Ultrasonographic Renal Subcapsular Thickening in Cats with Primary and Metastatic Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design and Patient Inclusion
2.2. Medical Records Extraction
2.3. Ultrasonographic Evaluation of Subcapsular Thickening
2.4. Ultrasonographic Evaluation of the Rest of the Kidney
2.5. Ultrasonographic Measurements of the Kidneys
3. Results
3.1. Study Population
3.2. Clinical Data
3.3. Ultrasonographic Findings of Subcapsular Thickening
3.4. Ultrasonographic Findings of the Rest of the Kidney
3.5. Kidney Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valdes-Martinez, A.; Cianciolo, R.; Mai, W. Association between renal hypoechoic subcapsular thickening and lymphosarcoma in cats. Vet. Radiol. Ultrasound 2007, 48, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; O’Brien, R.T. Abdominal ultrasonographic findings associated with feline infectious peritonitis: A retrospective review of 16 cases. J. Am. Anim. Hosp. Assoc. 2010, 46, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.R.; Penninck, D.G.; Webster, C.R.; Conrado, F.O. Abdominal ultrasonographic findings of cats with feline infectious peritonitis: An update. J. Feline Med. Surg. 2023, 25, 1098612X231216000. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Dobson, J. Small Animal Oncology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Meuten, D.J.; Meuten, T.L. Tumors of the urinary system. In Tumors in Domestic Animals; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 632–688. [Google Scholar]
- Baskin, G.B.; De Paoli, A. Primary renal neoplasms of the dog. Vet. Pathol. 1977, 14, 591–605. [Google Scholar] [CrossRef]
