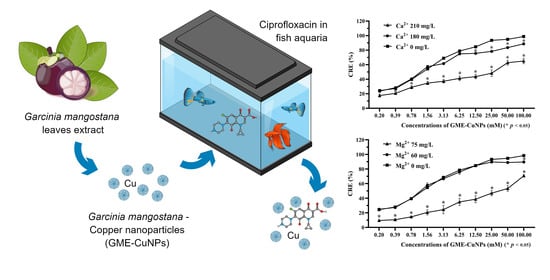
Effects of Salts and Other Contaminants on Ciprofloxacin Removal Efficiency of Green Synthesized Copper Nanoparticles
Abstract
Simple Summary
Abstract
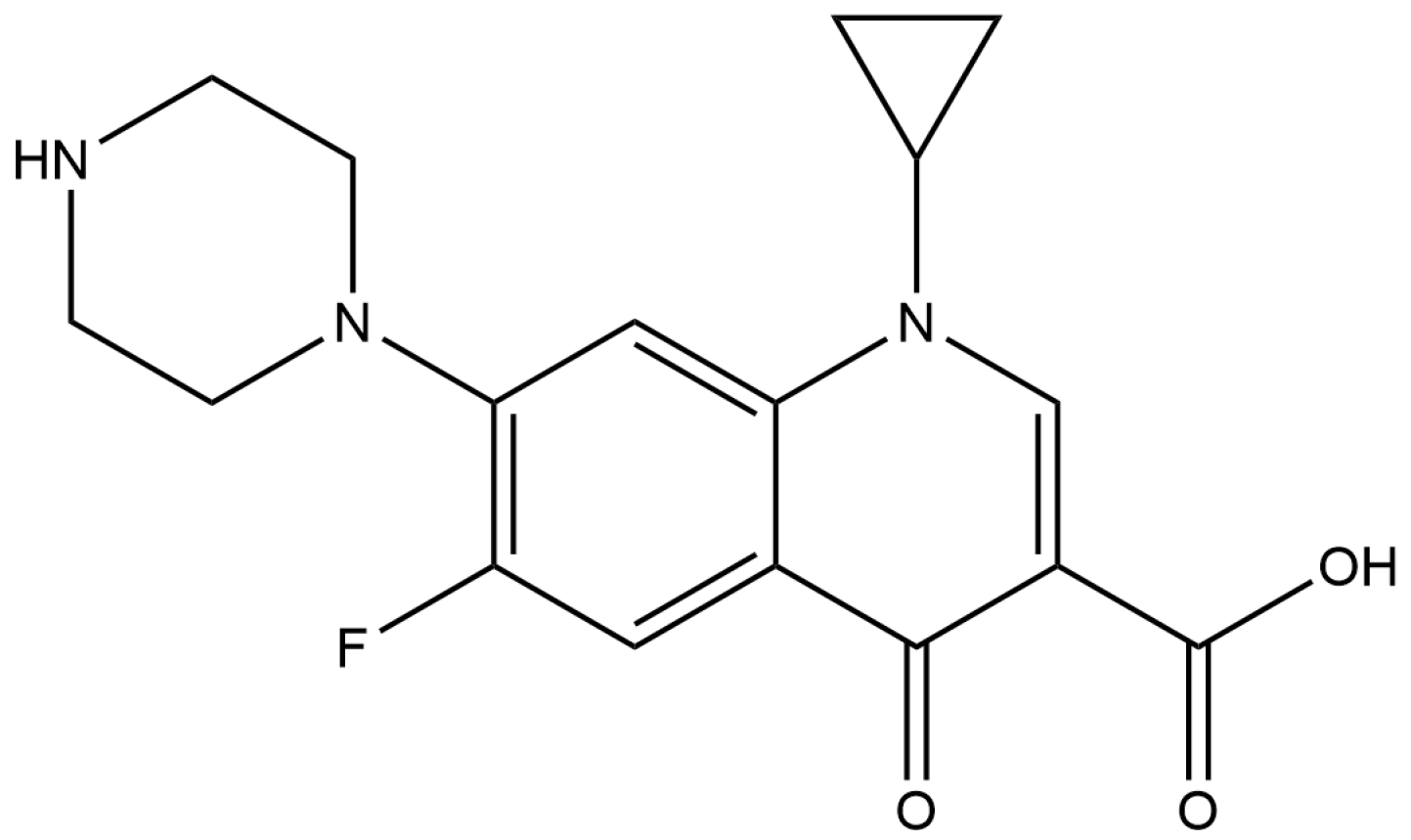
1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Plant and Extract Preparation
2.3. Chemical Analysis of GME
2.4. Green Synthesis of CuNPs
2.5. CIP Removal Efficiency (CRE) of GME-CuNPs
2.6. Effects of Salts on CRE
2.7. Effects of Fish Wastewater on CRE
2.8. Statistical Analysis
3. Results
3.1. Chemical Constituents of GME
3.2. Green Synthesis of GME-CuNPs
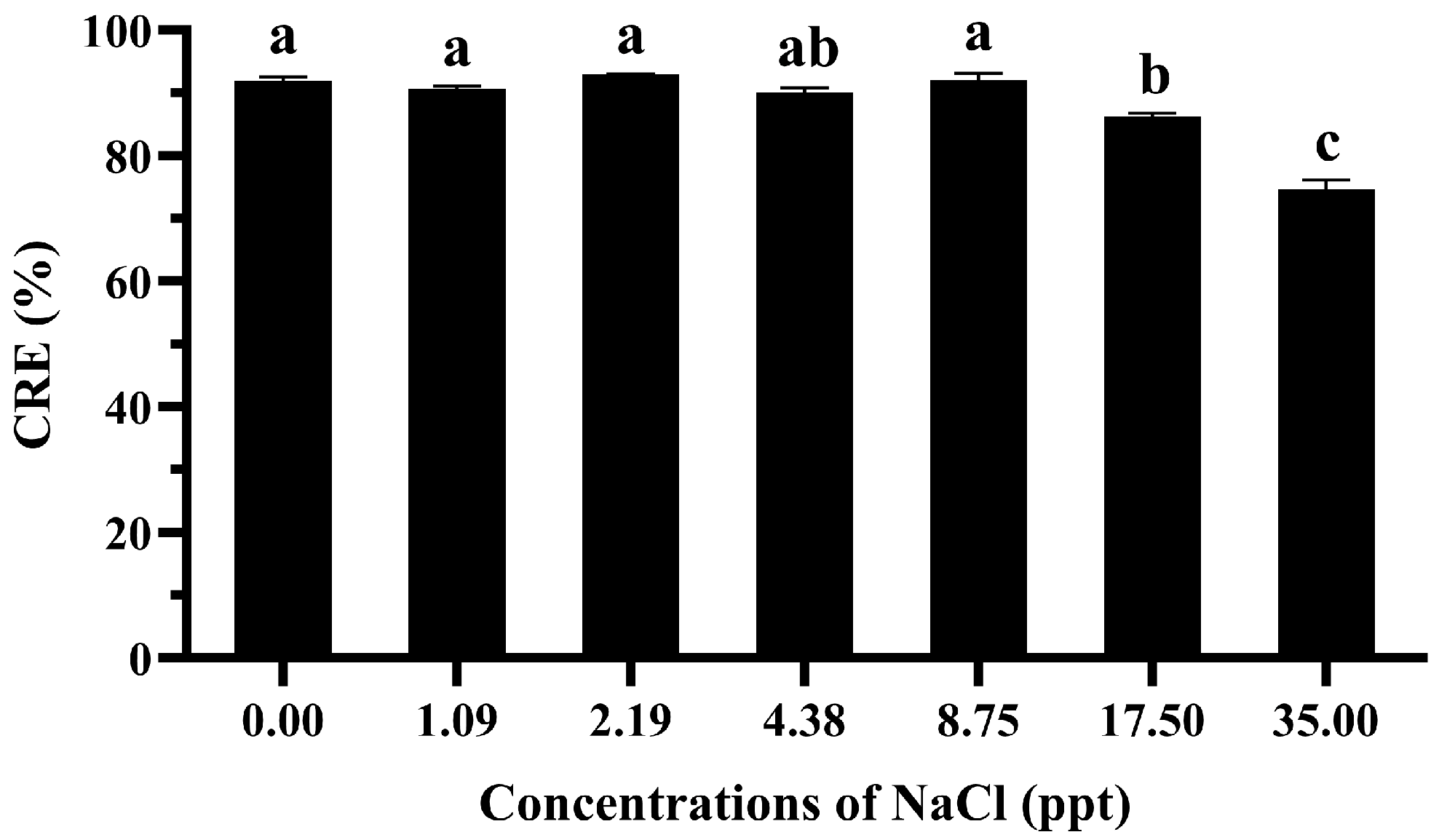
3.3. Effects of Sodium Chloride on CRE of GME-CuNPs
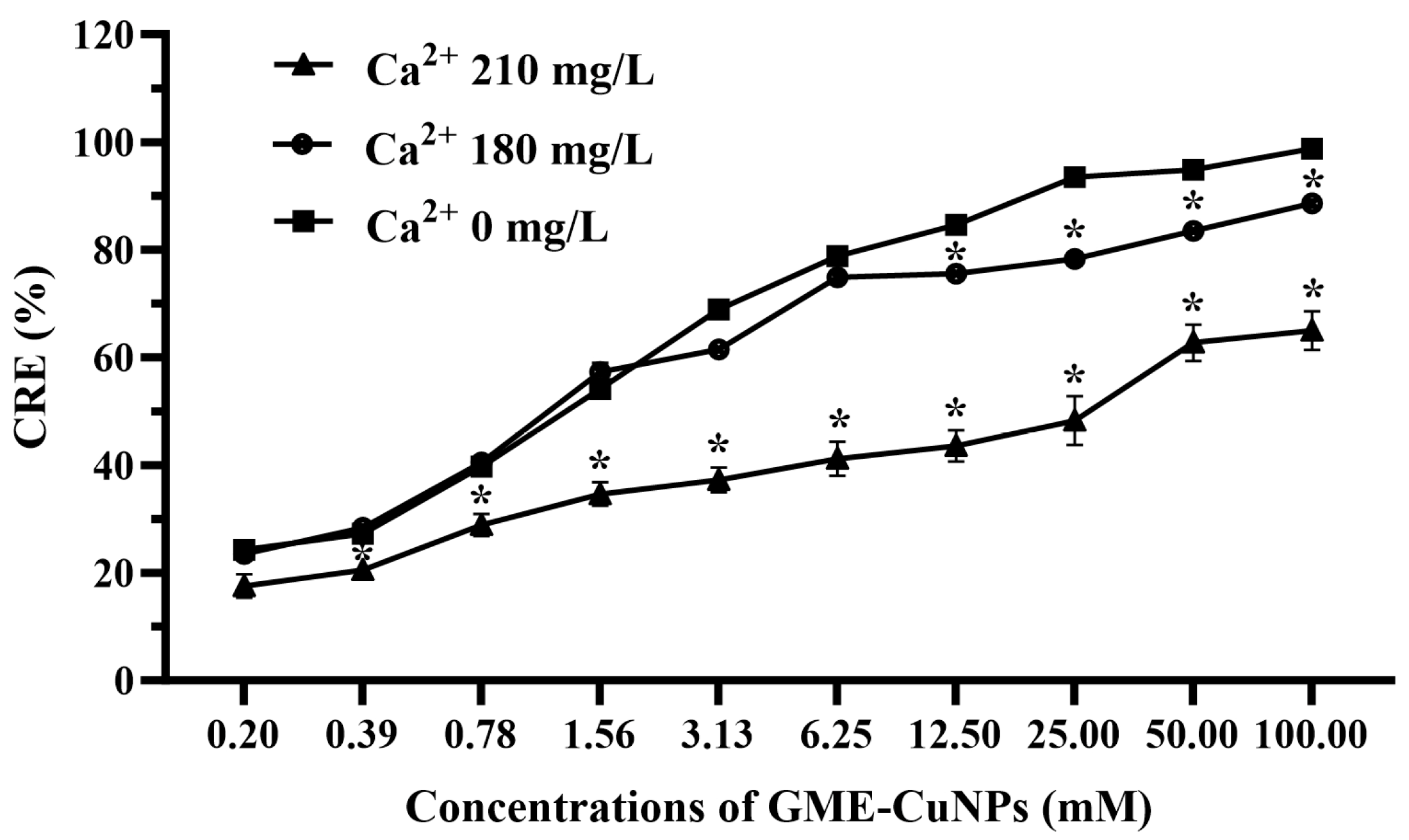
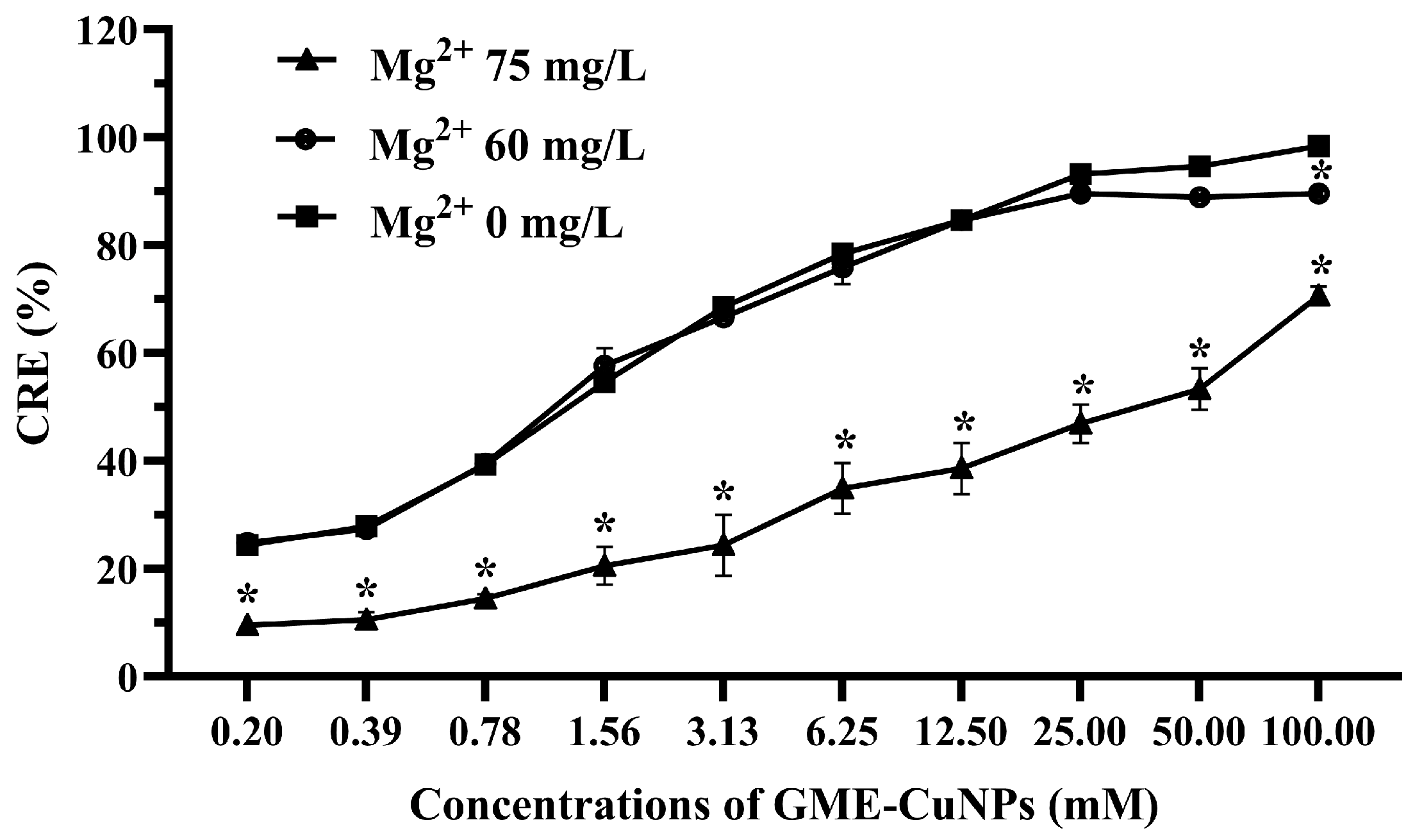
3.4. Effects of Divalent Salts on CRE of GME-CuNPs
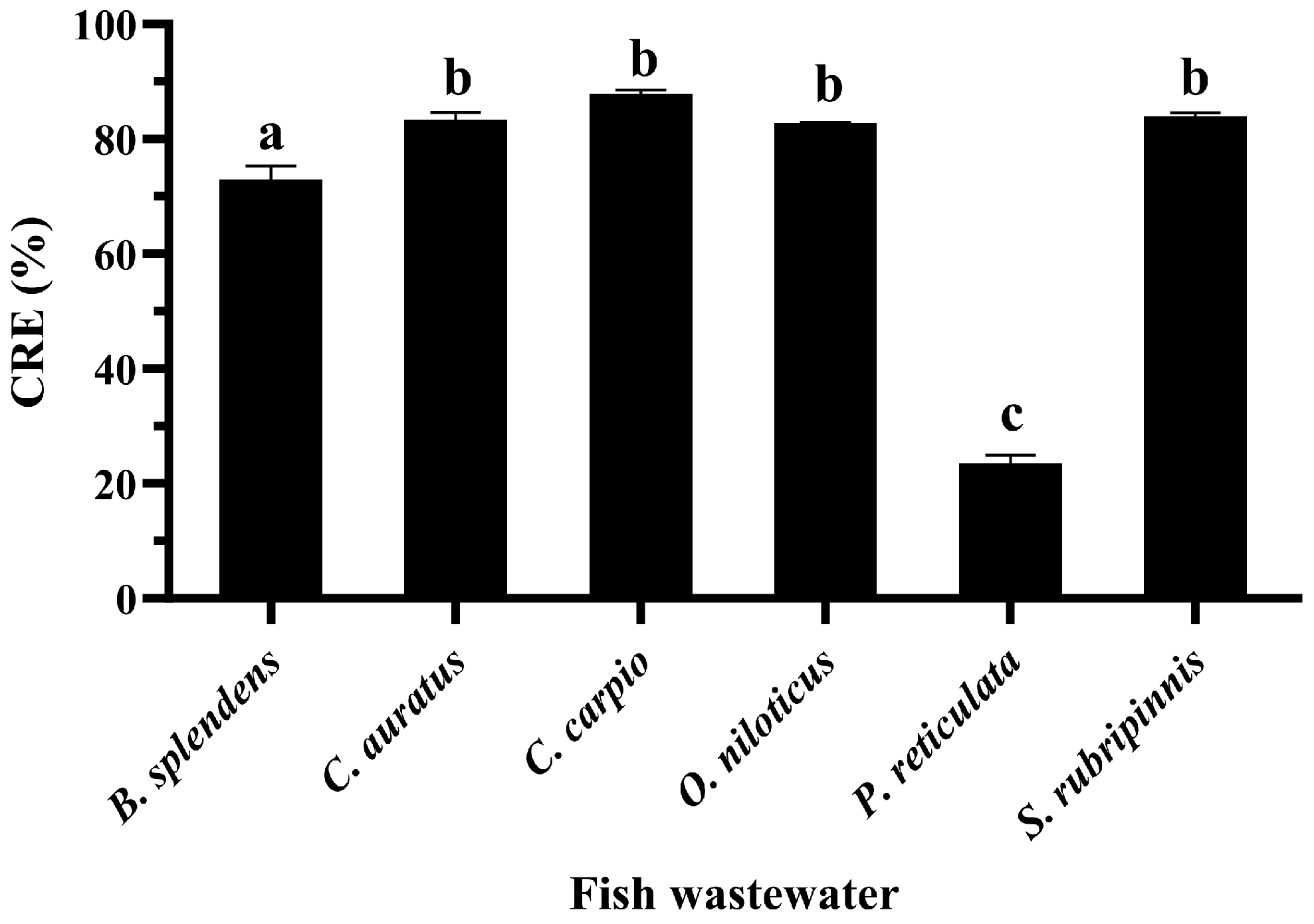
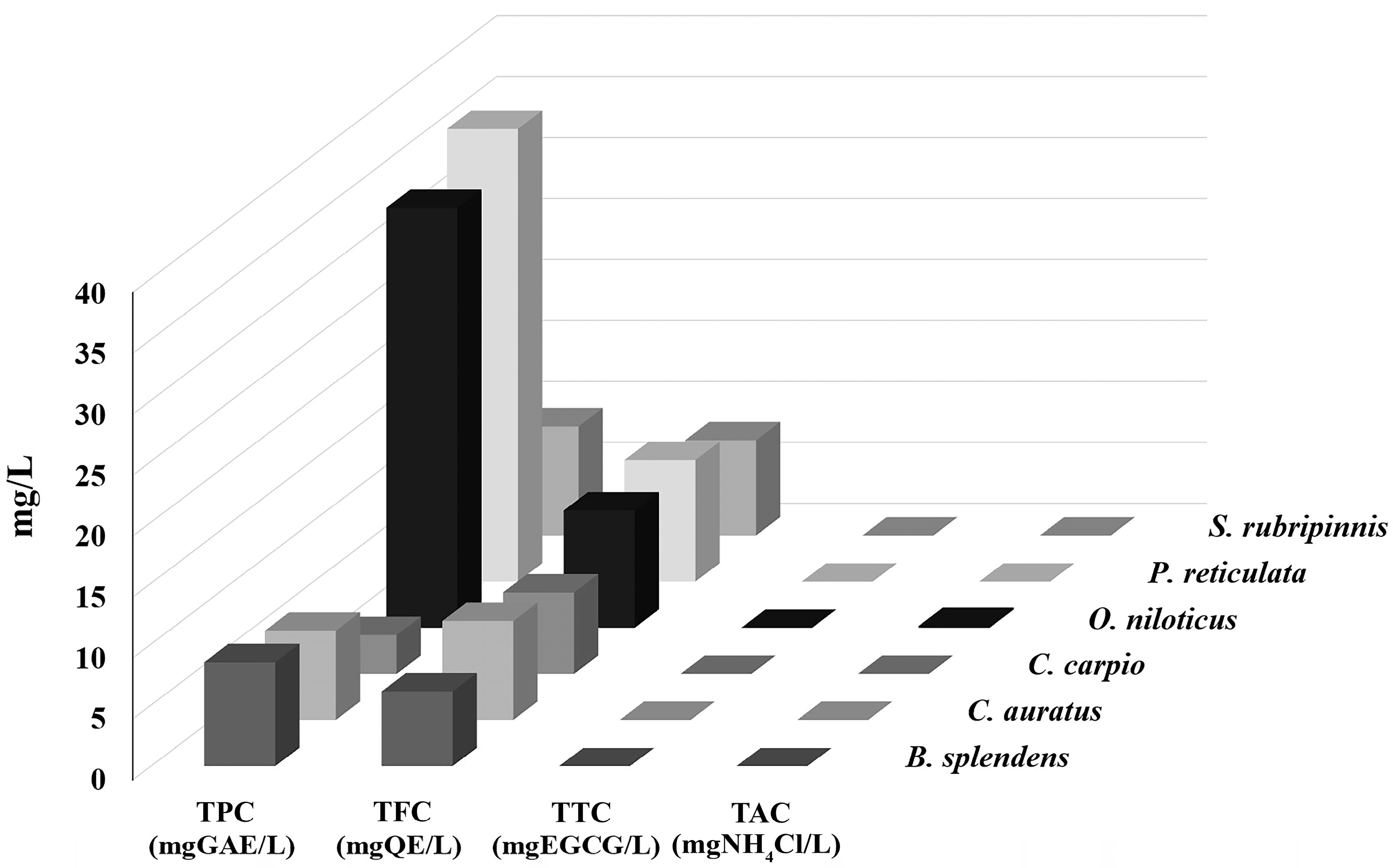
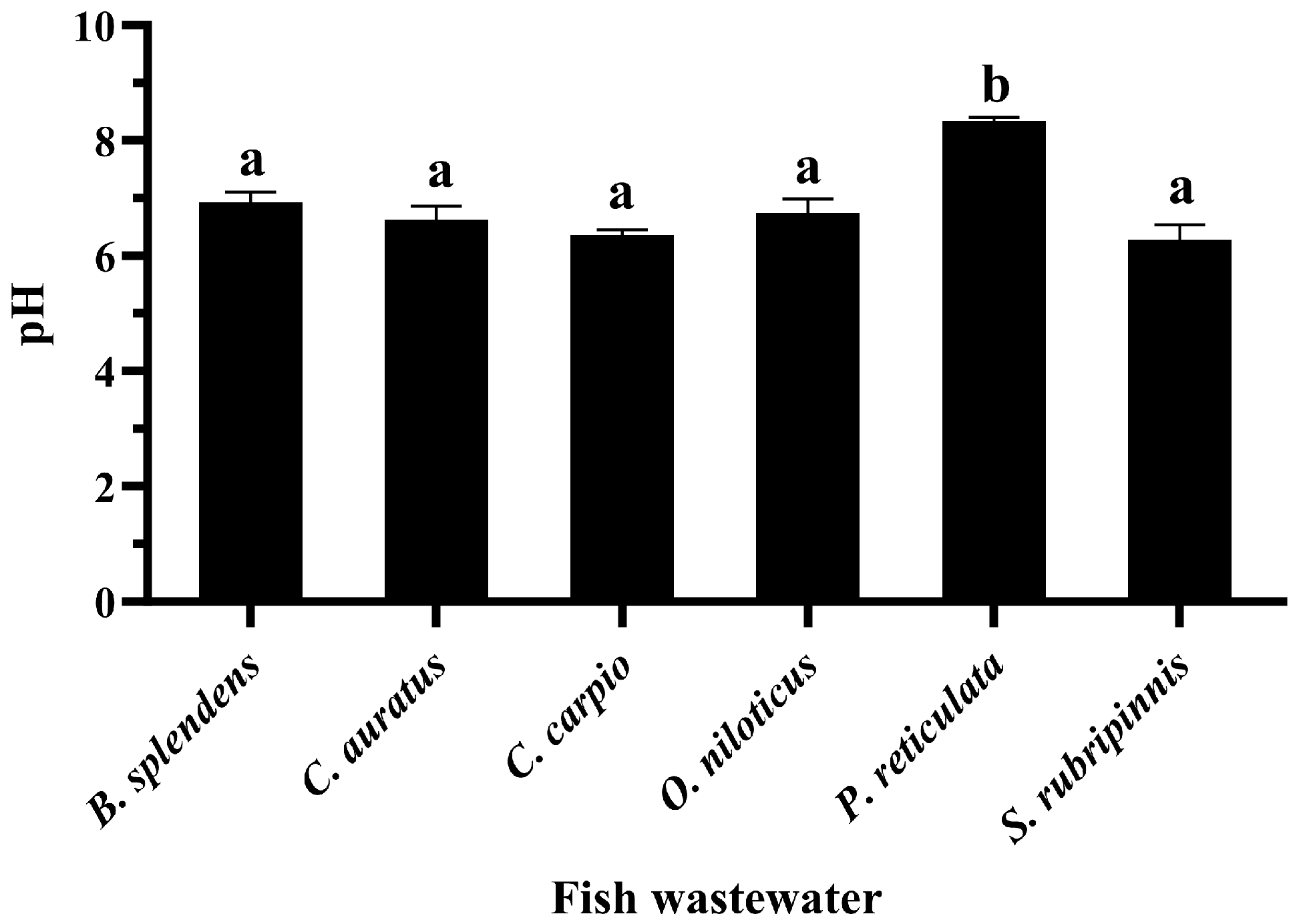
3.5. CRE of GME-CuNPs in Fish Aquaria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drlica, K.; Zhao, X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997, 61, 377–392. [Google Scholar] [PubMed]
- Garoff, L.; Huseby, D.L.; Praski Alzrigat, L.; Hughes, D. Effect of aminoacyl-tRNA synthetase mutations on susceptibility to ciprofloxacin in Escherichia coli. J. Antimicrob. Chemother. 2018, 73, 3285–3292. [Google Scholar] [CrossRef] [PubMed]
- Hal, A.M.; Manal, I. Effect of Nigella sativa oil and ciprofloxacin against bacterial infection on gene expression in Nile tilapia (Oreochromis niloticus) blood. Aquaculture 2021, 532, 736071. [Google Scholar] [CrossRef]
- Kuang, S.; Su, Y.; Wang, H.; Yu, W.; Lang, Q.; Matangi, R. Soil microbial community structure and diversity around the aging oil sludge in yellow river eelta as determined by high-throughput sequencing. Archaea 2018, 2018, 7861805. [Google Scholar] [CrossRef]
- Shariati-Rad, M.; Heidari, S. Classification and determination of total hardness of water using silver nanoparticles. Talanta 2020, 219, 121297. [Google Scholar] [CrossRef]
- Nwabuife, J.C.; Omolo, C.A.; Govender, T. Nano delivery systems to the rescue of ciprofloxacin against resistant bacteria “E. coli, P. aeruginosa, Saureus, and MRSA” and their infections. J. Control. Release 2022, 349, 338–353. [Google Scholar] [CrossRef]
