Ultrasound and Elastosonographic Features of the Patellar Ligament in Dogs Affected by Cranial Cruciate Ligament Disease
Abstract
Simple Summary
Abstract
1. Introduction
- Distinguishes between different tissues based on their rigidity. This characteristic permits an immediate visual distinction using a graphical representation. Moreover, the contrast between the different tissues is generally high.
- Allows the detection of pathologies or disorders within the same tissue, especially in tissues whose function is closely related to their structure.
- Avoids the artifacts caused by the presence of ossification or mineralization (acoustic shadowing) [29].
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
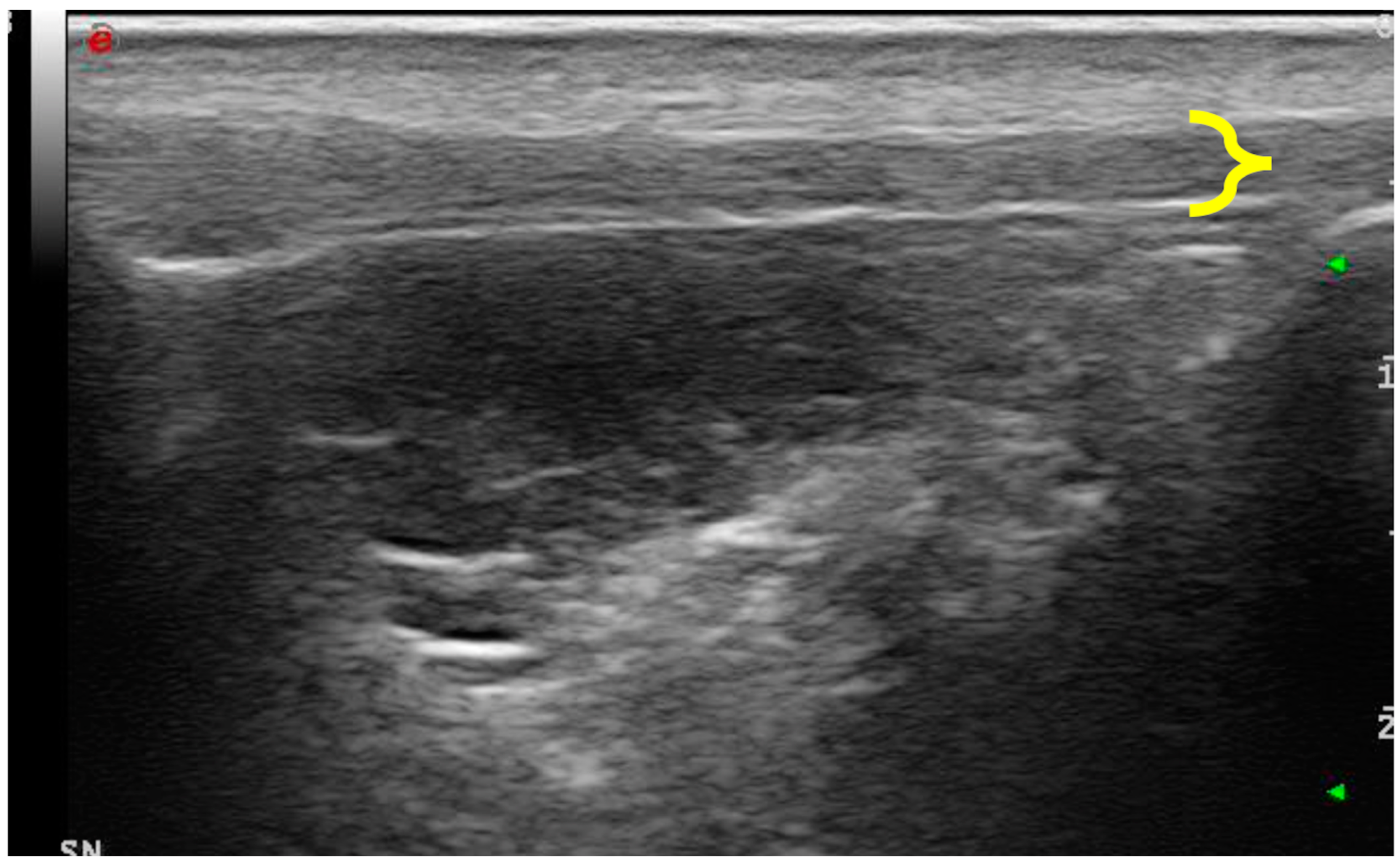
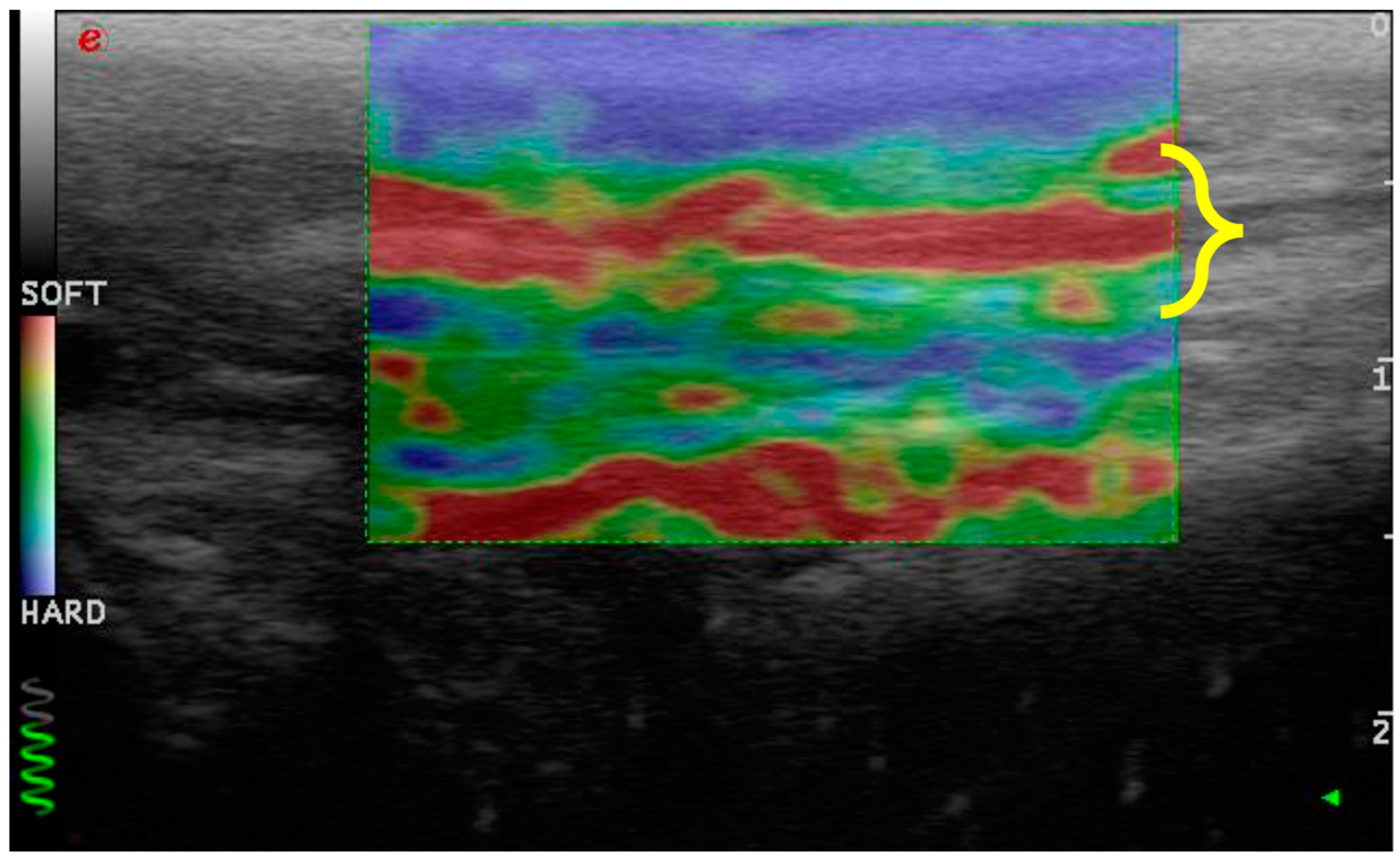
2.3. Ultrasound and Elastosonographic Examination
- Consistency between the elastogram and the underlying B-mode ultrasound image;
- Blue coloration of the skin and dermal tissue because they are harder than the patellar ligament;
- Green coloration of the marker (green coil). Thus represents real-time feedback on the quality of the strain image acquisition.
