Pathogens Associated with Bovine Mastitis: The Experience of Bosnia and Herzegovina
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Farms, and Sample Collection
2.2. Bacteriological Analysis
2.3. M. bovis Detection and Identification
2.3.1. Isolation
2.3.2. Real-Time PCR Detection and Identification
DNA Extraction from the Isolates
DNA Extraction from Milk Samples
M. bovis Real Time PCR
2.4. Isolation and Identification of Yeasts and Algae
2.5. Statistical Analysis
3. Results
3.1. Presence of Pathogens
3.1.1. Bacteria
3.1.2. Mycoplasma bovis
3.1.3. Yeasts and Algae
3.1.4. Bulk Tank Milk Results
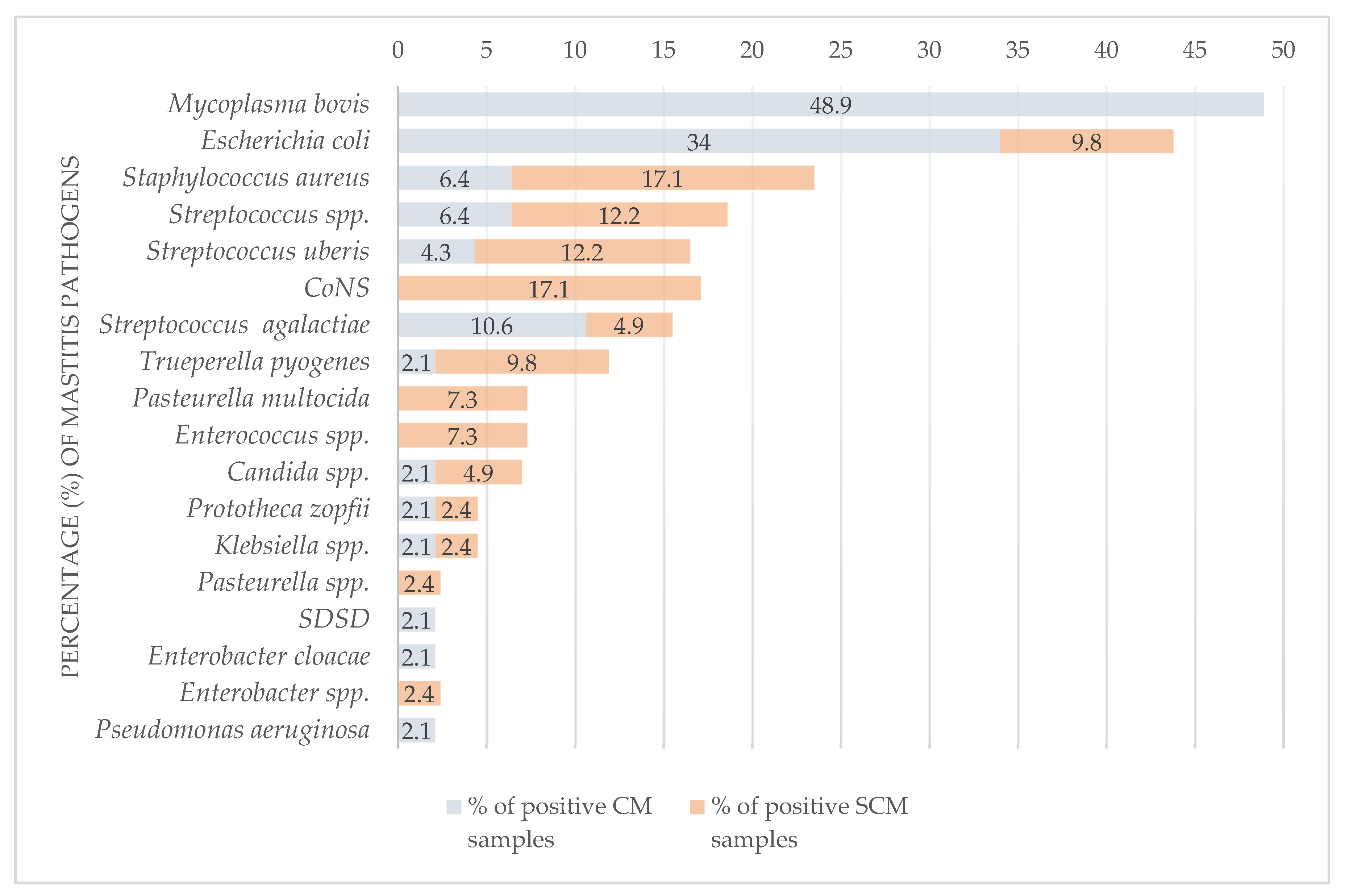
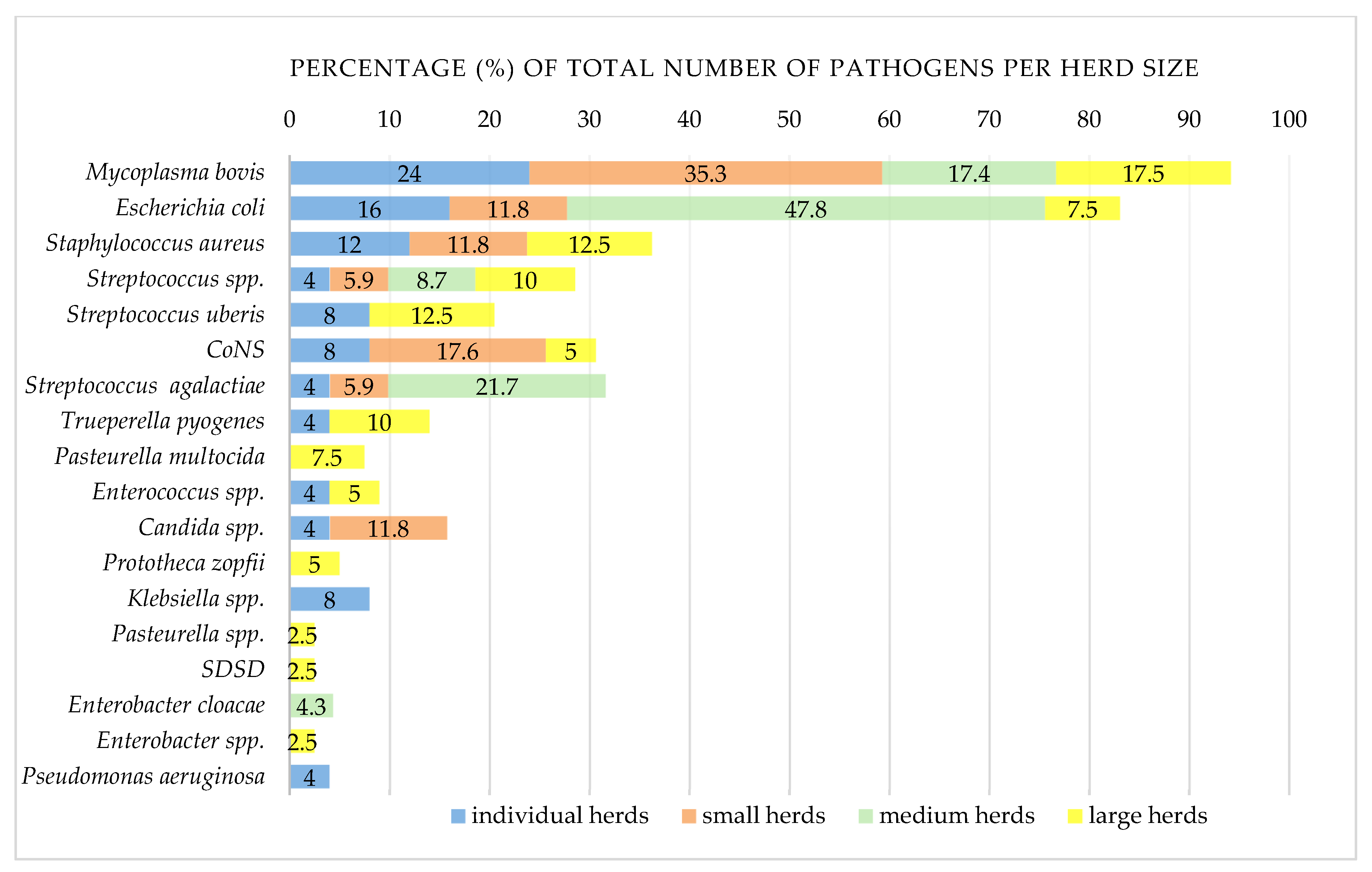
3.1.5. Distribution of Mastitis Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, A.J. Bovine Mastitis: An Evolving Disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, K.R.; Trajcev, M.; Buneski, G. A Review of the Factors Affecting the Costs of Bovine Mastitis. J. S. Afr. Vet. Assoc. 2006, 77, 52–60. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Egyedy, A.F.; Ametaj, B.N. Mastitis: Impact of Dry Period, Pathogens, and Immune Responses on Etiopathogenesis of Disease and Its Association with Periparturient Diseases. Dairy 2022, 3, 881–906. [Google Scholar] [CrossRef]
- The International Dairy Federation. Available online: https://fil-idf.org/ (accessed on 19 March 2023).