- Husein, D.Z.; Hassanien, R.; Al-Hakkani, M.F. Green-synthesized copper nano-adsorbent for the removal of pharmaceutical pollutants from real wastewater samples. Heliyon 2019, 5, e02339. [Google Scholar] [CrossRef]
- Sassa-deepaeng, T.; Yodthong, W.; Khumpirapang, N.; Anuchapreeda, S.; Okonogi, S. Effects of plant-based copper nanoparticles on the elimination of ciprofloxacin. Drug Discov. Ther. 2023, 17, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Lichak, M.R.; Barber, J.R.; Kwon, Y.M.; Francis, K.X.; Bendesky, A. Care and Use of Siamese Fighting Fish (Betta splendens) for Research. Comp. Med. 2022, 72, 169–180. [Google Scholar] [CrossRef]
- Dinh-Hung, N.; Dong, H.; Senapin, S.; Linh, N.; Shinn, A.; Pirarat, N.; Hirono, I.; Chatchaiphan, S.; Rodkhum, C. Infection and histopathological consequences in Siamese fighting fish (Betta splendens) due to exposure to a pathogenic Mycobacterium chelonae via different routes. Aquaculture 2024, 579, 740191. [Google Scholar] [CrossRef]
- Dong, H.; Senapin, S.; Phiwsaiya, K.; Techatanakitarnan, C.; Dokladda, K.; Ruenwongsa, P.B.P. Histopathology and culturable bacteria associated with “big belly” and “skin nodule” syndromes in ornamental Siamese fighting fish, Betta splendens. Microb. Pathog. 2018, 122, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Narendrakumar, L.; Sudhagar, A.; Preena, P.G.; Nithianantham, S.R.; Mohandas, S.P.; Swaminathan, T.R. Detection of Mycobacterium marinum and multidrug-resistant bacteria in a chronic progressive disease outbreak among Siamese fighting fish (Betta splendens) in India. Biologia 2022, 77, 2725–2733. [Google Scholar] [CrossRef]
- Lü, A.J.; Wang, R.X.; Hu, X.C.; Sun, J.F.; Li, L.; Pei, C.; Zhang, C.; Nie, G.X. First report of Rahnella aquatilis infection in crucian carp Carassius auratus in China. Dis. Aquat. Organ. 2017, 123, 205–212. [Google Scholar] [CrossRef]
- Rosidah, R.; Yunita, M.D.; Nurruhwati, I.; Rizal, A. Histopathological Changes in Gold Fish (Carassius auratus (Linnaeus, 1758)) Infected by Aeromonas hydrophila Bacteria with Various Densities. World Sci. News 2020, 142, 150–168. [Google Scholar]
