.2. E. coli F17 Adherence Assay
2.3. Cell Proliferation
2.4. RT-qPCR
2.5. Western Blot
2.6. Scratch Assay
2.7. Statistical Analysis
3. Results
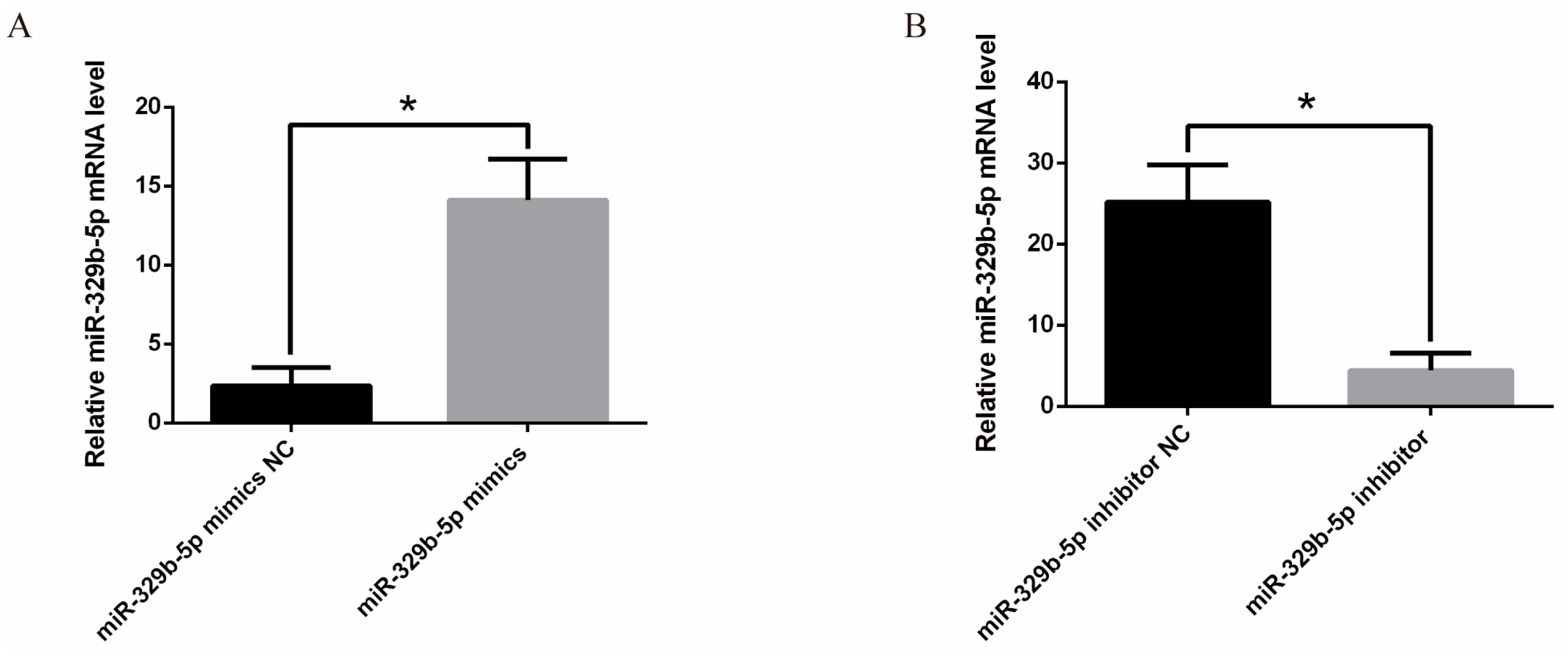
3.1. Effects of miR-329b-5p Mimics and miR-329b-5p Inhibitor
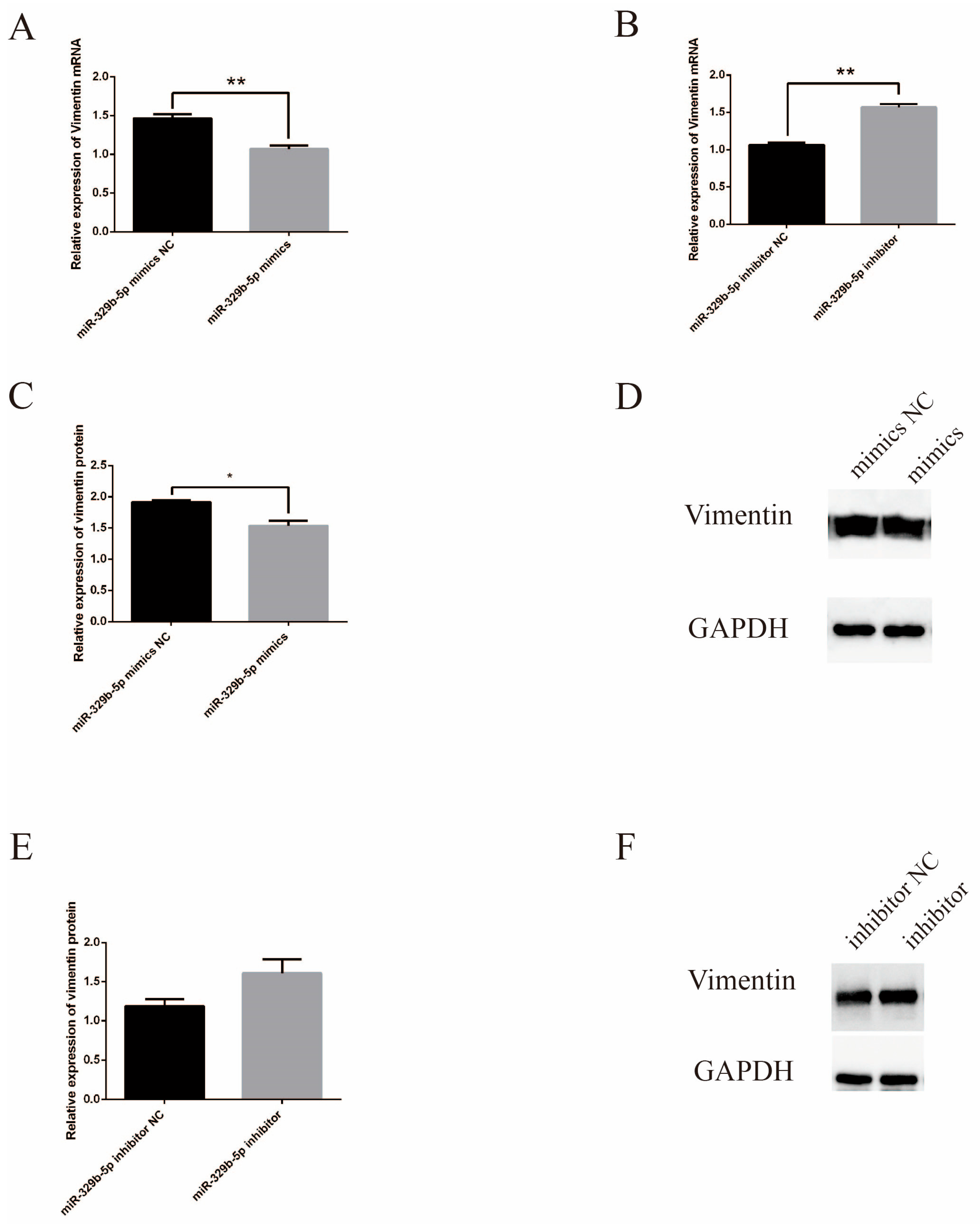
3.2. miR-329b-5p Influences the E. coli F17 Susceptibility of Sheep IEC s
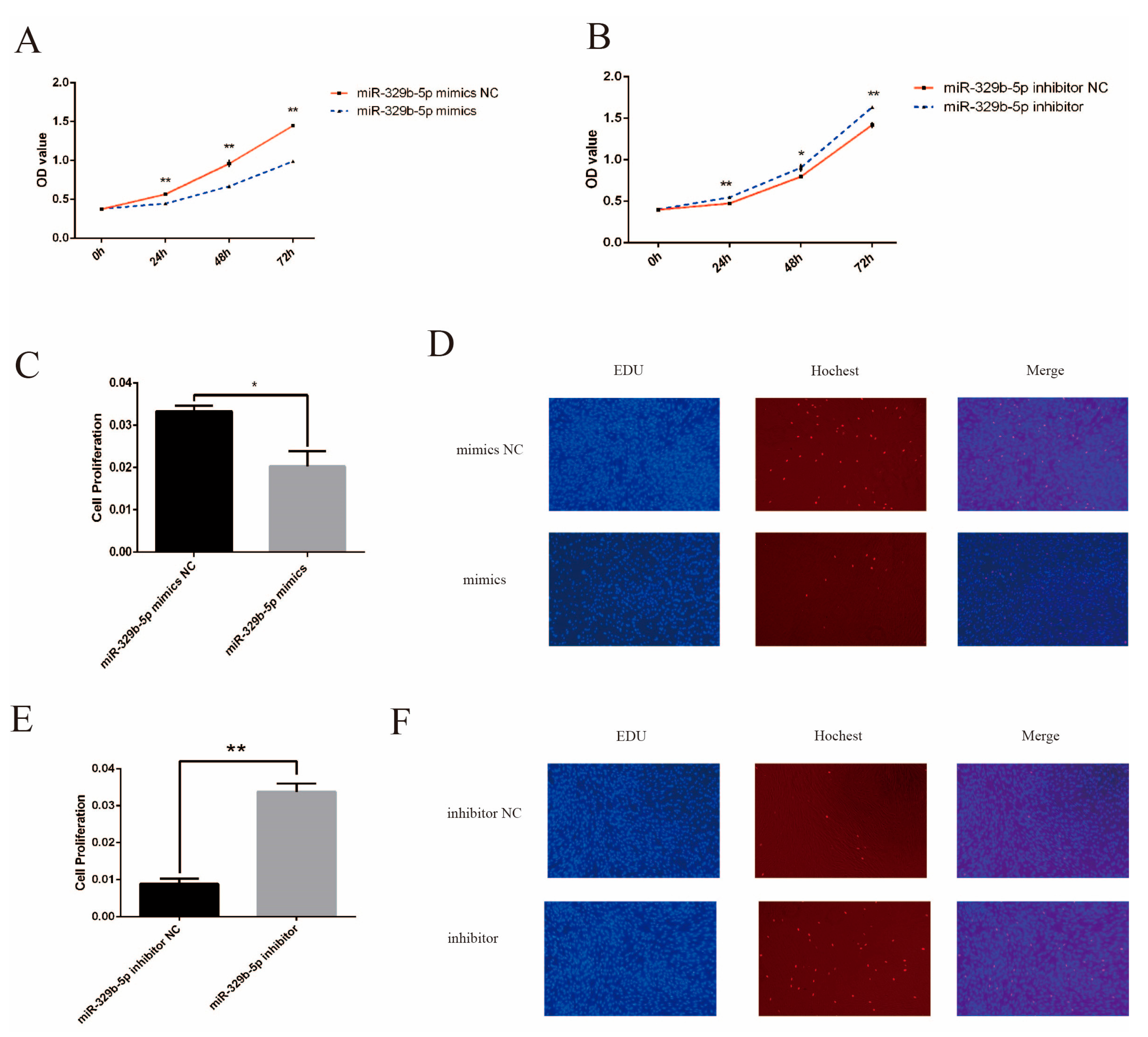
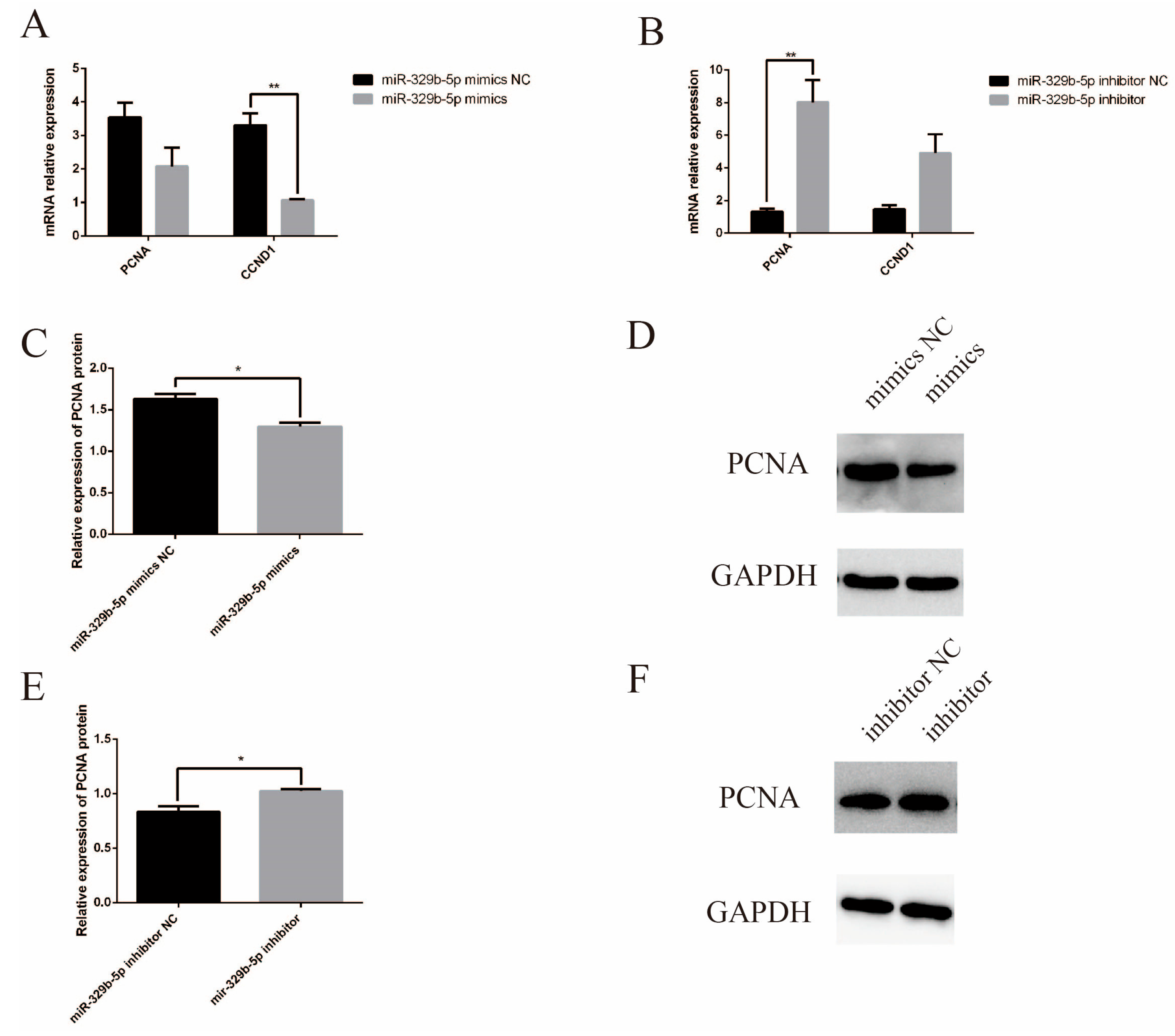
3.3. miR-329b-5p Suppress the Proliferation of IECs
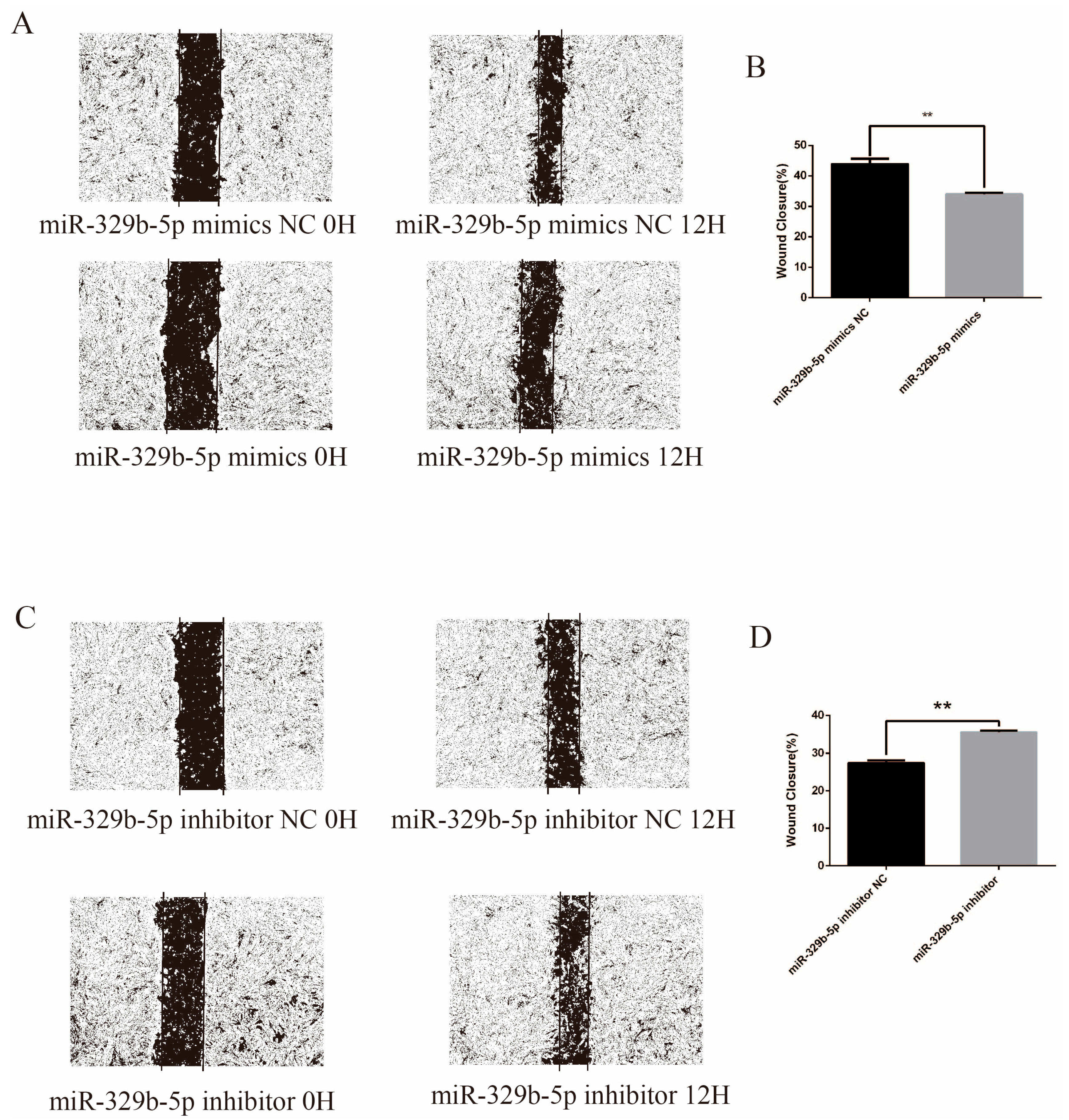
3.4. miR-329b-5p Suppress the Migration of IECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Bihannic, M.; Ghanbarpour, R.; Auvray, F.; Cavalié, L.; Châtre, P.; Boury, M.; Brugère, H.; Madec, J.Y.; Oswald, E. Identification and detection of three new F17 fimbrial variants in Escherichia coli strains isolated from cattle. Vet. Res. 2014, 45, 76. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, R.; Burdukiewicz, M.; Schierack, P. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Front. Cell. Infect. Microbiol. 2015, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, M.R.; Şahin, M. Prevalence of neonatal calf diarrhea caused by Escherichia coli and investigation of virulence factors, serotypes, and antibiotic susceptibility. Pol. J. Vet. Sci. 2023, 26, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Siuce, J.; Maturrano, L.; Wheeler, J.C.; Rosadio, R. Diarrheagenic Escherichia coli isolates from neonatal alpacas mainly display F17 fimbriae adhesion gene. Trop. Anim. Health Prod. 2020, 52, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Zouiten-Mekki, L.; Serghini, M.; Fekih, M.; Kallel, L.; Matri, S.; Ben Mustapha, N.; Boubaker, J.; Filali, A. Epithelial cell in intestinal homeostasis and inflammatory bowel diseases. Med. Sci. M/S 2013, 29, 1145–1150. [Google Scholar]
- Xiao, X.; Mao, X.; Chen, D.; Yu, B.; He, J.; Yan, H.; Wang, J. miRNAs Can Affect Intestinal Epithelial Barrier in Inflammatory Bowel Disease. Front. Immunol. 2022, 13, 868229. [Google Scholar] [CrossRef]
- Li, X.G.; Wang, Z.; Chen, R.Q.; Fu, H.L.; Gao, C.Q.; Yan, H.C.; Xing, G.X.; Wang, X.Q. LGR5 and BMI1 Increase Pig Intestinal Epithelial Cell Proliferation by Stimulating WNT/β-Catenin Signaling. Int. J. Mol. Sci. 2018, 19, 1036. [Google Scholar] [CrossRef]
