Correlation between Oxidative Stress Markers and Periodontal Disease in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Clinical Examination
2.3. Saliva Sampling Procedure
2.4. Biochemical Analyses of Saliva
- Determination of MMP-8 concentration
- Determination of total proteins
- Determination of Total antioxidants capacity (TAC)
- Determination of Total Superoxide Dismutase (T-SOD) activity
- Determination of 8-Hydroxydeoxyguanosine (8-OHdG)
- Determination of malondialdehyde (MDA)
2.5. Statistical Analysis
3. Results
3.1. Clinical Examination and Classification of the Dogs
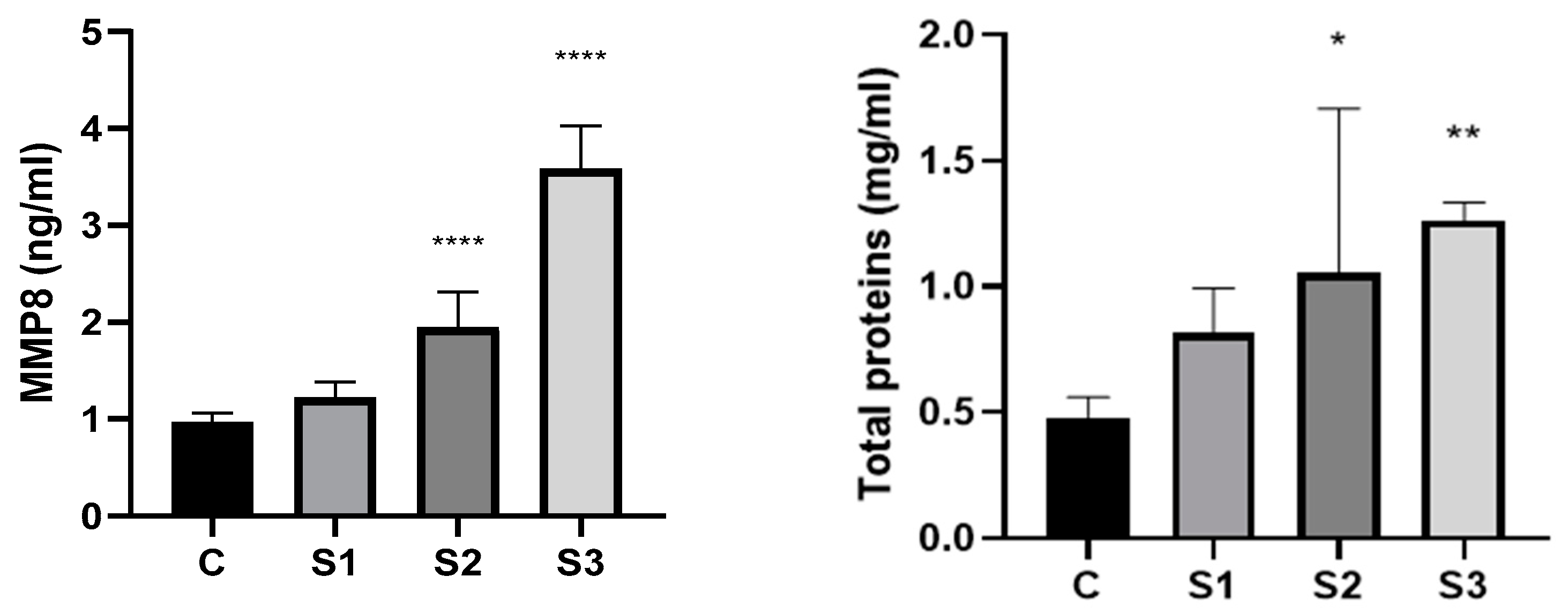
3.2. MMP8 and Total Proteins in the Dog’s Saliva
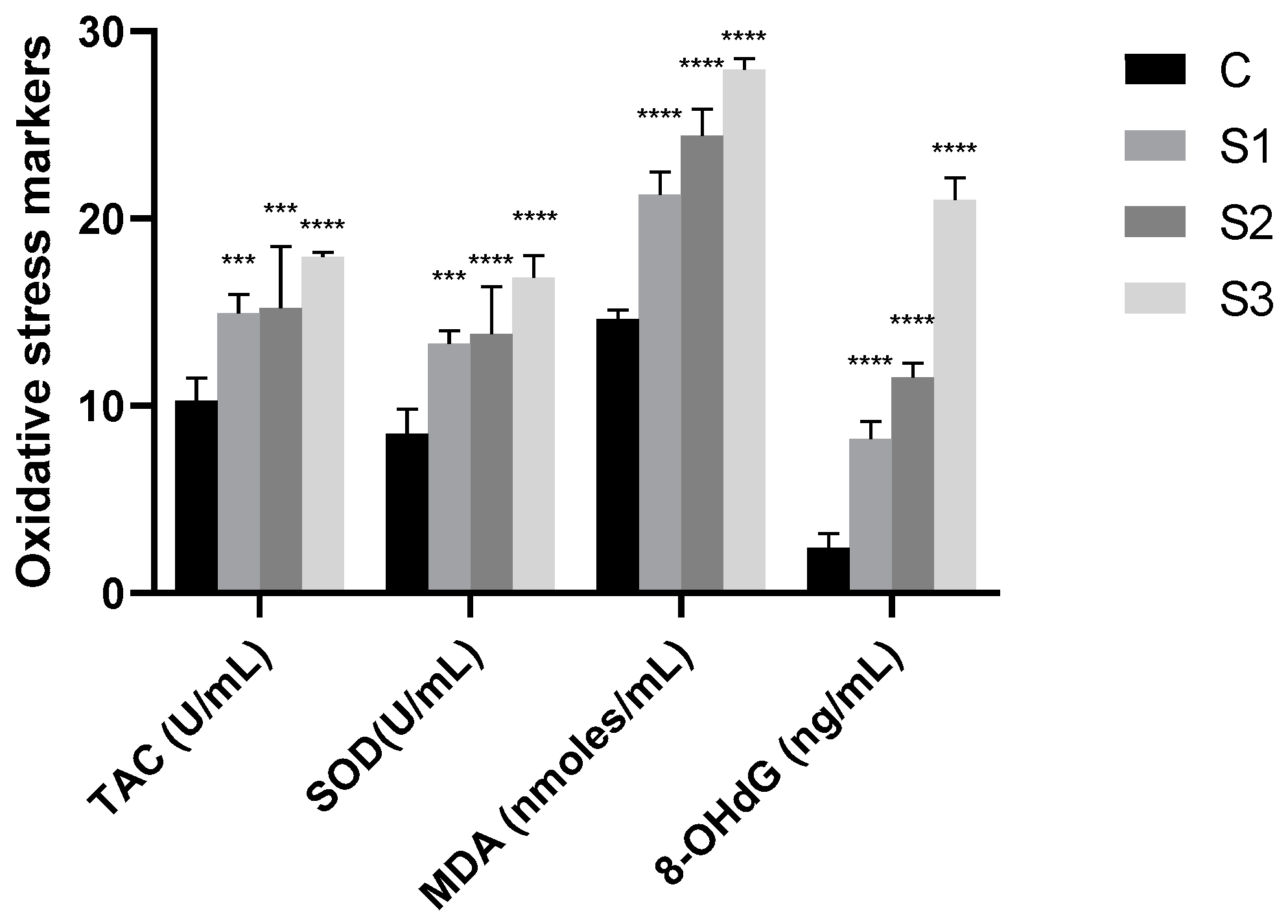
3.3. Oxidative Stress Markers in Dog’s Saliva
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, N.J.; Dean, R.S.; Cobb, M.; Brennan, M.L. Factors influencing common diagnoses made during first-opinion small-animal consultations in the United Kingdom. Prev. Vet. Med. 2016, 131, 87–94. [Google Scholar] [CrossRef]
- Wallis, C.; Holcombe, L.J. A review of the frequency and impact of periodontal disease in dogs. J. Small Anim. Pract. 2020, 61, 529–540. [Google Scholar] [CrossRef]
- Niemiec, B.A. Oral pathology. Top. Companion Anim Med. 2008, 23, 59–71. [Google Scholar] [CrossRef]
- Niemiec, B. Veterinary Periodontology; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 18–39, 41–69. [Google Scholar]
- Lobprise, H.; Dodd, J. Wiggs’s Veterinary Dentistry: Principles and Practice, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 463–481. [Google Scholar]
- Van Dyke, T.E. The etiology and pathogenesis of periodontitis revisited. J. Appl. Oral Sci. 2009, 17, i. [Google Scholar] [CrossRef]
- Harvey, C.E. Management of periodontal disease: Understanding the options. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 819–836. [Google Scholar] [CrossRef]