- Shan, Q.; Huang, H.; Zheng, G.; Yin, Y.; Zhu, X.; Ma, L.; Zhou, H.; Xie, W.; Li, L.; Liu, S. Pharmacokinetics and tissue residue profiles of enrofloxacin in crucian carp (Carassius auratus gibelio) following single and multiple oral administration. Front. Vet. Sci. 2022, 9, 872828. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M. Role of common carp (Cyprinus carpio) in aquaculture production systems. Front. Life Sci. 2015, 8, 399–410. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Zhang, D.; Xing, L.; Sun, W.; Sun, X.; Peng, J.; Zhang, Y.; Li, X. Pharmacokinetics of enrofloxacin and its metabolite in carp (Cyprinus carpio) after a single oral administration in medicated feed. J. Ocean Univ. China 2023, 22, 171–180. [Google Scholar] [CrossRef]
- Vajargah, M.F. A review of the physiology and biology of Nile tilapia (Oreochromis niloticus). J. Aquac. Mar. Biol. 2021, 10, 244–246. [Google Scholar] [CrossRef]
- Ali, S.E.; Mahana, O.; Mohan, C.V.; Delamare-Deboutteville, J.; Elgendy, M.Y. Genetic characterization and antimicrobial profiling of bacterial isolates collected from Nile tilapia (Oreochromis niloticus) affected by summer mortality syndrome. J. Fish Dis. 2022, 45, 1857–1871. [Google Scholar] [CrossRef]
- Hossain, S.; De Silva, B.C.J.; Dahanayake, P.S.; De Zoysa, M.; Heo, G.-J. Phylogenetic characteristics, virulence properties and antibiogram profile of motile Aeromonas spp. isolated from ornamental guppy (Poecilia reticulata). Arch. Microbiol. 2020, 202, 501–509. [Google Scholar] [CrossRef]
- Susilo, U.; Rachmawati, F.N.; Wibowo, E.S. Protease and Amylase Activities of Javaen barb (Systomus rubripinnis Val.). J. Biodjati 2022, 7, 45–55. [Google Scholar] [CrossRef]
- Shang, J.; Gao, X. Nanoparticle Counting: Towards Accurate Determination of the Molar Concentration. Chem. Soc. Rev. 2014, 43, 7267–7278. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Le, H.B.; Dong, T.O.; Pham, T.D. Determination of fluoroquinolones in pharmaceutical formulations by extractive spectrophotometric methods using ion-pair complex formation with bromothymol blue. J. Anal. Methods Chem. 2018, 2018, 8436948. [Google Scholar] [CrossRef] [PubMed]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Verlag Chemie GmbH: Weinheim, Germany, 1983; ISBN 3527295895. [Google Scholar]
- Khumpirapang, N.; Pikulkaew, S.; Anuchapreeda, S.; Okonogi, S. Alpinia galanga oil—A new natural source of fish anaesthetic. Aquac. Res. 2018, 49, 1546–1556. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Hardness in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Sasarom, M.; Wanachantararak, P.; Chaijareenont, P.; Okonogi, S. Biosynthesis of copper oxide nanoparticles using Caesalpinia sappan extract: In vitro evaluation of antifungal and antibiofilm activities against Candida albicans. Drug Discov. Ther. 2023, 17, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Jara-Cobos, L.; Peñafiel, M.E.; Menendez, M.; Abad-Delgado, D.; Ponce-Montalvo, J. Removal of ciprofloxacin from an aqueous medium by adsorption on natural and hydrolyzed bentonites. Front. Environ. Sci. 2023, 11, 1239754. [Google Scholar] [CrossRef]
- Atiya, M.A.; Hassan, A.K.; Kadhim, F.Q. Green synthesis of copper nanoparticles using tea leaves extract to remove ciprofloxacin (CIP) from aqueous media. Iraqi J. Sci. 2021, 62, 2832–2854. [Google Scholar] [CrossRef]
- Liu, W.; Sutton, N.B.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Pharmaceutical removal from water with iron-or manganese-based technologies: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1584–1621. [Google Scholar] [CrossRef]
- He, P.; Mao, T.; Wang, A.; Yin, Y.; Shen, J.; Chen, H.; Zhang, P. Enhanced reductive removal of ciprofloxacin in pharmaceutical wastewater using biogenic palladium nanoparticles by bubbling H2. RSC Adv. 2020, 10, 26067–26077. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Kareem, S.L. Enhancement of ciprofloxacin antibiotic removal from aqueous solution using ZnO nanoparticles coated on pistachio shell. Desalin Water Treat 2021, 213, 229–239. [Google Scholar] [CrossRef]
- Pham, T.D.; Vu, T.N.; Nguyen, H.L.; Le, P.H.P.; Hoang, T.S. Adsorptive removal of antibiotic ciprofloxacin from aqueous solution using protein-modified nanosilica. Polymers 2020, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Arshad, F.; Hussain, Z.; Mukhtar, M. Green adeptness in the synthesis and stabilization of copper nanoparticles: Catalytic, antibacterial, cytotoxicity, and antioxidant activities. Nanoscale Res. Lett. 2017, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.I.S.M.H.; Omar, A.F.; Rashid, M.; Hashim, U. VIS-NIR spectral and particles distribution of Au, Ag, Cu, Al and Ni nanoparticles synthesized in distilled water using laser ablation. Results Phys. 2019, 14, 102497. [Google Scholar] [CrossRef]
- Yodthong, W. Green synthesized copper nanoparticles and their anti-bacterial properties against bullfrog multidrug resistant gram negative bacteria. Vet. Integr. Sci. 2019, 17, 33–49. [Google Scholar]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Harshiny, M.; Iswarya, C.N.; Matheswaran, M. Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaf extract as a reducing agent. Powder Technol. 2015, 286, 744–749. [Google Scholar] [CrossRef]