- Mo, W.; Liu, G.; Wu, C.; Jia, G.; Zhao, H.; Chen, X.; Wang, J. STIM1 promotes IPEC-J2 porcine epithelial cell restitution by TRPC1 signaling. Anim. Biotechnol. 2022, 33, 1492–1503. [Google Scholar] [CrossRef]
- Wu, L.M.; Guo, R.; Hui, L.; Ye, Y.G.; Xiang, J.M.; Wan, C.Y.; Zou, M.; Ma, R.; Sun, X.Z.; Yang, S.J.; et al. Stanniocalcin-1 protects bovine intestinal epithelial cells from oxidative stress-induced damage. J. Vet. Sci. 2014, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.F.; Li, P.H.; Zhu, Y.; Zheng, S.S.; Liu, J.W.; Song, S.Q. MicroRNA-186 suppresses cell proliferation and metastasis in bladder cancer. Afr. Health Sci. 2022, 22, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lynam-Lennon, N.; Maher, S.G.; Reynolds, J.V. The roles of microRNA in cancer and apoptosis. Biol. Rev. Camb. Philos. Soc. 2009, 84, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.J.; Park, H.T. MicroRNA Mediated Regulation of Schwann Cell Migration and Proliferation in Peripheral Nerve Injury. BioMed Res. Int. 2018, 2018, 8198365. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, J.; Xu, T.; Yu, X. MiR-329 suppresses osteosarcoma development by downregulating Rab10. FEBS Lett. 2016, 590, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Zhang, T.; Wen, C.; Shi, M.; Tang, X.; Chen, H.; Peng, C.; Li, H.; Fang, Y.; et al. mir-329 restricts tumor growth by targeting grb2 in pancreatic cancer. Oncotarget 2016, 7, 21441–21453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, D.; Shi, Y.; Hu, Y.; Du, X.; Tu, G. miR-329-3p regulates neural stem cell proliferation by targeting E2F1. Mol. Med. Rep. 2019, 19, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pei, F.; Men, X.; Wang, K.; Ma, D. miR-329 inhibits papillary thyroid cancer progression via direct targeting WNT1. Oncol. Lett. 2018, 16, 3561–3568. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Wang, D.Y. Overexpression of miR-329-3p sensitizes osteosarcoma cells to cisplatin through suppression of glucose metabolism by targeting LDHA. Cell Biol. Int. 2021, 45, 766–774. [Google Scholar] [CrossRef]
- Xin, R.Q.; Li, W.B.; Hu, Z.W.; Wu, Z.X.; Sun, W. MiR-329-3p inhibits hepatocellular carcinoma cell proliferation and migration through USP22-Wnt/β-Catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9932–9939. [Google Scholar]
- Zhao, Y.; Jia, Y.; Wang, J.; Chen, X.; Han, J.; Zhen, S.; Yin, S.; Lv, W.; Yu, F.; Wang, J.; et al. circNOX4 activates an inflammatory fibroblast niche to promote tumor growth and metastasis in NSCLC via FAP/IL-6 axis. Mol. Cancer 2024, 23, 47. [Google Scholar] [CrossRef]