- Henry, C.J.; Turnquist, S.E.; Smith, A.; Graham, J.C.; Thamm, D.H.; O’Brien, M.; Clifford, C.A. Primary renal tumours in cats: 19 cases (1992–1998). J. Feline Med. Surg. 1999, 1, 165–170. [Google Scholar] [CrossRef]
- Cianciolo, R.E.; Mohr, F.C. Urinary System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2016; Volume 2, pp. 376–464.e371. [Google Scholar]
- Kenny, S.A.; Cook, M.R.; Lenz, J.A.; Maritato, K.C.; Skorupski, K.A.; Wustefeld-Janssens, B.G.; Pellin, M.A.; Silveira, C.J.; Veytsman, S.; Selmic, L.E.; et al. Clinical outcomes in cats with renal carcinoma undergoing nephrectomy: A retrospective study. Vet. Comp. Oncol. 2023, 21, 587–594. [Google Scholar] [CrossRef]
- Hahn, K.A.; McEntee, M.F. Primary lung tumors in cats: 86 cases (1979–1994). J. Am. Vet. Med. Assoc. 1997, 211, 1257–1260. [Google Scholar] [CrossRef]
- Santos, I.R.; Raiter, J.; Lamego, E.C.; Bandinelli, M.B.; Dal Pont, T.P.; Siqueira, K.F.; Almeida, B.A.; Panzeira, W.; Sonne, L.; Driemeier, D.; et al. Feline pulmonary carcinoma: Gross, histological, metastatic, and immunohistochemical aspects. Vet. Pathol. 2023, 60, 8–20. [Google Scholar] [CrossRef]
- Barr, F.; Gruffydd-Jones, T.; Brown, P.; Gibbs, C. Primary lung tumours in the cat. J. Small Anim. Pract. 1987, 28, 1115–1125. [Google Scholar] [CrossRef]
- Matsumoto, I.; Chambers, J.K.; Nibe, K.; Kinoshita, R.; Nishimura, R.; Nakayama, H.; Uchida, K. Histopathologic and immunohistochemistry findings in feline renal cell carcinoma. Vet. Pathol. 2018, 55, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.; Thomson, J. Bilateral renal carcinoma in a cat. Vet. Pathol. 1994, 31, 704–705. [Google Scholar] [CrossRef]