- Gorrel, C. Veterinary Dentistry for the General Practitioner, 2nd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; pp. 97–120. [Google Scholar]
- Lobprise, H.B. Blackwell’s Five-Minute Veterinary Consult Clinical Companion: Small Animal Dentistry, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 16–24. [Google Scholar]
- Wolf, H.F.; Rateitschak, E.M.; Rateitschak, K.H.; Klaus, H.; Edith, M.; Herbert, F.; Hassell, T.M. Color Atlas of Dental Medicine: Periodontology, 3rd ed.; Thieme-Stuttgart: New York, NY, USA, 2005; pp. 95–98. [Google Scholar]
- Kortegaard, H.E.; Eriksen, T.; Baelum, V. Periodontal disease in research beagle dogs—An epidemiological study. J. Small Anim. Pract. 2008, 49, 610–616. [Google Scholar] [CrossRef]
- Case, L.P.; Daristotle, L.; Hayek, M.G.; Foess Raasch, M. Section 5: Nutritionally Responsive Disorders, Chapter 34. Dental Health and Diet. In Canine and Feline Nutrition a Resource for Companion Animal Professionals, 3rd ed.; Mosby Elsevier: Maryland Heights, MO, USA, 2010; pp. 437–453. [Google Scholar]
- Albuquerque, C.; Morinha, F.; Requicha, J.; Martins, T.; Dias, I.; Guedes-Pinto, H.; Bastos, E.; Viegas, C. Canine periodontitis: The dog as an important model for periodontal studies. Vet. J. 2012, 191, 299–305. [Google Scholar] [CrossRef]
- Marshall, M.D.; Wallis, C.V.; Milella, L.; Colyer, A.; Tweedie, A.D.; Harris, S. A longitudinal assessment of periodontal disease in 52 miniature schnauzers. BMC Vet. Res. 2014, 10, 166. [Google Scholar] [CrossRef]
- Kyllar, M.; Witter, K. Prevalence of dental disorders in pet dogs. Vet. Med.-Czech. 2005, 11, 496–505. [Google Scholar] [CrossRef]
- Wallis, C.; Pesci, I.; Colyer, A.; Milella, L.; Southerden, P.; Holcombe, L.J.; Desforges, N. A longitudinal assessment of periodontal disease in Yorkshire terriers. BMC Vet. Res. 2019, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.; Patel, K.V.; Marshall, M.; Staunton, R.; Milella, L.; Harris, S.; Holcombe, L.J. A longitudinal assessment of periodontal health status in 53 Labrador retrievers. Small Anim. Pract. 2018, 59, 560–569. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Church, D.B.; Mcgreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. Vet. J. 2014, 202, 286–291. [Google Scholar] [CrossRef]