- Archanioti, P.; Gazouli, M.; Theodoropoulos, G.; Vaiopoulou, A.; Nikiteas, N. Micro-RNAs as regulators and possible diagnostic bio-markers in inflammatory bowel disease. J. Crohn’s Colitis 2011, 5, 520–524. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Xu, J.; Li, Y.; Zhao, R.; Du, S.; Lv, C.; Wu, W.; Liu, R.; Sheng, X.; Song, Y.; et al. MicroRNA-31 Reduces Inflammatory Signaling and Promotes Regeneration in Colon Epithelium, and Delivery of Mimics in Microspheres Reduces Colitis in Mice. Gastroenterology 2019, 156, 2281–2296.e6. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.C.; Sincavage, J.; Feinstein, S.; Mah, A.T.; Simmons, J.G.; Lund, P.K.; Sethupathy, P. miR-30 Family Controls Proliferation and Differentiation of Intestinal Epithelial Cell Models by Directing a Broad Gene Expression Program That Includes SOX9 and the Ubiquitin Ligase Pathway. J. Biol. Chem. 2016, 291, 15975–15984. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, Y.; Ge, W.; Zhou, K.; Wen, J.; Yan, W.; Wang, Y.; Wang, B.; Qu, C.; Wu, J.; et al. miR-200b inhibits TGF-β1-induced epithelial-mesenchymal transition and promotes growth of intestinal epithelial cells. Cell Death Dis. 2013, 4, e541. [Google Scholar] [CrossRef]
- Xiao, L.; Ma, X.X.; Luo, J.; Chung, H.K.; Kwon, M.S.; Yu, T.X.; Rao, J.N.; Kozar, R.; Gorospe, M.; Wang, J.Y. Circular RNA CircHIPK3 Promotes Homeostasis of the Intestinal Epithelium by Reducing MicroRNA 29b Function. Gastroenterology 2021, 161, 1303–1317.e3. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Ding, J.; Zhang, Y.; Cai, M.; Yang, J.; Cho, W.C.; Zheng, Y. microRNA-21: A key modulator in oncogenic viral infections. RNA Biol. 2021, 18, 809–817. [Google Scholar] [CrossRef]
- Poltronieri, P.; Sun, B.; Huang, K.Y.; Chang, T.H.; Lee, T.Y. State-of-the-Art on Viral microRNAs in HPV Infection and Cancer Development. MicroRNA 2018, 7, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Staedel, C.; Darfeuille, F. MicroRNAs and bacterial infection. Cell. Microbiol. 2013, 15, 1496–1507. [Google Scholar] [CrossRef]
- Yang, B.; Yang, R.; Xu, B.; Fu, J.; Qu, X.; Li, L.; Dai, M.; Tan, C.; Chen, H.; Wang, X. miR-155 and miR-146a collectively regulate meningitic Escherichia coli infection-mediated neuroinflammatory responses. J. Neuroinflammation 2021, 18, 114. [Google Scholar] [CrossRef]