- Brown, C.E.; Sola, M.F. Pathology in Practice. J. Am. Vet. Med. Assoc. 2022, 259, 1–3. [Google Scholar] [CrossRef]
- Keenihan, E.K.; Lynch, S.; Priestnall, S.L.; Harrington, N.T.; Benigni, L.; Lamb, C.R. Unusual rib metastasis in two cats with pulmonary carcinoma. J. Feline Med. Surg. 2013, 15, 1145–1148. [Google Scholar] [CrossRef]
- Bryan, J.N.; Henry, C.J.; Turnquist, S.E.; Tyler, J.W.; Liptak, J.M.; Rizzo, S.A.; Sfiligoi, G.; Steinberg, S.J.; Smith, A.N.; Jackson, T. Primary renal neoplasia of dogs. J. Vet. Intern. Med./Am. Coll. Vet. Intern. Med. 2006, 20, 1155–1160. [Google Scholar] [CrossRef]
- Miles, K.G. A review of primary lung tumors in the dog and cat. Vet. Radiol. 1988, 29, 122–128. [Google Scholar] [CrossRef]
- Rossi, F.; Gianni, B.; Marconato, L.; Sabattini, S.; Caleri, E.; Mattolini, M.; Camosci, V.; Carozzi, G. Comparison of sonographic and CT findings for the identification of renal nodules in dogs and cats. Vet. Radiol. Ultrasound 2023, 64, 439–447. [Google Scholar] [CrossRef]
- d’Anjou, M.-A.; Penninck, D. Kidneys and Ureters. In Atlas of Small Animal Ultrasonography, 2nd ed.; Penninck, D., d’Anjou, M.-A., Eds.; Wiley: New York, NY, USA, 2015; pp. 331–362. [Google Scholar]
- Aarsvold, S.; Reetz, J.A.; Reichle, J.K.; Jones, I.D.; Lamb, C.R.; Evola, M.G.; Keyerleber, M.A.; Marolf, A.J. Computed tomographic findings in 57 cats with primary pulmonary neoplasia. Vet. Radiol. Ultrasound 2015, 56, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Nunley, J.; Sutton, J.; Culp, W.; Wilson, D.; Coleman, K.; Demianiuk, R.; Schechter, A.; Moore, G.; Donovan, T.; Schwartz, P. Primary pulmonary neoplasia in cats: Assessment of computed tomography findings and survival. J. Small Anim. Pract. 