- Sarip, N.A.; Aminudin, N.I.; Danial, W.H. Green synthesis of metal nanoparticles using garcinia extracts: A review. Environ. Chem. Lett. 2021, 20, 469–493. [Google Scholar] [CrossRef]
- Okonogi, S.; Duangrat, C.; Anuchpreeda, S.; Tachakittirungrod, S.; Chowwanapoonpohn, S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007, 103, 839–846. [Google Scholar] [CrossRef]
- da Demenciano, S.C.; Silva, M.C.B.L.; Alexandrino, C.A.F.; Kato Junior, W.H.; de Figueiredo, P.O.; Garcez, W.S.; Campos, R.P.; de Guimarães, R.C.A.; Sarmento, U.C.; Bogo, D. Antiproliferative activity and antioxidant potential of extracts of Garcinia gardneriana. Molecules 2020, 25, 3201. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- do Espirito Santo, B.L.S.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.O.; Bogo, D.; de Freitas, K.C.; de Guimarães, R.C.A.; Hiane, P.A.; Pott, A.; de Filiú, W.F.O. Medicinal potential of Garcinia species and their compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef] [PubMed]
- Sungpud, C.; Panpipat, W.; Sae-Yoon, A.; Chaijan, M. Polyphenol extraction from mangosteen (Garcinia mangostana Linn) pericarp by bio-based solvents. Int. Food Res. J. 2020, 27, 111–120. [Google Scholar]
- Hasheminya, S.M.; Dehghannya, J. Green synthesis and characterization of copper nanoparticles using Eryngium caucasicum Trautv aqueous extracts and its antioxidant and antimicrobial properties. Part. Sci. Technol. 2020, 38, 1019–1026. [Google Scholar] [CrossRef]
- Magondu, E.W.; Rasowo, J.; Oyoo-Okoth, E.; Charo-Karisa, H. Evaluation of sodium chloride (NaCl) for potential prophylactic treatment and its short-term toxicity to African catfish Clarias gariepinus (Burchell 1822) yolk-sac and swim-up fry. Aquaculture 2011, 319, 307–310. [Google Scholar] [CrossRef]
- Peixoto, P.G.; Oliveira, R.V.; Lima, I.M.; Pelli, A. Sodium chloride as reducing agent of stress induced in guppy Poecillia reticulata Peters, 1859. J. Health Sci. Inst. 2014, 32, 12–16. [Google Scholar]
- Ijmtst, E. Ocean Salinity. Encycl. Astrobiol. 2015, 1753. [Google Scholar]