- Sun, L.; Wu, S.; Dai, C.H.; Sun, S.Y.; Zhu, G.Q.; Wu, S.L.; Bao, W.B. Insight into the molecular mechanism of miR-192 regulating Escherichia coli resistance in piglets. Biosci. Rep. 2018, 38, BSR20171160. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.H.; Wang, F.; Wang, S.Q.; Wu, Z.C.; Wu, S.L.; Bao, W.B. miR-215 Targeting Novel Genes EREG, NIPAL1 and PTPRU Regulates the Resistance to E.coli F18 in Piglets. Genes 2020, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zou, S.; Yuan, Z.; Chen, W.; Wang, S.; Cao, X.; Lv, X.; Getachew, T.; Mwacharo, J.M.; Haile, A.; et al. Sheep β-Defensin 2 Regulates Escherichia coli F17 Resistance via NF-κB and MAPK Signaling Pathways in Ovine Intestinal Epithelial Cells. Biology 2021, 10, 1356. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Zhou, X.; Kaper, J.B. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect. Immun. 2005, 73, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Ivanov, P.A.; Senchenkova, S.N.; Naumenko, O.I.; Ovchinnikova, O.O.; Shashkov, A.S.; Golomidova, A.K.; Babenko, V.V.; Kulikov, E.E.; Letarov, A.V. Structure and gene cluster of the O antigen of Escherichia coli F17, a candidate for a new O-serogroup. Int. J. Biol. Macromol. 2019, 124, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Buts, L.; Bouckaert, J.; De Genst, E.; Loris, R.; Oscarson, S.; Lahmann, M.; Messens, J.; Brosens, E.; Wyns, L.; De Greve, H. The fimbrial adhesin F17-G of enterotoxigenic Escherichia coli has an immunoglobulin-like lectin domain that binds N-acetylglucosamine. Mol. Microbiol. 2003, 49, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Umpiérrez, A.; Ernst, D.; Fernández, M.; Oliver, M.; Casaux, M.L.; Caffarena, R.D.; Schild, C.; Giannitti, F.; Fraga, M.; Zunino, P. Virulence genes of Escherichia coli in diarrheic and healthy calves. Rev. Argent. Microbiol. 2021, 53, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xiao, J.; Wu, N.; Liu, C.; Xu, J.; Liu, F.; Wu, L. MicroRNA-223 Regulates the Differentiation and Function of Intestinal Dendritic Cells and Macrophages by Targeting C/EBPβ. Cell Rep. 2015, 13, 1149–1160. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, H.; Hao, Y.; Xu, F.; Yang, J.; Zhang, R.; Lu, G.; Zheng, Z.; Cui, M.; Qi, C.F.; et al. Reprogramming macrophage orientation by microRNA 146b targeting transcription factor IRF5. EBioMedicine 2016, 14, 83–96. [Google Scholar] [CrossRef]
