Body Weight as a Preferred Method for Normalizing the Computed Tomography-Derived Liver Volume in Dogs without Hepatic Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Case Selection
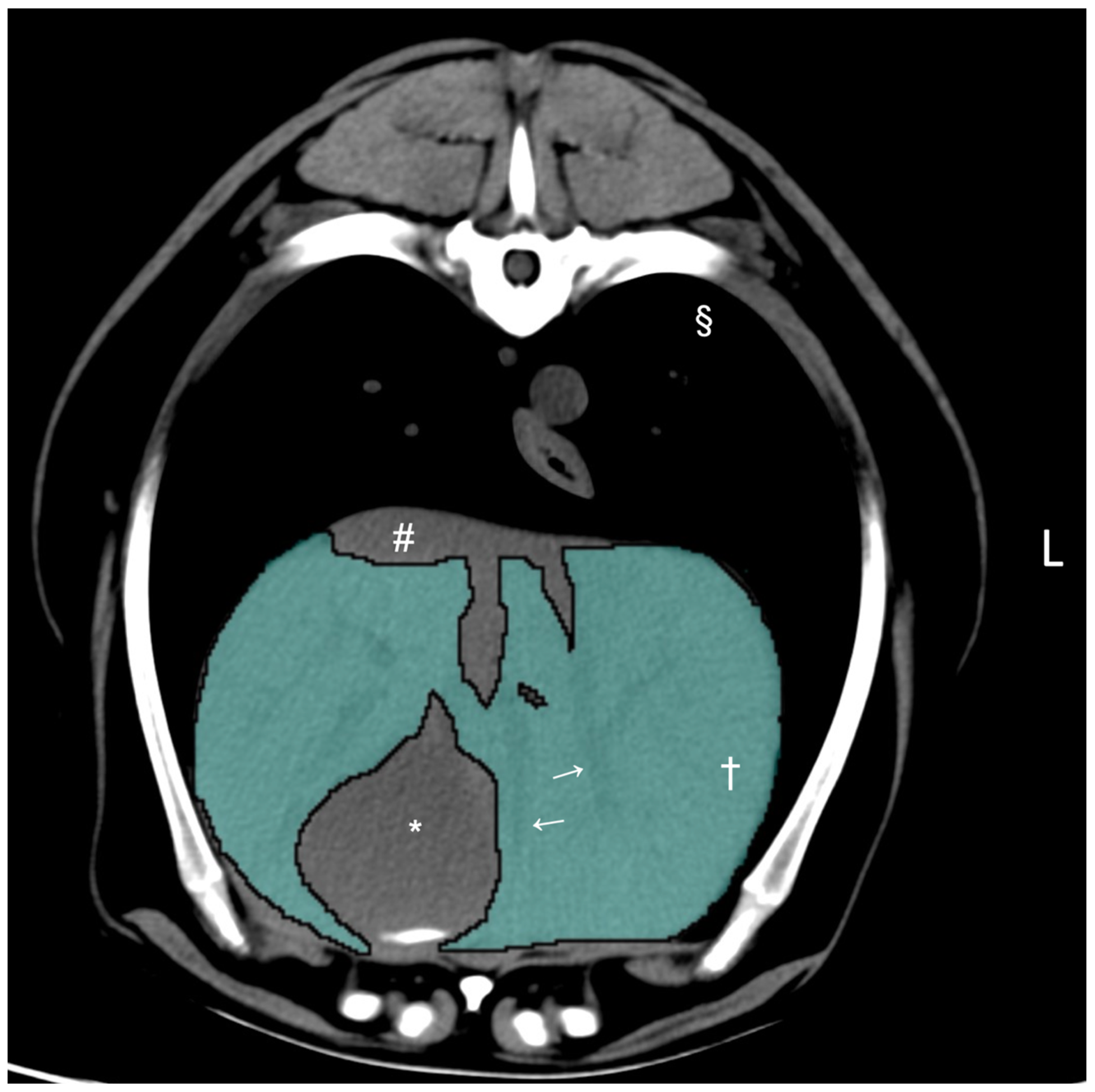
2.2. Computed Tomographic Hepatic Volumetry
2.3. Variables for Evaluation
2.4. Statistical Methods
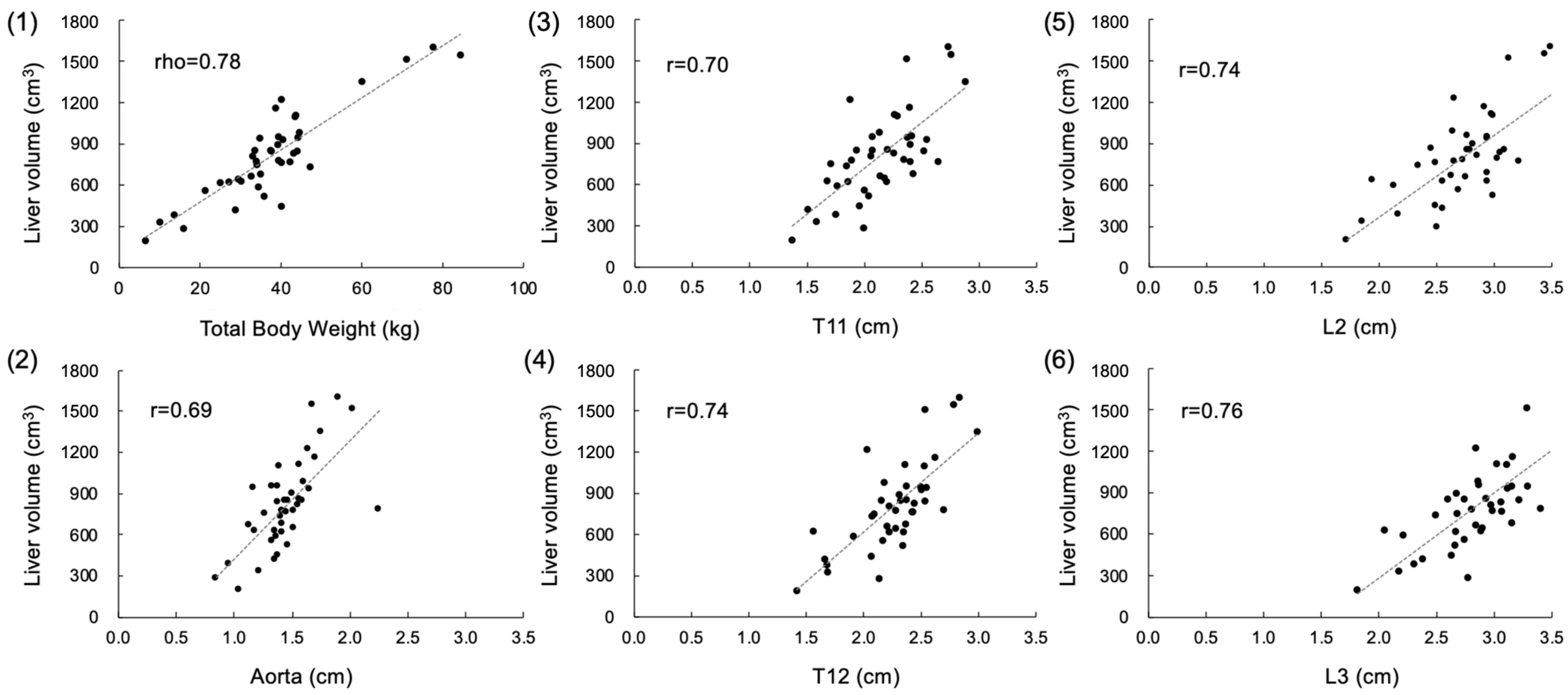
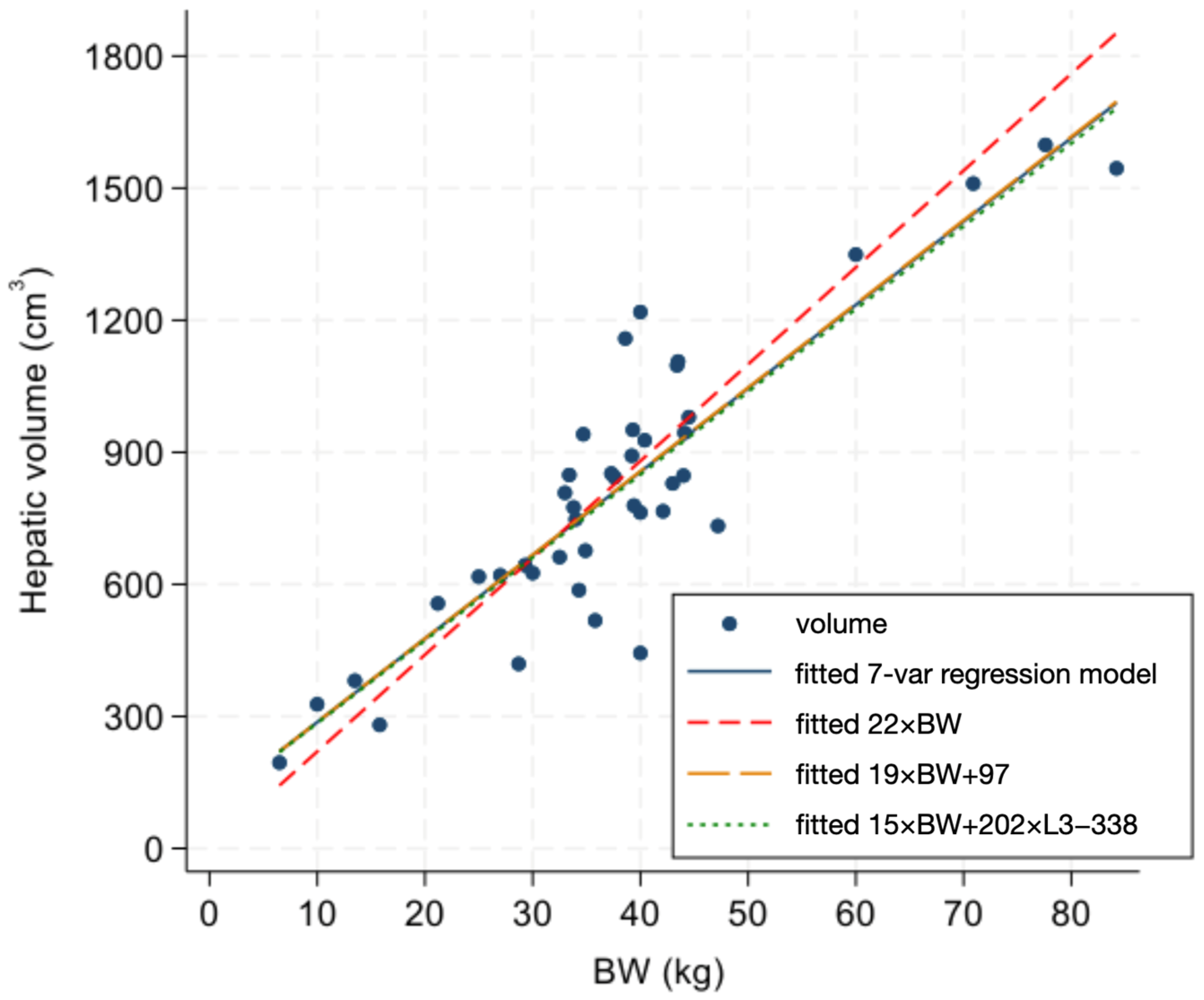
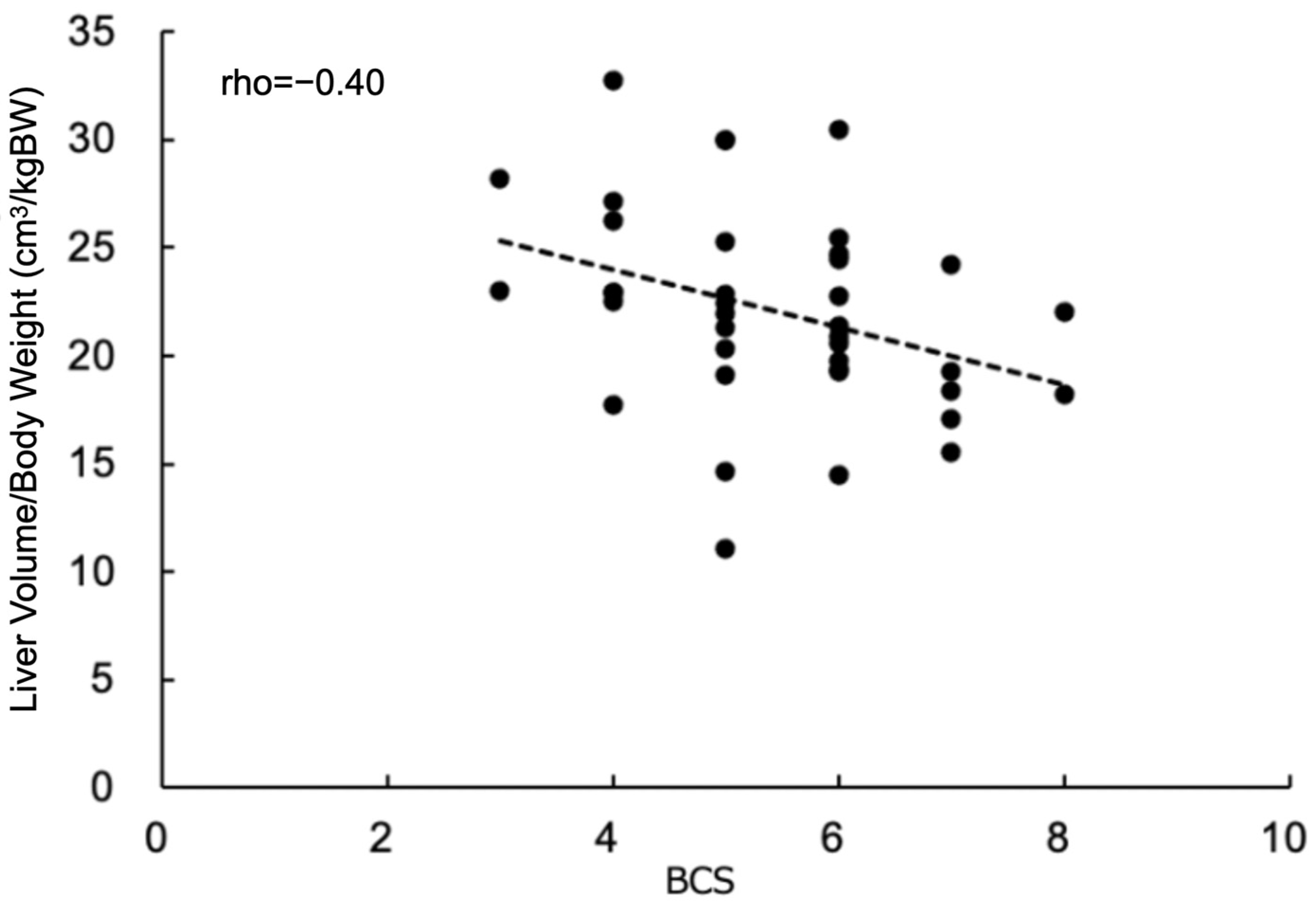
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suter, P.F. Radiographic diagnosis of liver disease in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 1982, 12, 153–173. [Google Scholar] [CrossRef]
- Wrigley, R.H. Radiographic and ultrasonographic diagnosis of liver diseases in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 1985, 15, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.D.; Panciera, D.L.; Larson, M.M.; Saunders, G.K.; Werre, S.R. A comparison of hepatic sonographic features and histopathologic diagnosis in canine liver disease: 138 cases. J. Vet. Intern. Med. 2013, 27, 806–813. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Kwon, D.; Yoon, H.; Yu, J.; Bang, S.; Lee, Y.; Jeon, S.; Jung, J.; Chang, J.; Chang, D. Evaluation of the radiographic liver length/11th thoracic vertebral length ratio as a method for quantifying liver size in cats. Vet. Radiol. Ultrasound. 2019, 60, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Keh, S.; Kim, H.; Kim, J.; Yoon, J. Radiographic liver size in Pekingese dogs versus other dog breeds. Vet. Radiol. Ultrasound. 