- Kyllar, M.; Doskarova, B.; Paral, V. Morphometric assessment of periodontal tissues in relation to periodontal disease in dogs. J. Vet. Dent. 2013, 30, 146–149. [Google Scholar] [CrossRef]
- Gawor, J.P.; Reiter, A.M.; Jodkowska, K.; Kurski, G.; Wojtacki, M.P.; Kurek, A. Influence of diet on oral health in cats and dogs. J. Nutr. 2006, 136, 2021S–2023S. [Google Scholar] [CrossRef]
- Ray, J.D., Jr.; Eubanks, D.L. Dental homecare: Teaching your clients to care for their pet’s teeth. J. Vet. Dent. 2009, 26, 57–60. [Google Scholar] [CrossRef]
- Holcombe, L.J.; Patel, N.; Colyer, A.; Deusch, O.; O’Flynn, C.; Harris, S. Early canine plaque biofilms: Characterization of key bacterial interactions involved in initial colonization of enamel. PLoS ONE 2014, 9, e113744. [Google Scholar] [CrossRef]
- Quest, B.W. Oral health benefits of a daily dental chew in dogs. J. Vet. Dent. 2013, 30, 84–87. [Google Scholar] [CrossRef]
- Lobprise, H. Canine periodontal disease: Overview. Vet. Tech. 2006, 27, 168–173. [Google Scholar]
- de Sousa-Pereira, P.; Cova, M.; Abrantes, J.; Ferreira, R.; Trindade, F.; Barros, A.; Gomes, P.; Colaço, B.; Amado, F.; Esteves, P.J.; et al. Cross-species comparison of mammalian saliva using an LC-MALDI based proteomic approach. Proteomics 2015, 15, 1598–1607. [Google Scholar] [CrossRef]
- Sanguansermsri, P.; Jenkinson, H.F.; Thanasak, J.; Chairatvit, K.; Roytrakul, S.; Kittisenachai, S.; Puengsurin, D.; Surarit, R. Comparative proteomic study of dog and human saliva. PLoS ONE 2018, 13, e0208317. [Google Scholar] [CrossRef]