- Al-Mustafa, J.; Taha, Z.A. Thermodynamics of the complexation of ciprofloxacin with calcium and magnesium perchlorate. Thermochim. Acta 2011, 521, 9–13. [Google Scholar] [CrossRef]
- Saana, S.B.B.M.; Fosu, S.A.; Sebiawu, G.E.; Jackson, N.; Karikari, T. Assessment of the quality of groundwater for drinking purposes in the upper West and Northern regions of Ghana. SpringerPlus 2016, 5, 2001. [Google Scholar] [PubMed]
- Hu, R.; Huang, T.; Wen, G.; Tang, Z.; Liu, Z.; Li, K. Pilot study on the softening rules and regulation of water at various hardness levels within a chemical crystallization circulating pellet fluidized bed system. J. Water Process Eng. 2021, 41, 102000. [Google Scholar] [CrossRef]
- El-Bendary, N.; El-Etriby, H.K.; Mahanna, H. Reuse of adsorption residuals for enhancing removal of ciprofloxacin from wastewater. Environ. Technol. 2022, 43, 4438–4454. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of ciprofloxacin from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]

| Peak No. | RT (min) | Detected m/z | Assigned Ion | MS/MS | Tentative Identification | Formula | Error (ppm) |
|---|---|---|---|---|---|---|---|
| 1 | 2.303 | 185.0668 | [M+H]+ | 127.0251, 84.9603 | Pentane-1,2,2,3,3,4,4-heptol | C5H12O7 | −6.60 |
| 2 | 3.145 | 118.0865 | [M+NH4]+ | 97.0291, 59.0497 | Isopropenyl acetate | C5H8O2 | −2.07 |
| 3 | 3.285 | 209.0309 | [M-H]− | 191.0170, 129.0176, 85.0288, 59.0136 | Glucaric acid | C6H10O8 | −2.91 |
| 4 | 3.428 | 193.0722 | [M+H]+ | 129.0552, 111.0447, 83.0496, 69.0340, 55.0544 | Quinic acid | C7H12O6 | −7.95 |
| 191.0554 | [M-H]− | 127.0384, 85.0288, 59.0136 | 3.72 | ||||
| 5 | 3.761 | 300.1258 | [M+H]+ | 173.0454, 93.0553, 75.0445, 57.0341 | Unidentified | - | - |
| 6 | 3.989 | 133.0138 | [M-H]− | 115.0024, 71.0134 | Malic acid | C4H6O5 | 3.36 |
| 7 | 4.546 | 189.0035 | [M-H]− | 127.0021, 96.9592, 83.0132, 57.0341 | Oxalosuccinic acid | C6H6O7 | 3.05 |
| 8 | 4.770 | 222.0611 | [M+NH4]+ | 169.0147, 113.0239, 97.0290, 58.0655 | Daucic acid | C7H8O7 | −1.22 |
| 9 | 4.863 | 191.0209 | [M-H]− | 111.0078.87.0084 | Citric acid | C6H8O7 | −6.15 |
| 10 | 4.906 | 236.0774 | [M+NH4]+ | 201.0410, 183.0302, 155.0348, 109.0291, 81.0333, 59.0499 | Ascorbic acid-6-acetate | C8H10O7 | −3.90 |
| 217.0347 | [M-H]− | 199.0220, 155.0326, 127.0388, 83.0496 | 3.12 | ||||
| 11 | 7.097 | 166.0867 | [M+H]+ | 120.0816, 103.0548, 77.0387 | Phenyl alanine | C9H11NO2 | −2.68 |