2013, 54, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.A.; Friedman, M.A.; Brito, A.C. Determination of liver, kidney, and spleen volumes by computed tomography: An experimental study in dogs. J. Comput. Assist. Tomogr. 1981, 5, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Schiano, T.D.; Bodian, C.; Schwartz, M.E.; Glajchen, N.; Min, A.D. Accuracy and significance of computed tomographic scan assessment of hepatic volume in patients undergoing liver transplantation. Transplantation 2000, 69, 545–550. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; De Robertis, R.; Demozzi, E.; Crosara, S.; Canestrini, S.; Pozzi Mucelli, R. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World J. Radiol. 2014, 6, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Gotra, A.; Sivakumaran, L.; Chartrand, G.; Vu, K.N.; Vandenbroucke-Menu, F.; Kauffmann, C.; Kadoury, S.; Gallix, B.; de Guise, J.A.; Tang, A. Liver segmentation: Indications, techniques and future directions. Insights Imaging 2017, 8, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Vigano, L.; Polastri, R.; Muratore, A.; Eminefendic, H.; Regge, D.; Capussotti, L. Postoperative liver dysfunction and future remnant liver: Where is the limit? Results of a prospective study. World J. Surg. 2007, 31, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Itano, O.; Shinoda, M.; Kitago, M.; Yagi, H.; Abe, Y.; Takeuchi, A.; Takemura, Y.; Kitagawa, Y. Predictive factors for liver volume and function recovery after resection using three-dimensional analysis. HPB 2020, 22, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Xu, X.; Liu, C.; Zhang, T.; Qu, K. Assessment of liver volume variation to evaluate liver function. Front. Med. 2012, 6, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kummeling, A.; Vrakking, D.J.; Rothuizen, J.; Gerritsen, K.M.; van Sluijs, F.J. Hepatic volume measurements in dogs with extrahepatic congenital portosystemic shunts before and after surgical attenuation. J. Vet. Intern. Med. 2010, 24, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Stieger, S.M.; Zwingenberger, A.; Pollard, R.E.; Kyles, A.E.; Wisner, E.R. Hepatic volume estimation using quantitative computed tomography in dogs with portosystemic shunts. Vet. Radiol. Ultrasound. 2007, 48, 409–413. [Google Scholar] [CrossRef]
- Zwingenberger, A.L.; Daniel, L.; Steffey, M.A.; Mayhew, P.D.; Mayhew, K.N.; Culp, W.T.; Hunt, G.B. Correlation between liver volume, portal vascular anatomy, and hepatic perfusion in dogs with congenital portosystemic shunt before and after placement of ameroid constrictors. Vet. Surg. 2014, 43, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Kurihara, H.; Moore, G.E.; Murakami, M. Pilot Study: The Effects of Slice Parameters and the Interobserver Measurement Variability in Computed Tomographic Hepatic Volumetry in Dogs without Hepatic Disease. Vet. Sci. 2023, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, H.; Eom, K. Retrospective quantitative assessment of liver size by measurement of radiographic liver area in small-breed dogs. Am. J. Vet. Res. 2019, 80, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Cockett, P.A. Radiographic anatomy of the canine liver: Simple measurements determined from the lateral radiograph. J. Small Anim. Pract. 1986, 27, 577–589. [Google Scholar] [CrossRef]