- Pasha, S.; Inui, T.; Chapple, I.; Harris, S.; Holcombe, L.; Grant, M.M. The Saliva Proteome of Dogs: Variations within and between Breeds and between Species. Proteomics 2018, 18, 1700293. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Kononen, E.; Pradhan-Palikhe, P.; Tervahartiala, T.; Pussinen, P.J.; Suominen-Taipale, L.; Sorsa, T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 2010, 37, 487–493. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Todorova, I.; Simeonova, G.; Kyuchukova, D.; Dinev, D.; Gadjeva, V. Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp. Clin. Pathol. 2005, 13, 190–194. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Vasconcellos, R.S.; Pedreira, R.S.; Silva, F.L.; Sá, F.C.; Kroll, F.S.A.; Maria, A.P.J.; Venturini, K.S.; Carciofi, A.C. Alterations to oxidative stress markers in dogs after a short-term stress during transport. J. Nutr. Sci. 2014, 3, e27. [Google Scholar] [CrossRef]
- McMichael, M.A. Oxidative stress, antioxidants, and assessment of oxidative stress in dogs and cats. J. Am. Vet. Med. Assoc. 2007, 231, 714–720. [Google Scholar] [CrossRef]
- Scott, B.C.; Aruoma, O.I.; Evans, P.J.; O’Neill, C.; Van der Vliet, A.; Cross, C.E.; Tritschler, H.; Halliwell, B. Lipoic and dihydrolipoic acids as antioxidants. A critical evaluation. Free Radic. Res. 1994, 20, 119–133. [Google Scholar] [CrossRef]
- Andersson, H.; Karlsen, A.; Blomhoff, R.; Raastad, T.; Kadi, F. Plasma antioxidant responses and oxidative stress following a soccer game in elite female players. Scand. J. Med. Sci. Sports 2010, 20, 600–608. [Google Scholar] [CrossRef]
- Martinez-Haro, M.; Green, A.J.; Mateo, R. Effects of lead exposure on oxidative stress biomarkers and plasma biochemistry in waterbirds in the field. Environ. Res. 2011, 111, 530–538. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef]
- Pavlica, Z.; Petelin, M.; Nemec, A.; Erzen, D.; Skaleric, U. Measurement of total antioxidant capacity in gingival crevicular fluid and serum in dogs with periodontal disease. Am. J. Vet. Res. 2004, 65, 1584–1588. [Google Scholar] [CrossRef]