2.4. Statistical Analysis
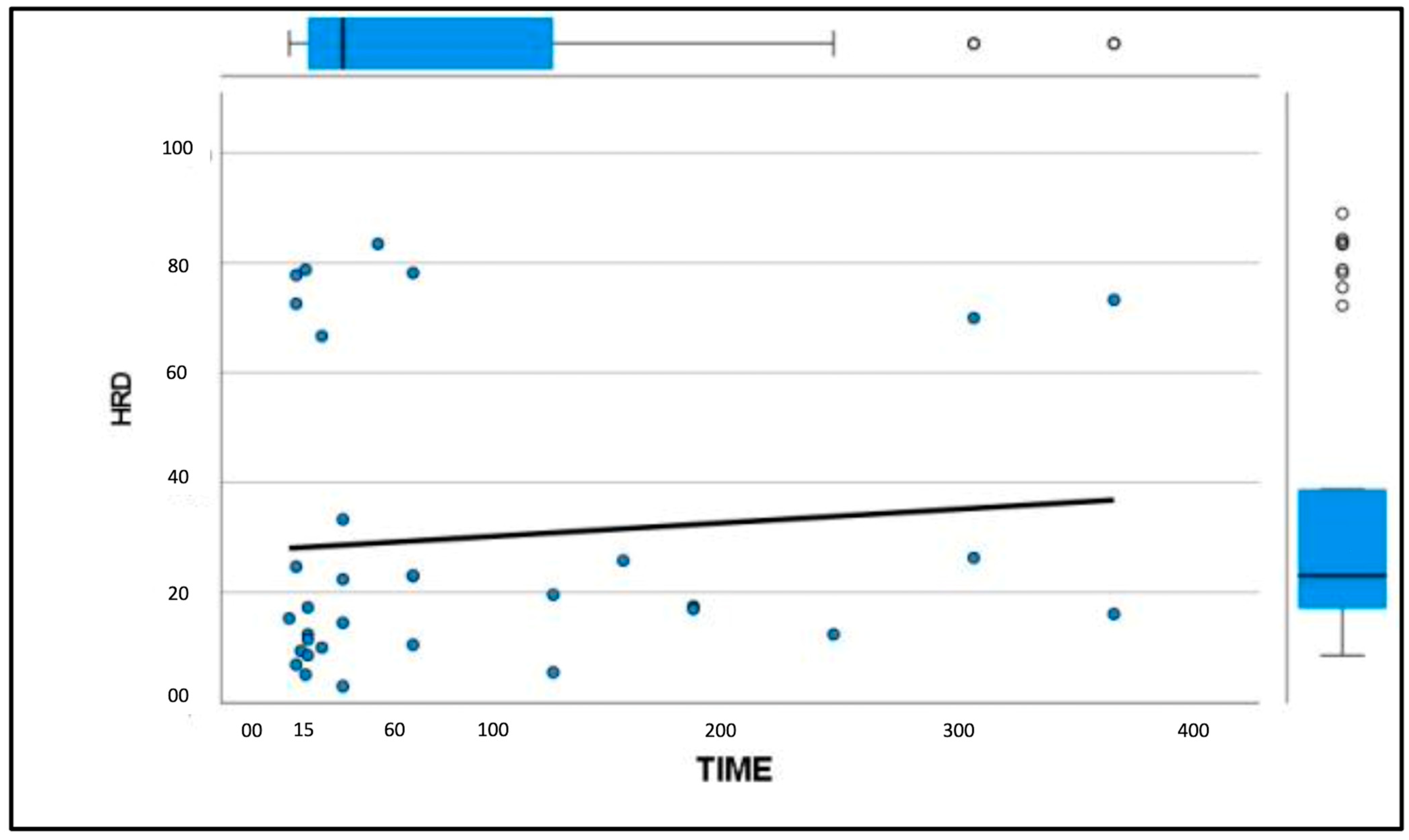
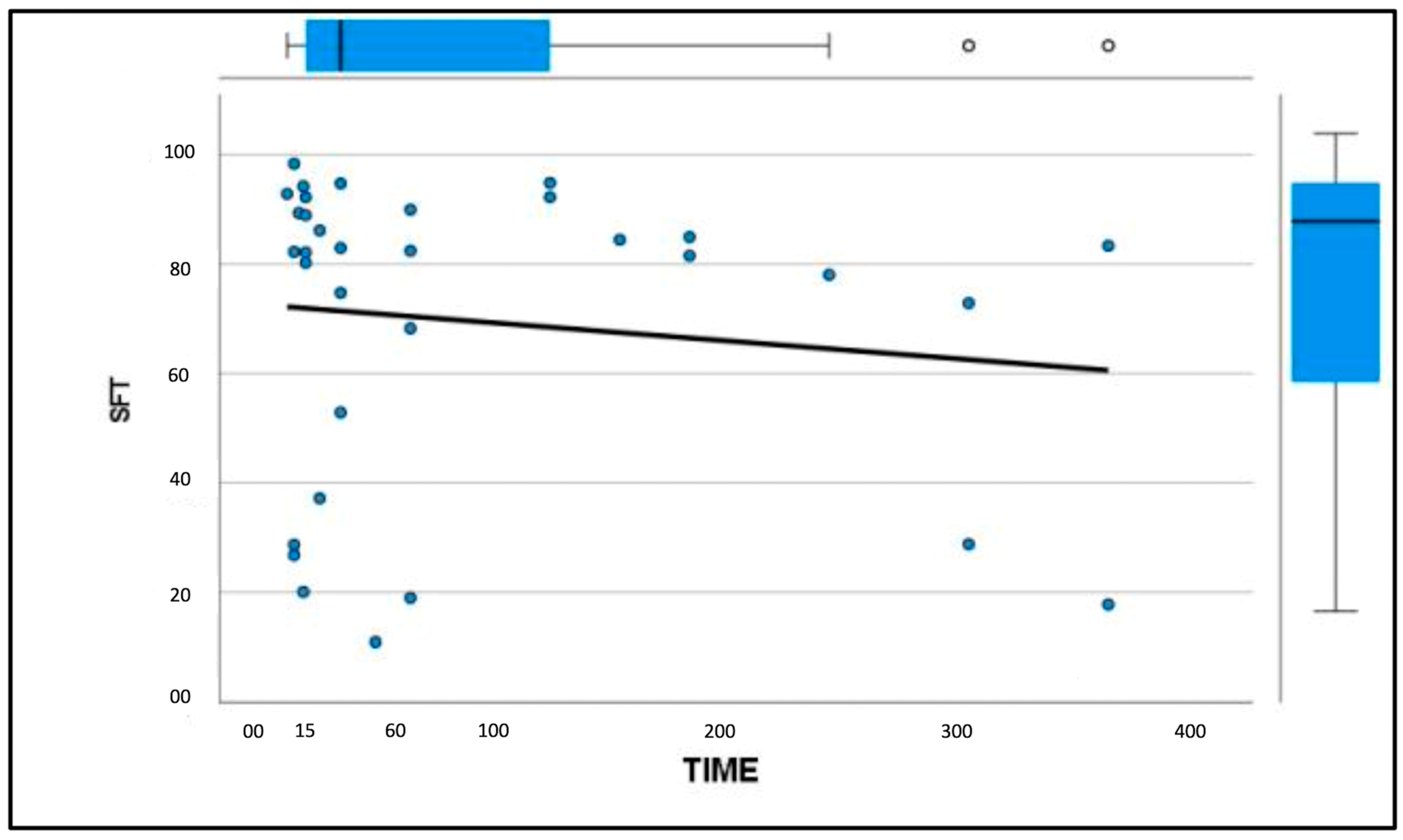
3. Results
3.1. Enrolled Patients
3.2. Ultrasonographic Examination
3.3. Elastosonographic Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, H.E.; De Lahunta, A. Miller’s Anatomy of the Dog, 4th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2012; pp. 180–181. [Google Scholar]
- de Rooster, H.; Comerford, E. Morphology and function of the cruciate ligaments. In Advances in the Canine Cranial Cruciate Ligament, 2nd ed.; Muir, P., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 3–11. [Google Scholar]
- Witsberger, T.H.; Villamil, J.A.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Sellon, D.C.; Marcellin-Little, D.J. Risk factors for cranial cruciate ligament rupture in dogs participating in canine agility. BMC Vet. Res. 2022, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Griffon, D.J. A review of the pathogenesis of canine cranial cruciate ligament disease as a basis for future preventive strategies. Vet. Surg. 2010, 39, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Kyllar, M.; Cížek, P. Cranial cruciate ligament structure in relation to the tibial plateau slope and intercondylar notch width in dogs. J. Vet. Sci. 2018, 19, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Comerford, E.J.; Smith, K.; Hayashi, K. Update on the aetiopathogenesis of canine cranial cruciate ligament disease. Vet. Comp. Orthop. Traumatol. 2011, 24, 91–98. [Google Scholar]
- Spinella, G.; Arcamone, G.; Valentini, S. Cranial cruciate ligament rupture in dogs: Review on biomechanics, etiopathogenetic factors and rehabilitation. Vet Sci. 2021, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; May, C. Meniscal damage associated with cruciate disease in the dog. J. Small Anim. Pract. 1991, 32, 111. [Google Scholar] [CrossRef]
- Ralphs, S.C.; Whitney, W.O. Arthroscopic evaluation of menisci in dogs with cranial cruciate ligament injuries: 100 cases (1999–2000). J. Am. Vet. Med. Assoc. 2002, 221, 1601. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Solano, M. Predictive variables for complications after tibial plateau leveling osteotomy with stifle inspection by arthrotomy in 1000 consecutive dogs. Vet. Surg. 2010, 39, 460. [Google Scholar] [CrossRef]