2015, 56, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Mount, D.B.; Yu, A. Brenner and Rector’s The Kidney; Elsevier: Philadelphia, PA, USA, 2008. [Google Scholar]
- Allan, P.L. Medical diseases of the kidney. In Clinical Ultrasound, 3rd ed.; Allan, P.L., Baxter, G.M., Weston, M.J., Eds.; Churchill Livingstone: Edinburgh, UK, 2011; pp. 445–459. [Google Scholar]
- Cadman, P.J.; Lindsell, D.R.; Golding, S.J. An unusual appearance of renal lymphoma. Clin. Radiol. 1988, 39, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Cruz Villalon, F.; Escribano Fernandez, J.; Ramirez Garcia, T. The hypoechoic halo: A finding in renal lymphoma. J. Clin. Ultrasound 1995, 23, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Gorg, C.; Weide, R.; Schwerk, W.B. Unusual perirenal sonographic pattern in malignant lymphoma of the kidney. Clin. Radiol. 1995, 50, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Spiesecke, P.; Munch, F.; Fischer, T.; Hamm, B.; Lerchbaumer, M.H. Multiparametric ultrasound findings in acute kidney failure due to rare renal cortical necrosis. Sci. Rep. 2021, 11, 2060. [Google Scholar] [CrossRef] [PubMed]
- Spies, J.B.; Hricak, H.; Slemmer, T.M.; Zeineh, S.; Alpers, C.E.; Zayat, P.; Lue, T.F.; Kerlan, R.K., Jr.; Madrazo, B.L.; Sandler, M.A. Sonographic evaluation of experimental acute renal arterial occlusion in dogs. AJR Am. J. Roentgenol. 