- Rathnayake, N.; Gieselmann, D.R.; Heikkinen, A.M.; Tervahartiala, T.; Sorsa, T. Salivary Diagnostics-Point-of-Care diagnostics of MMP-8 in dentistry and medicine. Diagnostics 2017, 7, 7. [Google Scholar] [CrossRef]
- Bringel, M.; Jorge, P.K.; Francisco, P.A.; Lowe, C.; Sabino-Silva, R.; Colombini-Ishikiriama, B.L.; Machado, M.A.A.M.; Siqueira, W.L. Salivary proteomic profile of dogs with and without dental calculus. BMC Vet. Res. 2020, 16, 298. [Google Scholar] [CrossRef]
- Torres, S.M.F.; Furrow, E.; Souza, C.P.; Granick, J.L.; de Jong, E.P.; Griffin, T.J.; Wang, X. Salivary proteomics of healthy dogs: An in depth catalog. PLoS ONE 2018, 13, e0191307. [Google Scholar] [CrossRef]
- Turunen, S.; Puurunen, J.; Auriola, S.; Kullaa, A.M.; Kärkkäinen, O.; Lohi, H.; Hanhineva, K. Metabolome of canine and human saliva: A non-targeted metabolomics study. Metabolomics 2020, 16, 90. [Google Scholar] [CrossRef]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef]
- Toczewska, J.; Maciejczyk, M.; Konopka, T.; Zalewska, A. Total Oxidant and Antioxidant Capacity of Gingival Crevicular Fluid and Saliva in Patients with Periodontitis: Review and Clinical Study. Antioxidants 2020, 9, 450. [Google Scholar] [CrossRef]
- Peluso, I.; Raguzzini, A. Salivary and Urinary Total Antioxidant Capacity as Biomarkers of Oxidative Stress in Humans. Pathol. Res. Int. 2016, 2016, 5480267. [Google Scholar] [CrossRef]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Hendi, S.S.; Kasraei, S.; Moghimbeigi, A. Total antioxidant capacity of saliva and dental caries. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e553–e556. [Google Scholar] [CrossRef]
- Araujo, H.C.; Nakamune, A.C.M.S.; Garcia, W.G.; Pessan, J.P.; Antoniali, C. Carious Lesion Severity Induces Higher Antioxidant System Activity and Consequently Reduces Oxidative Damage in Children’s Saliva. Oxidative Med. Cell. Longev. 2020, 2020, 3695683. [Google Scholar] [CrossRef]