- Hayes, G.M.; Langley-Hobbs, S.J.; Jeffery, N.D. Risk factors for medial meniscal injury in association with cranial cruciate ligament rupture. J. Small Anim. Pract. 2010, 51, 630–634. [Google Scholar] [CrossRef]
- Franklin, S.P.; Gilley, R.S.; Palmer, R.H. Meniscal injury in dogs with cranial cruciate ligament rupture. Compend Contin. Educ. Vet. 2010, 32, E1–E10. [Google Scholar] [PubMed]
- Krupkova, O.; Smolders, L.; Wuertz-Kozak, K.; Cook, J.; Pozzi, A. The pathobiology of the meniscus: A comparison between the human and dog. Front. Vet. Sci. 2018, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Das, S.; Matthews, G.; Cantatore, M.; Czopowicz, M.; Silva, L.; McCarthy, J.; Fernandez-Salesa, N.; Lafuente, P.; Allan, R.; et al. Multiligament stifle injury, a multicenter retrospective study in 26 dogs. Vet. Med. Sci. 2023, 9, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Bruce, W.J. Multiple ligamentous injuries of the canine stifle joint: A study of 12 cases. J. Small Anim. Pract. 1998, 39, 333–340. [Google Scholar] [CrossRef]
- Laing, E.J. Collateral ligament injury and stifle luxation. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 845–853. [Google Scholar] [CrossRef]
- Palierne, S.; Blondel, M.; Vié, K.; Autefage, A. Morphometric assessment of the medial collateral ligament of the canine stifle joint. Res. Vet. Sci. 2022, 10, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Aron, D. Traumatic dislocation of the stifle joint: Treatment of 12 dogs and one cat. J. Am. Anim. Hosp. Assoc. 1998, 24, 333. [Google Scholar]
- Haut, R.C.; Lancaster, R.L.; DeCamp, C.E. Mechanical properties of the canine patellar tendon: Some correlations with age and the content of collagen. J. Biomech. 1992, 25, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Cardenas, L.; Soslowsky, L.J. Biomechanics of tendon injury and repair. J. Biomech. 2004, 37, 865–877. [Google Scholar] [CrossRef]
- Ricciardi, M.; Lenoci, D. Comparative diagnostic imaging of a partial patellar ligament tear in a dog. Open Vet. J. 2017, 8, 160–167. [Google Scholar] [CrossRef]
- Slocum, B.; Slocum, T.D. Tibial plateau leveling osteotomy for repair of cranial cruciate ligament rupture in the canine. Vet. Clin. N. Am. Small Anim. 1993, 23, 777–795. [Google Scholar] [CrossRef]