| 12 | 7.579 | 595.1456 | [M+H]+ | 443.0991, 425.0889, 289.0719, 127.0394 | Epicatechin-(4beta->8)- gallocatechin | C30H26O13 | −1.65 |
| 593.1317 | [M-H]− | 441.0767, 315.0464, 153.0181, 125.0227 | |||||
| 13 | 9.820 | 355.1042 | [M+H]+ | 163.0402, 145.0297, 117.0330, 89.0389 | Caffeoylquinic acid | C16H18O9 | −5.19 |
| 353.0875 | [M-H]− | 191.0541, 93.0333 | 0.87 | ||||
| 14 | 10.103 | 579.1536 | [M+H]+ | 427.1049, 409.0944, 333.0998, 291.0881, 247.0616, 163.0398, 127.0395 | Procyanidin B2 | C30H26O12 | −6.73 |
| 577.1356 | [M-H]− | 425.0830, 407.0724, 289.0690, 125.0242 | −0.78 | ||||
| 15 | 10.706 | 867.2184 | [M+H]+ | 579.1529, 425.0882, 289.0724, 247.0612, 127.0396 | Procyanidin trimer | C45H38O18 | −6.12 |
| 865.1987 | [M-H]− | 797.1475, 713.1401, 575.1106, 407.0715, 287.0526, 125.0223 | −1.90 | ||||
| 16 | 10.726 | 291.0879 | [M+H]+ | 207.0667, 139.0399, 123.0448 | Catechin | C15H14O6 | −5.45 |
| 289.0713 | [M-H]− | 245.0796, 151.0388, 125.0236, 57.0349 | 1.60 | ||||
| 17 | 11.207 | 337.0931 | [M-H]− | 191.0544, 145.0282, 93.0341 | Coumaroylquinic acid | C16H18O8 | −0.62 |
| 339.1102 | [M+H]+ | 147.0452, 91.0541 | −8.13 | ||||
| 18 | 11.671 | 611.1634 | [M+H]+ | 465.1035, 303.0517, 85.0288 | Rutin | C27H30O16 | −4.48 |
| 609.1461 | [M-H]− | 0.01 | |||||
| 19 | 11.862 | 741.1857 | [M+H]+ | 571.1276, 451.1049, 289.0719, 179.0348, 123.0446 | Cinchonain IIa | C39H32O15 | −5.81 |
| 739.1679 | [M-H]− | 587.1122, 289.0695, 125.0265 | −1.43 | ||||
| 20 | 11.990 | 433.1158 | [M+H]+ | 313.0726, 283.0620 | Pueraria glycoside | C21H20O10 | −6.64 |
| 431.0989 | [M-H]− | 311.0522, 217.0108, 151.0025, 59.0137 | −1.23 | ||||
| 21 | 12.798 | 451.1243 | [M+H]+ | 305.0676, 191.0348, 129.0553, 85.0290 | Astilbin | C21H22O11 | −1.80 |
| 449.1087 | [M-H]− | 303.0476, 285.0374, 151.0025, 65.0035 | 0.52 | ||||
| 22 | 13.083 | 451.1252 | [M+H]+ | 305.0677, 259.0614, 195.0300, 129.0555, 85.0290, 71.0497 | Astilbin derivative | C21H22O11 | −3.79 |
| 449.1083 | [M-H]− | 303.0481, 285.0372, 151.0025 | 1.41 | ||||
| 23 | 13.135 | 449.1083 | [M-H]− | 407.0721, 303.0478, 285.0377, 151.0025 | Astilbin derivative | C21H22O11 | 1.41 |
| 24 | 13.321 | 373.2236 | [M+H]+ | 211.1711, 193.1601, 175.1499, 135.1180, 109.1017, 69.0703 | 9-Hydroxy-7-megastigmen- 3-one glucoside | C19H32O7 | −4.07 |
| 25 | 13.697 | 300.2552 | [M+H]+ | 282.2446, 251.2010, 135.1167, 95.0860 | N-Lauroyl Valine | C17H33NO3 | −6.26 |