- Trivedi, S.; Lal, N.; Mahdi, A.A.; Singh, B.; Pandey, S. Association of salivary lipid peroxidation levels, antioxidant enzymes, and chronic periodontitis. Int. J. Periodontics Restor. Dent. 2015, 35, e14–e19. [Google Scholar] [CrossRef]
- Sezer, U.; Ciçek, Y.; Canakçi, C.F. Increased salivary levels of 8-hydroxydeoxyguanosine may be a marker for disease activity for periodontitis. Dis. Markers 2012, 32, 165–172. [Google Scholar] [CrossRef]
- Canakçi, C.F.; Canakçi, V.; Tatar, A.; Eltas, A.; Sezer, U.; Ciçek, Y.; Oztas, S. Increased salivary level of 8-hydroxydeoxyguanosine is a marker of premature oxidative mitochondrial DNA damage in gingival tissue of patients with periodontitis. Arch. Immunol. Et Ther. Exp. 2009, 57, 205–211. [Google Scholar] [CrossRef]
- Harvey, C.E.; Shofer, F.S.; Laster, L. Association of age and body weight with periodontal disease in North American dogs. J. Vet. Dent. 1994, 11, 94–105, Erratum in J. Vet. Dent. 1994, 11, 133. [Google Scholar] [CrossRef]
- Mendoza-Núñez, V.M.; Sánchez-Rodríguez, M.A.; Retana-Ugalde, R.; Vargas-Guadarrama, L.A.; Altamirano-Lozano, M.A. Total antioxidant levels, gender, and age as risk factors for DNA damage in lymphocytes of the elderly. Mech. Ageing Dev. 2001, 122, 835–847. [Google Scholar] [CrossRef]
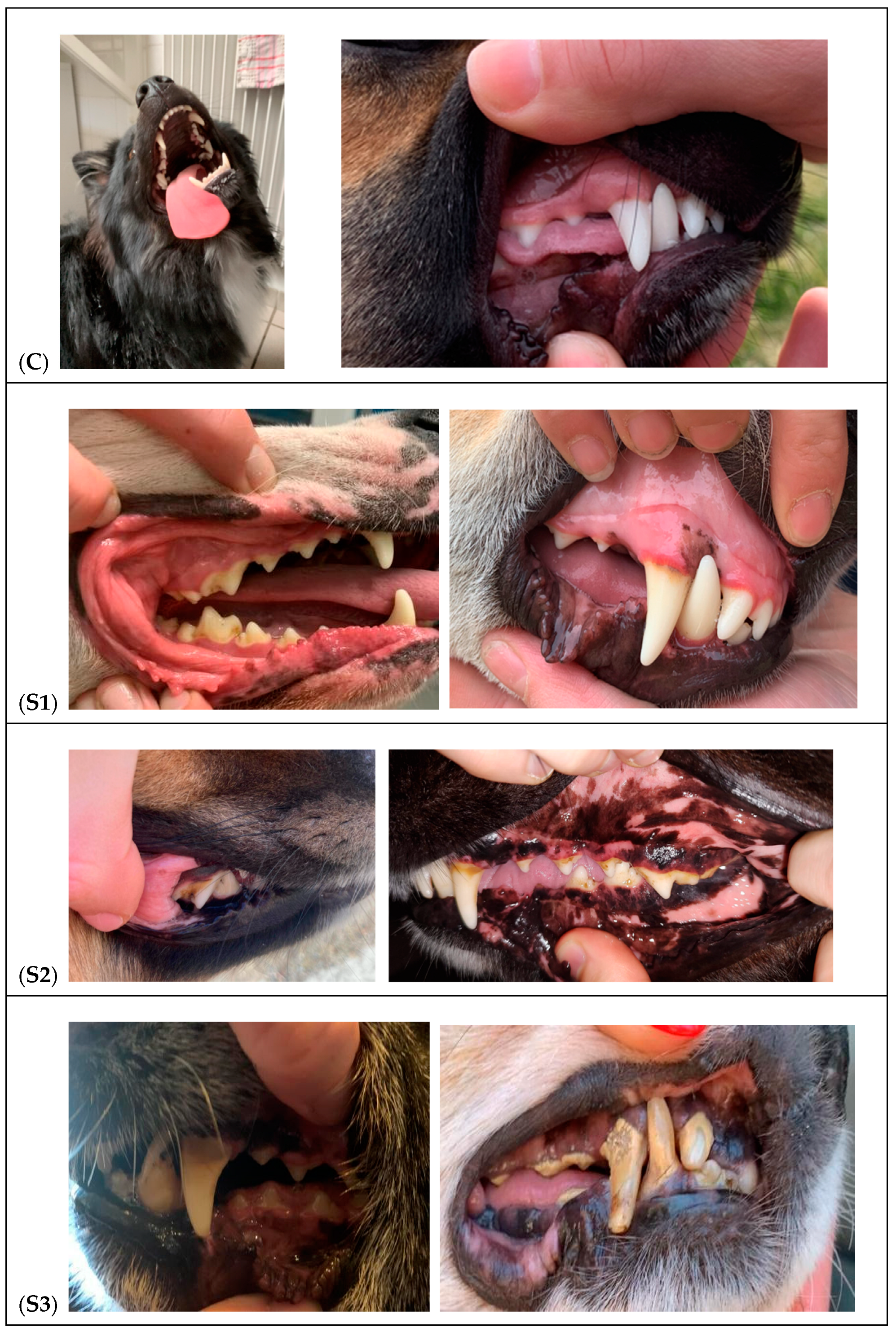
| Stage | Description |
|---|---|
| O | No accumulation of tartar. Gums are normal. |
| I | Small accumulation of tartar. Gums are slightly inflamed. |
| II | Moderate accumulation of tartar. The gums are painful, swollen, red, and bleed easily. |
| III | Heavy tartar is present. Severe inflammation of the gums. Evidence of infection or tooth loss. |
| Groups | Estimation of Tartar Amount | Estimation of the Degree of Inflammation |
|---|---|---|
| Control group C (n = 6) | – | – |
| Study group S1 (n = 6) | + | + |
| Study group S2 (n = 5) | + + | + + |
| Study group S3 (n = 5) | + + + | + + + |
| Groups | Age | Breed |
|---|---|---|