- Mikami, Y.; Philips, R.L.; Sciumè, G.; Petermann, F.; Meylan, F.; Nagashima, H.; Yao, C.; Davis, F.P.; Brooks, S.R.; Sun, H.W.; et al. MicroRNA-221 and -222 modulate intestinal inflammatory Th17 cell response as negative feedback regulators downstream of interleukin-23. Immunity 2021, 54, 514–525.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Zhou, M.; Chen, Y.; McAllister, T.A.; Plastow, G.; Stanford, K.; Selinger, B.; Guan, L.L. MicroRNAomes of Cattle Intestinal Tissues Revealed Possible miRNA Regulated Mechanisms Involved in Escherichia coli O157 Fecal Shedding. Front. Cell. Infect. Microbiol. 2021, 11, 634505. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.; Hadlich, F.; Kemper, N.; Lübke-Becker, A.; Muráni, E.; Wimmers, K.; Ponsuksili, S. MicroRNA expression profiling of porcine mammary epithelial cells after challenge with Escherichia coli in vitro. BMC Genom. 2017, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lv, X.; Zhang, W.; Hu, T.; Cao, X.; Ren, Z.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Sun, W. Non-Coding Transcriptome Provides Novel Insights into the Escherichia coli F17 Susceptibility of Sheep Lamb. Biology 2022, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Fang, R.H.; Wu, J.; Si, Y.; Jia, S.Q.; Li, Q.; Bai, J.Z.; She, X.N.; Wang, J.Q. MicroRNA-329 upregulation impairs the HMGB2/β-catenin pathway and regulates cell biological behaviors in melanoma. J. Cell Physiol. 2019, 234, 23518–23527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.; Fang, J. Progress on the mechanisms of Lactobacillus plantarum to improve intestinal barrier function in ulcerative colitis. J. Nutr. Biochem. 2024, 124, 109505. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Wang, P.; Wang, F. Intestinal barrier dysfunction in severe burn injury. Burn. Trauma 2019, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Y.; Hu, W.; Li, F.; Sheng, H.; Huang, C.; Gou, X.; Hou, J.; Zheng, J.; Xiao, Y. Luminol-conjugated cyclodextrin biological nanoparticles for the treatment of severe burn-induced intestinal barrier disruption. Burn. Trauma 2024, 12, tkad054. [Google Scholar] [CrossRef]