- Morgan, J.P. Congenital Anomalies of the Vertebral Column of the Dog: A Study of the Incidence and Significance Based on a Radiographic and Morphologic Study. Vet. Radiol. 1968, 9, 21–29. [Google Scholar] [CrossRef]
- Kawasaki, S.; Makuuchi, M.; Matsunami, H.; Hashikura, Y.; Ikegami, T.; Chisuwa, H.; Ikeno, T.; Noike, T.; Takayama, T.; Kawarazaki, H. Preoperative measurement of segmental liver volume of donors for living related liver transplantation. Hepatology 1993, 18, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, T.; Kasahara, M.; Uryuhara, K.; Inomata, Y.; Uemoto, S.; Asonuma, K.; Egawa, H.; Fujita, S.; Hayashi, M.; Tanaka, K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 1999, 67, 321–327. [Google Scholar] [CrossRef]
- Proctor, N.J.; Tucker, G.T.; Rostami-Hodjegan, A. Predicting drug clearance from recombinantly expressed CYPs: Intersystem extrapolation factors. Xenobiotica 2004, 34, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.E.; Rostami-Hodjegan, A.; Burn, J.L.; Tooley, A.; Boyle, J.; Ellis, S.W.; Tucker, G.T. Inter-individual variability in levels of human microsomal protein and hepatocellularity per gram of liver. Br. J. Clin. Pharmacol. 2003, 56, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, A.; Wischhusen, F.; Puschel, K.; Rogiers, X. Standard liver volume in the Caucasian population. Liver Transpl. Surg. 1999, 5, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Murry, D.J.; Crom, W.R.; Reddick, W.E.; Bhargava, R.; Evans, W.E. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metab. Dispos. 1995, 23, 1110–1116. [Google Scholar] [PubMed]