1984, 142, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.E.; Roubidoux, M.A.; Dunnick, N.R. Secondary renal neoplasms. Abdom. Imaging 1998, 23, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Gumeler, E.; Onur, M.R.; Karaosmanoglu, A.D.; Ozmen, M.; Akata, D.; Karcaaltincaba, M. Computed tomography and magnetic resonance imaging of peripelvic and periureteric pathologies. Abdom. Radiol. 2018, 43, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, N.; Gallo, G.; Majluf, R.; Diez, B.; Arias, E.; Riudavets, M.A.; Sevlever, G. Extranodal Rosai-Dorfman disease presenting as a solitary mass with human Herpesvirus 6 detection in a pediatric patient. Pediatr. Dev. Pathol. 2012, 15, 324–328. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Song, D.-W.; Ro, W.-B.; Kim, H.-S.; Lee, G.-W.; Cho, J.-H.; Jeong, W.-C.; Kim, S.-H.; Sur, J.-H.; Park, H.-M. Renal lymphoma with mesenteric lymphomatosis in a cat. J. Vet. Clin. 2020, 37, 208–212. [Google Scholar] [CrossRef]
- Bound, N.J.; Priestnall, S.L.; Cariou, M.P. Lingual and renal lymphoma in a cat. J. Feline Med. Surg. 2011, 13, 272–275. [Google Scholar] [CrossRef]
- Williams, A.G.; Hohenhaus, A.E.; Lamb, K.E. Incidence and treatment of feline renal lymphoma: 27 cases. J. Feline Med. Surg. 2021, 23, 936–944. [Google Scholar] [CrossRef]
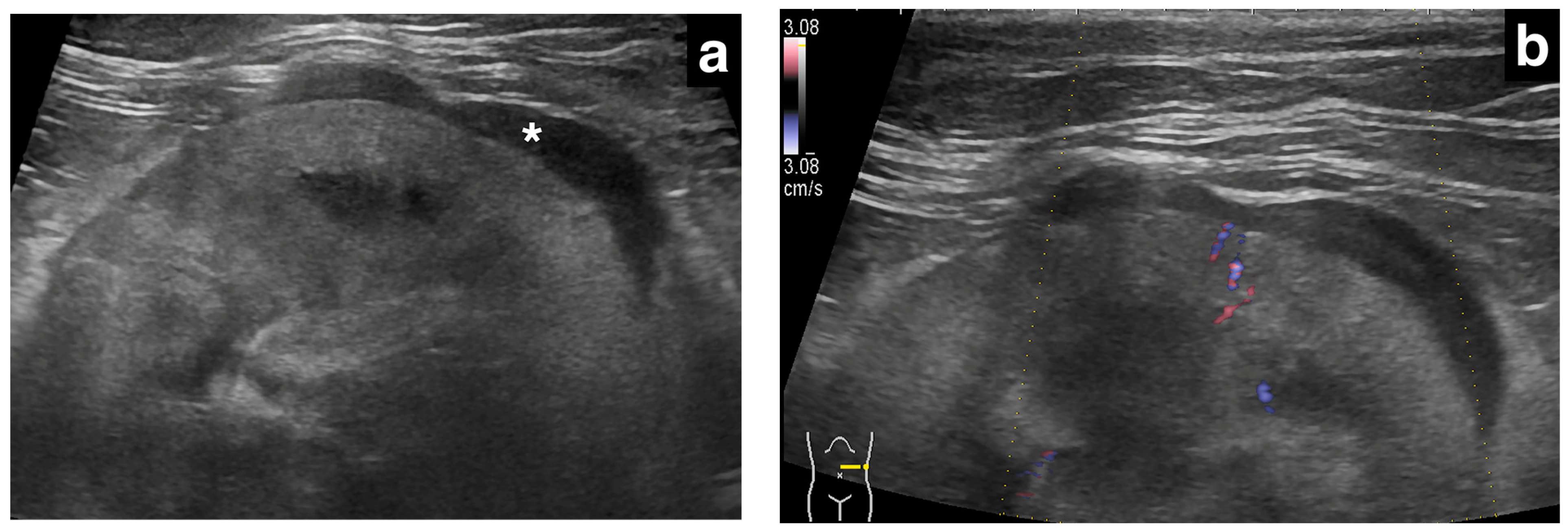
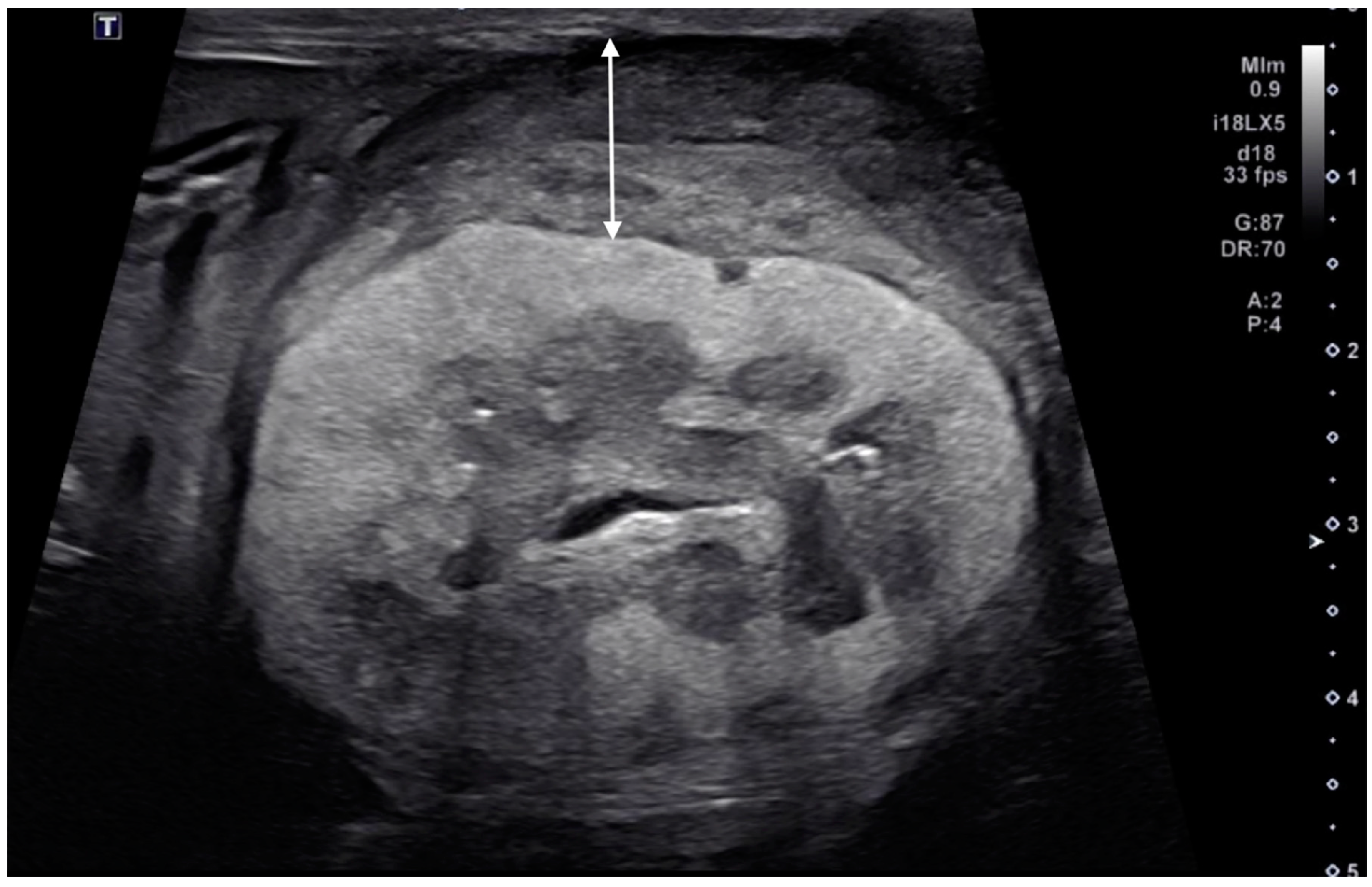
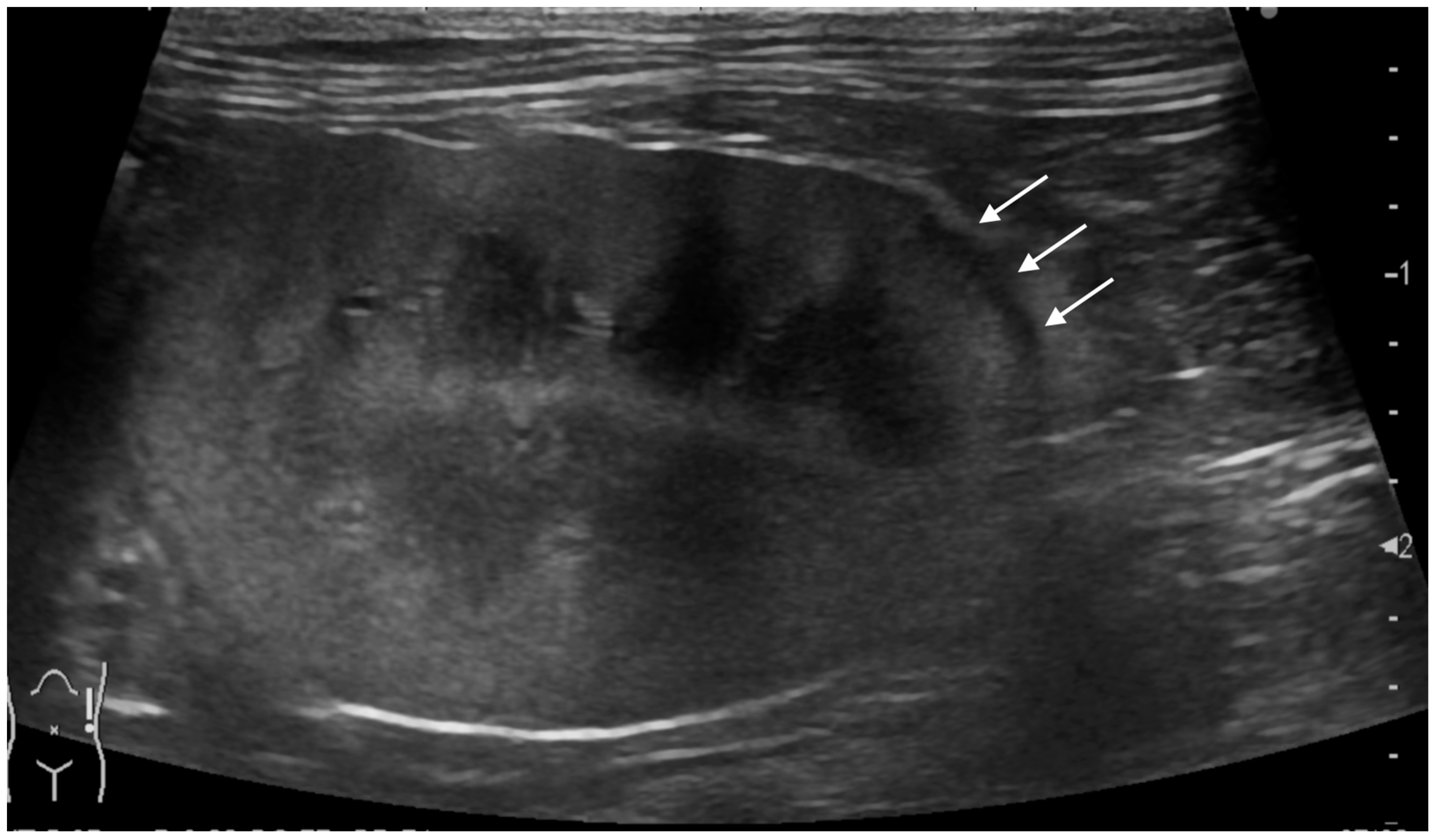
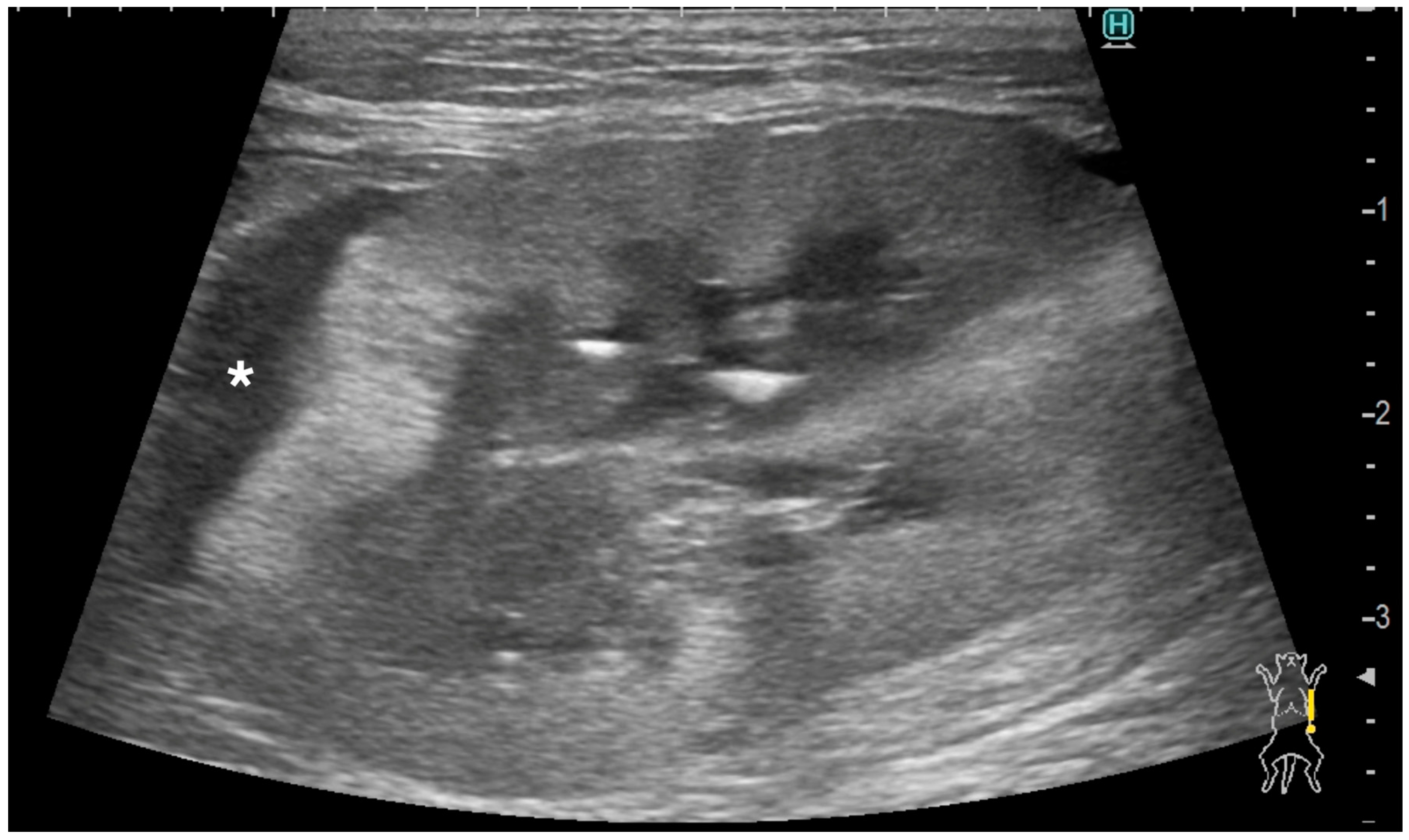