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Cairns, C.A.; Xiao, L.; Wang, J.Y. Posttranscriptional Regulation of Intestinal Mucosal Growth and Adaptation by Noncoding RNAs in Critical Surgical Disorders. J. Investig. Surg. Off. J. Acad. Surg. Res. 2024, 37, 2308809. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ling, P.; Li, Y.; Wang, Y.; Li, G.; Qiu, C.; Wang, J.; Gong, K. miR-138-5p ameliorates intestinal barrier disruption caused by acute superior mesenteric vein thrombosis injury by inhibiting the NLRP3/HMGB1 axis. PeerJ 2024, 12, e16692. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, Y.; Gao, J.; Zheng, M.; Wang, P.; Zu, G. miR-379-5P inhibition enhances intestinal epithelial proliferation and barrier function recovery after ischemia/reperfusion by targeting EIF4G2. Shock 2023, 60, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Cardano, M.; Tribioli, C.; Prosperi, E. Targeting Proliferating Cell Nuclear Antigen (PCNA) as an Effective Strategy to Inhibit Tumor Cell Proliferation. Curr. Cancer Drug Targets 2020, 20, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Paunesku, T.; Mittal, S.; Protić, M.; Oryhon, J.; Korolev, S.V.; Joachimiak, A.; Woloschak, G.E. Proliferating cell nuclear antigen (PCNA): Ringmaster of the genome. Int. J. Radiat. Biol. 2001, 77, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Bologna-Molina, R.; Mosqueda-Taylor, A.; Molina-Frechero, N.; Mori-Estevez, A.D.; Sánchez-Acuña, G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e174–e179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Luo, F.; Fan, X.; Chen, J.; Zhang, C.; Hui, R. KCTD10 interacts with proliferating cell nuclear antigen and its down-regulation could inhibit cell proliferation. J. Cell. Biochem. 2009, 106, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.G.; Zhu, B.; Lv, S.Q.; Zhu, H.; Tang, J.; Huang, C.; Li, Q.; Zhou, P.; Wang, D.L.; Li, G.H. Inhibition of EGR1 inhibits glioma proliferation by targeting CCND1 promoter. J. Exp. Clin. Cancer Res. CR 2017, 36, 186. [Google Scholar] [CrossRef]
- Nardone, V.; Barbarino, M.; Angrisani, A.; Correale, P.; Pastina, P.; Cappabianca, S.; Reginelli, A.; Mutti, L.; Miracco, C.; Giannicola, R.; et al. CDK4, CDK6/cyclin-D1 Complex Inhibition and Radiotherapy for Cancer Control: A Role for Autophagy. Int. J. Mol. Sci. 2021, 22, 8391. [Google Scholar] [CrossRef]
- Chen, Z.M.; Yu, Q.; Chen, G.; Tang, R.X.; Luo, D.Z.; Dang, Y.W.; Wei, D.M. MiR-193a-3p inhibits pancreatic ductal adenocarcinoma cell proliferation by targeting CCND1. Cancer Manag. Res. 2019, 11, 4825–4837. [Google Scholar] [CrossRef]