- Dennler, R.; Kipfer, N.M.; Tepic, S.; Hassig, M.; Montavon, P.M. Inclination of the patellar ligament in relation to flexion angle in stifle joints of dogs without degenerative joint disease. Am. J. Vet. Res. 2006, 67, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Carey, K.; Aiken, S.W.; Di Resta, G.R.; Herr, L.G.; Monette, S. Radiographic and clinical changes of the patellar tendon after tibial plateau leveling osteotomy 94 cases (2000–2003). Vet. Comp. Orthop. Traumatol. 2005, 18, 235–242. [Google Scholar] [PubMed]
- Pownder, S.L.; Hayashi, K.; Lin, B.Q.; Meyers, K.N.; Caserto, B.G.; Breighner, R.E.; Potter, H.G.; Koff, M.F. Differences in the magnetic resonance imaging parameter T2* may be identified during the course of canine patellar tendon healing: A pilot study. Quant Imaging Med. Surg. 2021, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Malmgaard-Clausen, N.M.; Tran, P.; Svensson, R.B.; Hansen, P.; Nybing, J.D.; Magnusson, S.P.; Kjaer, M. Magnetic Resonance T2 * Is Increased in Patients with Early-Stage Achilles and Patellar Tendinopathy. J. Magn. Reson. Imaging 2021, 54, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Kiss, Z.S.; Malara, F.A.; Ooi, A.B.; Cook, J.L.; Crossley, K.M. Comparative accuracy of magnetic resonance imaging and ultrasonography in confirming clinically diagnosed patellar tendinopathy. Am. J. Sports Med. 2007, 35, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.S.; Miyamoto, H.; Tamegger, M.; Faschingbauer, R.; Moriggl, B.; Klima, G.; Feutcher, G.M.; Kustlunger, M.; Jaschke, W.R. Achilles tendon assessed with sonoelastography: Histologic agreement. Radiology 2013, 267, 837–842. [Google Scholar] [CrossRef]
- Sigrist, R.M.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303. [Google Scholar] [CrossRef]
- Winnicki, K.; Ochała-Kłos, A.; Rutowicz, B.; Pękala, P.A.; Tomaszewski, K.A. Functional anatomy, histology and biomechanics of the human Achilles tendon—A comprehensive review. Ann. Anat. 2020, 229, 151461. [Google Scholar] [CrossRef]
- Palumbo Piccionello, A.; Serrani, D.; Busoni, V.; Salvaggio, A.; Bonazzi, M.; Bergamino, C.; Volta, A. Sonoelastographic features of the patellar ligament in clinically normal dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, J.F.; Pedersen, M.R.; Ewertsen, C.; Săftoiu, A.; Lönn, L.; Rafaelsen, S.R.; Nielsen, M.B. A comparative study of strain and shear-wave elastography in an elasticity phantom. Am. J. Roentgenol. 2015, 204, W236–W242. [Google Scholar] [CrossRef]