| Control group (C) | 2–3 years (4 dogs) 4–5 years (2 dogs) | Icelandic sheepdog; Rottweiler; Belgian Tervuren; mixed breed |
| Study group S1 | 2–3 years (1 dogs) 4–5 years (2 dogs) 8–9 years (3 dogs) | French bulldog; Rottweiler; mixed-breed |
| Study group S2 | 2–3 years (1 dogs) 4–5 years (2 dogs) 6–7 years (2 dogs) | Australian shepherd; Border collie; Rottweiler; mixed breed |
| Study group S3 | 6–7 years (2 dogs) 8–9 years (1 dogs) 14 years (2 dogs) | Mixed-breed |
| Groups | TAC (U/mL) | SOD (U/mL) | MDA (nmoles/mL) | 8-OHdG (ng/mL) |
|---|---|---|---|---|
| Control group (C) | 10.253 ± 1.138 | 8.515 ± 1.024 | 14.629 ± 0.443 | 2.418 ± 0.696 |
| Study group S1 | 14.942 ± 0.949 | 13.307 ± 0.644 | 21.268 ± 1.120 | 8.233 ± 0.481 |
| Study group S2 | 15.239 ± 2.936 | 13.845 ± 2.251 | 24.444 ± 1.243 | 11.541 ± 0.644 |
| Study group S3 | 17.970 ± 0.216 | 16.854 ± 1.047 | 27.951 ± 0.528 | 21.024 ± 1.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peștean, C.P.; Pocquet, H.; Dumitraș, D.A.; Morohoschi, A.G.; Ștefănuț, L.C.; Andrei, S. Correlation between Oxidative Stress Markers and Periodontal Disease in Dogs. Vet. Sci. 2024, 11, 99. https://doi.org/10.3390/vetsci11030099
Peștean CP, Pocquet H, Dumitraș DA, Morohoschi AG, Ștefănuț LC, Andrei S. Correlation between Oxidative Stress Markers and Periodontal Disease in Dogs. Veterinary Sciences. 2024; 11(3):99. https://doi.org/10.3390/vetsci11030099
Chicago/Turabian StylePeștean, Cosmin Petru, Hélène Pocquet, Daria Antonia Dumitraș, Andreea Georgiana Morohoschi, Laura Cristina Ștefănuț, and Sanda Andrei. 2024. "Correlation between Oxidative Stress Markers and Periodontal Disease in Dogs" Veterinary Sciences 11, no. 3: 99. https://doi.org/10.3390/vetsci11030099
APA StylePeștean, C. P., Pocquet, H., Dumitraș, D. A., Morohoschi, A. G., Ștefănuț, L. C., & Andrei, S. (2024). Correlation between Oxidative Stress Markers and Periodontal Disease in Dogs. Veterinary Sciences, 11(3), 99. https://doi.org/10.3390/vetsci11030099