- Urata, K.; Kawasaki, S.; Matsunami, H.; Hashikura, Y.; Ikegami, T.; Ishizone, S.; Momose, Y.; Komiyama, A.; Makuuchi, M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995, 21, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Gondolesi, G.E.; Bodian, C.A.; Jeon, H.; Schwartz, M.E.; Fishbein, T.M.; Miller, C.M.; Emre, S. A simple new formula to assess liver weight. Transplant. Proc. 2003, 35, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.N.; Tucker, G.T.; Tanner, M.S.; Rostami-Hodjegan, A. Changes in liver volume from birth to adulthood: A meta-analysis. Liver Transpl. 2005, 11, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Hosey-Cojocari, C.; Chan, S.S.; Friesen, C.S.; Robinson, A.; Williams, V.; Swanson, E.; O’Toole, D.; Radford, J.; Mardis, N.; Johnson, T.N.; et al. Are body surface area based estimates of liver volume applicable to children with overweight or obesity? An in vivo validation study. Clin. Transl. Sci. 2021, 14, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.L.; Bailey, D.B. Cancer Chemotherapy. In Withrow and MacEwen’s Small Animal Clinical Oncology; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; pp. 182–208. [Google Scholar]
- Price, G.S.; Frazier, D.L. Use of body surface area (BSA)-based dosages to calculate chemotherapeutic drug dose in dogs: I. Potential problems with current BSA formulae. J. Vet. Intern. Med. 1998, 12, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef]