- DeSandre-Robinson, D.M.; Tano, C.A.; Fiore, K.L.; Prytherch, B. Radiographic evaluation and comparison of the patellar ligament following tibial plateau leveling osteotomy and tibial tuberosity advancement in dogs: 106 cases (2009–2012). J. Am. Vet. Med. Ass. 2017, 250, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Brukner, P. Patellar tendinopathy. Clin. Sports Med. 2003, 22, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Domenichini, R.; Pialat, J.B.; Podda, A.; Aubry, S. Ultrasound elastography in tendon pathology: State of the art. Skeletal. Radiol. 2017, 46, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Van der Worp, H.; Van Ark, M.; Roerink, S.; Pepping, G.J.; Van Den Akker-Scheek, I.; Zwerver, J. Risk factors for patellar tendinopathy: A systematic review of the literature. Br. J. Sports Med. 2011, 45, 446–452. [Google Scholar] [CrossRef]
- Serrani, D.; Volta, A.; Cingolani, F.; Pennasilico, L.; Di Bella, C.; Bonazzi, M.; Salvaggio, A.; Palumbo, A.P. Serial Ultrasonographic and Real- Time Elastosonographic Assessment of the Ovine Common Calcaneal Tendon, after an Experimentally Induced Tendinopathy. Vet. Sci. 2021, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Del Signore, F.; De Dominicis, S.; Mastromatteo, G.; Simeoni, F.; Scapolo, P.A.; Tamburro, R.; Vignoli, M. Sonoelastography of Normal Canine Common Calcaneal Tendon: Preliminary Results. Vet. Comp. Orthop. Traum. 2020, 34, 200–205. [Google Scholar] [CrossRef] [PubMed]
- McCagherty, J.; Longo, M.; Pennington, C.; Liuti, T.; Morrison, L.R.; Brown, H.; Clements, D.N. Effect of Stifle Flexion Angle on the Repeatability of Real-Time Elastosonography of the Patellar Ligament in Medium-to Large-Breed Dogs. Vet. Comp. Orthop. Traumatol. 2020, 33, 391–397. [Google Scholar] [CrossRef]
- Porta, F.; Damjanov, N.; Galluccio, F.; Iagnocco, A.; Matucci-Cerinic, M. Ultrasound elastography is a reproducible and feasible tool for the evaluation of the patellar tendon in healthy subjects. Int. J. Rheu. 2014, 17, 762–766. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, L.; Liu, Q.; Zhang, W. Application of shear wave elastography and B-mode ultrasound in patellar tendinopathy after extracorporeal shockwave therapy. J. Med. Ultrason. 2020, 47, 469–476. [Google Scholar] [CrossRef]