| Case | Signalment | Affected Kidney | US Findings of Subcapsular Thickening Lesion | US Findings of the Kidney | Diagnosis of Renal Lesion | Diagnosis of Other Organs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distribution | Maximum Thickness (mm) | Echotexture | Echogenicity | Renal Cortical Margin | Renal Capsular Margin | Vascularity | Perirenal Effusion | Architecture | Characteristic Pattern | Corticomedullary Distinction | Renal Pelvis Diameter (mm) | Mineralization | Cystic Lesion | Nodule | |||||
| 1 | 10 year-old, MN, DSH | left | focal | 3.5 | homo | hypo | irregular | irregular | no | no | normal | ill-defined | 1.3 | no | no | yes | Metastatic Carcinoma and Suspected Metastatic Carcinoma (sample obtained from either right or left kidney) | Pulmonary Carcinoma (lung, muscle) | |
| right | focal | 3.5 | homo | hypo | irregular | irregular | no | no | abnormal | hypoechoic striations within hyperechoic areas | ill-defined | no dilation | no | no | no | ||||
| 2 | 15 year-old, FN, DSH | left | focal | 1 | homo | hypo | smooth | smooth | NA | no | abnormal | ill-defined | no dilation | no | no | no | Suspected Metastatic Carcinoma | Pulmonary Carcinoma (lung, muscle) | |
| 3 | 11 year-old, MN, DSH | right | focal | 2.8 | homo | hypo | irregular | smooth | yes | no | abnormal | ill-defined | no dilation | no | no | yes | Suspected Metastatic Carcinoma | Pulmonary Carcinoma (lung) | |
| 4 | 9 year-old, FN, DSH | left | focal | 4.8 | homo | hypo | irregular | irregular | no | no | abnormal | hypoechoic striations within hyperechoic areas | ill-defined | no dilation | no | no | no | Metastatic Carcinoma | Pulmonary Carcinoma (lung) |
| right | focal | 1.5 | homo | hypo | smooth | smooth | NA | no | abnormal | well-defined | no dilation | no | no | no | Suspected Metastatic Carcinoma | ||||