| 26 | 13.959 | 314.2712 | [M+H]+ | 296.2604, 135.1181, 72.0814 | Palmitoylglycine | C18H35NO3 | −7.09 |
| 27 | 14.392 | 340.1049 | [M+NH4]+ | 173.0459, 105.0342, 77.0383 | Dulcisflavan | C15H14O8 | −6.49 |
| 321.0608 | [M-H]− | 297.0306, 199.0225, 155.0335, 127.0391 | 2.46 | ||||
| 28 | 14.569 | 453.1204 | [M+H]+ | 411.1099, 343.0837, 301.0729, 191.0355, 165.0563, 123.0450 | Cinchonain Ia | C24H20O9 | −5.28 |
| 451.1032 | [M-H]− | 341.0627, 217.0118, 109.0289 | 0.57 | ||||
| 29 | 17.257 | 274.2756 | [M+H]+ | 230.2494, 149.0244, 88.0764, 57.0703 | Unidentified | - | - |
| 30 | 17.455 | 505.1385 | [M-H]− | 447.0674, 343.0748, 240.9990, 96.9593 | Caryatin glucoside | C24H26O12 | −6.63 |
| 31 | 32.277 | 403.2354 | [M+H]+ | 259.1557, 217.0356, 185.0823, 157.0139, 129.0188, 57.0699 | 5S,15S-dihydroxy-9S,11R,8S,12S-diperoxy-6E,13E-eicosadienoic acid | C20H34O8 | −6.83 |
| 32 | 32.714 | 379.1558 | [M-H]− | 324.0949, 281.0411, 180.9696, 112.9845 | 6-Deoxy-gamma-mangostin | C23H24O5 | −1.85 |
| 33 | 33.817 | 395.1501 | [M-H]− | 351.0819, 283.0207, 152.9937, 78.9583 | Gamma-mangostin | C23H24O6 | −0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassa-deepaeng, T.; Khumpirapang, N.; Yodthong, W.; Myat, Y.Y.; Anuchapreeda, S.; Okonogi, S. Effects of Salts and Other Contaminants on Ciprofloxacin Removal Efficiency of Green Synthesized Copper Nanoparticles. Vet. Sci. 2024, 11, 179. https://doi.org/10.3390/vetsci11040179
Sassa-deepaeng T, Khumpirapang N, Yodthong W, Myat YY, Anuchapreeda S, Okonogi S. Effects of Salts and Other Contaminants on Ciprofloxacin Removal Efficiency of Green Synthesized Copper Nanoparticles. Veterinary Sciences. 2024; 11(4):179. https://doi.org/10.3390/vetsci11040179
Chicago/Turabian StyleSassa-deepaeng, Tanongsak, Nattakanwadee Khumpirapang, Wachira Yodthong, Yin Yin Myat, Songyot Anuchapreeda, and Siriporn Okonogi. 2024. "Effects of Salts and Other Contaminants on Ciprofloxacin Removal Efficiency of Green Synthesized Copper Nanoparticles" Veterinary Sciences 11, no. 4: 179. https://doi.org/10.3390/vetsci11040179
APA StyleSassa-deepaeng, T., Khumpirapang, N., Yodthong, W., Myat, Y. Y., Anuchapreeda, S., & Okonogi, S. (2024). Effects of Salts and Other Contaminants on Ciprofloxacin Removal Efficiency of Green Synthesized Copper Nanoparticles. Veterinary Sciences, 11(4), 179. https://doi.org/10.3390/vetsci11040179