| Variable | Mean ± SD | Median (Range) |
|---|---|---|
| Liver volume (cm3) | 813.8 ± 326.5 | 779.0 (194.9–1598.5) |
| T11 (cm) | 2.14 ± 0.35 | 2.1 (1.4–2.9) |
| T12 (cm) | 2.28 ± 0.34 | 2.3 (1.4–3.0) |
| L2 (cm) | 2.75 ± 0.40 | 2.8 (1.7–3.5) |
| L3 (cm) | 2.87 ± 0.41 | 2.9 (1.8–3.6) |
| Aorta (cm) | 1.46 ± 0.27 | 1.4 (0.8–2.3) |
| BW (kg) | nonparametric | 37.7 (6.5–84.2) |
| BCS | nonparametric | 5 (3–8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, K.; Moore, G.; Murakami, M. Body Weight as a Preferred Method for Normalizing the Computed Tomography-Derived Liver Volume in Dogs without Hepatic Disease. Vet. Sci. 2024, 11, 153. https://doi.org/10.3390/vetsci11040153
Kinoshita K, Moore G, Murakami M. Body Weight as a Preferred Method for Normalizing the Computed Tomography-Derived Liver Volume in Dogs without Hepatic Disease. Veterinary Sciences. 2024; 11(4):153. https://doi.org/10.3390/vetsci11040153
Chicago/Turabian StyleKinoshita, Kosuke, George Moore, and Masahiro Murakami. 2024. "Body Weight as a Preferred Method for Normalizing the Computed Tomography-Derived Liver Volume in Dogs without Hepatic Disease" Veterinary Sciences 11, no. 4: 153. https://doi.org/10.3390/vetsci11040153
APA StyleKinoshita, K., Moore, G., & Murakami, M. (2024). Body Weight as a Preferred Method for Normalizing the Computed Tomography-Derived Liver Volume in Dogs without Hepatic Disease. Veterinary Sciences, 11(4), 153. https://doi.org/10.3390/vetsci11040153