| 5 | 14 year-old, FN, DSH | left | focal | 1.2 | homo | hypo | smooth | smooth | NA | no | abnormal | hypoechoic striations within hyperechoic areas | ill-defined | no dilation | no | no | no | Metastatic Carcinoma | Pulmonary Carcinoma (lung) |
| 6 | 11 year-old, MN, DSH | left | focal | 3 | homo | hypo | smooth | smooth | NA | yes | normal | ill-defined | no dilation | yes | no | no | Metastatic Carcinoma | Metastatic Carcinoma (lung) | |
| right | circumferential | 13.2 | hetero | hetero | irregular | irregular | yes | yes | abnormal | completely effaced | 1.9 | yes | yes | no | Primary Renal Carcinoma | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuyama, A.; Toshima, A.; Nakajima, A.; Murakami, M. Ultrasonographic Renal Subcapsular Thickening in Cats with Primary and Metastatic Carcinoma. Vet. Sci. 2024, 11, 134. https://doi.org/10.3390/vetsci11030134
Masuyama A, Toshima A, Nakajima A, Murakami M. Ultrasonographic Renal Subcapsular Thickening in Cats with Primary and Metastatic Carcinoma. Veterinary Sciences. 2024; 11(3):134. https://doi.org/10.3390/vetsci11030134
Chicago/Turabian StyleMasuyama, Ayano, Atsushi Toshima, Asami Nakajima, and Masahiro Murakami. 2024. "Ultrasonographic Renal Subcapsular Thickening in Cats with Primary and Metastatic Carcinoma" Veterinary Sciences 11, no. 3: 134. https://doi.org/10.3390/vetsci11030134
APA StyleMasuyama, A., Toshima, A., Nakajima, A., & Murakami, M. (2024). Ultrasonographic Renal Subcapsular Thickening in Cats with Primary and Metastatic Carcinoma. Veterinary Sciences, 11(3), 134. https://doi.org/10.3390/vetsci11030134