- Zang, Y.; Li, J.; Wan, B.; Tai, Y. circRNA circ-CCND1 promotes the proliferation of laryngeal squamous cell carcinoma through elevating CCND1 expression via interacting with HuR and miR-646. J. Cell. Mol. Med. 2020, 24, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, T.; Li, Z.; Lu, S. FGFR1 regulates proliferation and metastasis by targeting CCND1 in FGFR1 amplified lung cancer. Cell Adhes. Migr. 2020, 14, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.; Kuczmarski, E.R.; Herrmann, H.; Goldman, R.D. Intermediate Filaments Play a Pivotal Role in Regulating Cell Architecture and Function. J. Biol. Chem. 2015, 290, 17145–17153. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Viedma-Poyatos, Á.; Navarro-Carrasco, E.; Martínez, A.E.; Pajares, M.A.; Pérez-Sala, D. Vimentin filaments interact with the actin cortex in mitosis allowing normal cell division. Nat. Commun. 2019, 10, 4200. [Google Scholar] [CrossRef] [PubMed]
- Karoii, D.H.; Azizi, H.; Amirian, M. Signaling Pathways and Protein-Protein Interaction of Vimentin in Invasive and Migration Cells: A Review. Cell. Reprogramming 2022, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.M.; Bayless, K.J. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation 2014, 21, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, 1796. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Ostrowska-Podhorodecka, Z.; McCulloch, C.A. Vimentin regulates the assembly and function of matrix adhesions. Wound Repair Regen. 2021, 29, 602–612. [Google Scholar] [CrossRef]
- Bornheim, R.; Müller, M.; Reuter, U.; Herrmann, H.; Büssow, H.; Magin, T.M. A dominant vimentin mutant upregulates Hsp70 and the activity of the ubiquitin-proteasome system, and causes posterior cataracts in transgenic mice. J. Cell Sci. 2008, 121 Pt 22, 3737–3746. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Y.; Ruan, Y.; Lu, Y.; Lin, D.; Xie, Y.; Dong, B.; Dang, Q.; Quan, C. CLDN6 enhances chemoresistance to ADM via AF-6/ERKs pathway in TNBC cell line MDAMB231. Mol. Cell Biochem. 2018, 443, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.K.; Yeh, K.T.; Hsieh, M.J.; Yeh, C.M.; Lin, C.C.; Kao, C.Y.; Huang, L.R.; Lin, S.H. UNC13C Suppress Tumor Progression via Inhibiting EMT Pathway and Improves Survival in Oral Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Lee, Y.S.; Yun, N.H.; Shin, C.H.; Hong, H.K.; Kim, H.H.; Cho, Y.B. MicroRNA-17-5p