- Mattern, K.L.; Berry, C.R.; Peck, J.N.; De Haan, J.J. Radiographic and ultrasonographic evaluation of the patellar ligament following tibial plateau leveling osteotomy. Vet. Radiol. Ultrasound. 2006, 47, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zann, G.J.; Kim, S.E.; Tinga, S.; Pozzi, A.; Banks, S.A. The effect of tibial plateau leveling osteotomy on patellofemoralkinematics in dogs: An in vivo study. Vet. Surg. 2020, 49, 207–213. [Google Scholar] [CrossRef]
- Guénégo, L.; Vezzoni, A.; Vezzoni, L. Comparison of tibial anatomical-mechanical axis angles and patellar positions between tibial plateau levelling osteotomy (TPLO) and modified cranial closing wedge osteotomy (AMA-based CCWO) for the treatment of cranial cruciate ligament disease in larjge dogs with tibial plateau slopes greater than 30° and clinically normal Labradors retrievers. BMC Vet. Res. 2021, 17, 318. [Google Scholar]
- Sundararajan, S.R.; Srikanth, K.P.; Rajasekaran, S. Neglected patellar tendon ruptures—A simple modified reconstruction using hamstrings tendon graft. Intern. Orthop. 2013, 37, 2159–2164. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennasilico, L.; Volta, A.; Sassaroli, S.; Di Bella, C.; Riccio, V.; Pilati, N.; Tambella, A.M.; Dini, F.; Palumbo Piccionello, A. Ultrasound and Elastosonographic Features of the Patellar Ligament in Dogs Affected by Cranial Cruciate Ligament Disease. Vet. Sci. 2024, 11, 126. https://doi.org/10.3390/vetsci11030126
Pennasilico L, Volta A, Sassaroli S, Di Bella C, Riccio V, Pilati N, Tambella AM, Dini F, Palumbo Piccionello A. Ultrasound and Elastosonographic Features of the Patellar Ligament in Dogs Affected by Cranial Cruciate Ligament Disease. Veterinary Sciences. 2024; 11(3):126. https://doi.org/10.3390/vetsci11030126
Chicago/Turabian StylePennasilico, Luca, Antonella Volta, Sara Sassaroli, Caterina Di Bella, Valentina Riccio, Nicola Pilati, Adolfo Maria Tambella, Fabrizio Dini, and Angela Palumbo Piccionello. 2024. "Ultrasound and Elastosonographic Features of the Patellar Ligament in Dogs Affected by Cranial Cruciate Ligament Disease" Veterinary Sciences 11, no. 3: 126. https://doi.org/10.3390/vetsci11030126
APA StylePennasilico, L., Volta, A., Sassaroli, S., Di Bella, C., Riccio, V., Pilati, N., Tambella, A. M., Dini, F., & Palumbo Piccionello, A. (2024). Ultrasound and Elastosonographic Features of the Patellar Ligament in Dogs Affected by Cranial Cruciate Ligament Disease. Veterinary Sciences, 11(3), 126. https://doi.org/10.3390/vetsci11030126