regulates EMT by targeting vimentin in colorectal cancer. Br. J. Cancer 2020, 123, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [PubMed]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122 Pt 18, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fu, L.; Deng, J.; Zhang, J.; Zou, Y.; Liao, L.; Ma, X.; Li, Z.; Xu, Y.; Xu, Y.; et al. miR-301a Deficiency Attenuates the Macrophage Migration and Phagocytosis through YY1/CXCR4 Pathway. Cells 2022, 11, 3952. [Google Scholar] [CrossRef]
- Liu, W.G.; Zhuo, L.; Lu, Y.; Wang, L.; Ji, Y.X.; Guo, Q. miR-874-3p inhibits cell migration through targeting RGS4 in osteosarcoma. J. Gene Med. 2020, 22, e3213. [Google Scholar] [CrossRef]

| Gene Name | Sequences (5′→3′) | Product Length/bp | Accession No. |
|---|---|---|---|
| F17b-A | F: CAACTAACGGGATGTACAGTTTC R: CTGATAAGCGATGGTGTAATTAAC | 323 | L14318.1 |
| F17b-G | F: CGTGGGAAATTATCTATCAACG R: TGTTGATATTCCGTTAACCGTAC | 615 | L14319.1 |
| Vimentin | F: CTGCTAACCGCAACAACGAC R: TAGTCCCTTTGAGCGCATCC | 108 | XM_004014247.6 |
| PCNA | F: TCTGCAAGTGGAGAACTTGGAA R: AGGAGACAGTGGAGTGGCTT | 162 | XM_004014340.5 |
| CCND1 | F: CCGAGGAGAACAAGCAGATC R: GAGGGTGGGTTGGAAATG | 91 | XM_027959928.2 |
| GAPDH | F: TCTCAAGGGCATTCTAGGCTAC R: GCCGAATTCATTGTCGTACCAG | 151 | XM_060411593.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Chen, W.; Yang, H.; Song, Z.; Wang, Y.; Su, R.; Mwacharo, J.M.; Lv, X.; Sun, W. miR-329b-5p Affects Sheep Intestinal Epithelial Cells against Escherichia coli F17 Infection. Vet. Sci. 2024, 11, 206. https://doi.org/10.3390/vetsci11050206
Xu Y, Chen W, Yang H, Song Z, Wang Y, Su R, Mwacharo JM, Lv X, Sun W. miR-329b-5p Affects Sheep Intestinal Epithelial Cells against Escherichia coli F17 Infection. Veterinary Sciences. 2024; 11(5):206. https://doi.org/10.3390/vetsci11050206
Chicago/Turabian StyleXu, Yeling, Weihao Chen, Huiguo Yang, Zhenghai Song, Yeqing Wang, Rui Su, Joram M. Mwacharo, Xiaoyang Lv, and Wei Sun. 2024. "miR-329b-5p Affects Sheep Intestinal Epithelial Cells against Escherichia coli F17 Infection" Veterinary Sciences 11, no. 5: 206. https://doi.org/10.3390/vetsci11050206
APA StyleXu, Y., Chen, W., Yang, H., Song, Z., Wang, Y., Su, R., Mwacharo, J. M., Lv, X., & Sun, W. (2024). miR-329b-5p Affects Sheep Intestinal Epithelial Cells against Escherichia coli F17 Infection. Veterinary Sciences, 11(5), 206. https://doi.org/10.3390/vetsci11050206