- Fredebeul-Krein, F.; Schmenger, A.; Wente, N.; Zhang, Y.; Krömker, V. Factors Associated with the Severity of Clinical Mastitis. Pathogens 2022, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Velázquez-Ordoñez, V.; Delgadillo-Ruiz, L.; Zaragoza-Bastida, A. Bovine Mastitis, a Worldwide Impact Disease: Prevalence, Antimicrobial Resistance, and Viable Alternative Approaches. Vet. Anim. Sci. 2023, 21, 100306. [Google Scholar] [CrossRef] [PubMed]
- Markey, B.; Leonard, F.; Archambault, M.; Cullinane, A.; Maguire, D. Clinical Veterinary Microbiology, 2nd ed.; Mosby Elsevier: Edinburgh, Scotland, 2013. [Google Scholar]
- Klaas, I.C.; Zadoks, R.N. An Update on Environmental Mastitis: Challenging Perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Suresh, K.P.; Jayamma, K.S.; Shome, B.R.; Patil, S.S.; Amachawadi, R.G. An Understanding of the Global Status of Major Bacterial Pathogens of Milk Concerning Bovine Mastitis: A Systematic Review and Meta-Analysis (Scientometrics). Pathogens 2021, 10, 545. [Google Scholar] [CrossRef]
- Dworecka-Kaszak, B.; Krutkiewicz, A.; Szopa, D.; Kleczkowski, M.; Biegańska, M. High Prevalence of Candida Yeast in Milk Samples from Cows Suffering from Mastitis in Poland. Sci. World J. 2012, 2012, 196347. [Google Scholar] [CrossRef]
- Gaudie, C.M.; Wragg, P.N.; Barber, A.M. Outbreak of Disease Due to Candida Krusei in a Small Dairy Herd in the UK. Vet. Rec. 2009, 165, 535–537. [Google Scholar] [CrossRef]
- Jagielski, T.; Krukowski, H.; Bochniarz, M.; Piech, T.; Roeske, K.; Bakuła, Z.; Wlazło, Ł.; Woch, P. Prevalence of Prototheca Spp. on Dairy Farms in Poland—A Cross-Country Study. Microb. Biotechnol. 2019, 12, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Milanov, D.; Petrović, T.; Polaček, V.; Suvajdžić, L.; Bojkovski, J. Mastitis Associated with Prototheca Zopfii—An Emerging Health and Economic Problem on Dairy Farms. J. Vet. Res. 2016, 60, 373–378. [Google Scholar] [CrossRef][Green Version]
- Libisch, B.; Picot, C.; Ceballos-Garzon, A.; Moravkova, M.; Klimesová, M.; Telkes, G.; Chuang, S.T.; Le Pape, P. Prototheca Infections and Ecology from a One Health Perspective. Microorganisms 2022, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.A.J.; Fox, L.K.; Lysnyansky, I. Mycoplasma Mastitis in Cattle: To Cull or Not to Cull. Vet. J. 2016, 216, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.; Ayling, R.; McAuliffe, L. Mycoplasma Mastitis. Vet. Rec. 2007, 160, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Gelgie, A.E.; Korsa, M.G.; Kerro Dego, O. Mycoplasma Bovis Mastitis. Curr. Res. Microb. Sci. 2022, 3, 100123. [Google Scholar] [CrossRef]
- United States Department of Agriculture Foreign Agricultural Service. Available online: https://fas.usda.gov/data/ (accessed on 27 March 2023).
- Veterinary Office of Bosnia and Herzegovina. Available online: https://www.vet.gov.ba/ (accessed on 8 November 2023).
- Maksimović, Z.; Bačić, A.; Rifatbegović, M. Mycoplasmas Isolated from Ruminants in Bosnia and Herzegovina between 1995 and 2015. Veterinaria 2016, 65, 27–31. [Google Scholar]
- Burović, J. Isolation of bovine clinical mastitis bacterial pathogens and their antimicrobial susceptibility in the Zenica region in 2017. Vet. Stanica 2020, 51, 47–52. [Google Scholar] [CrossRef]
- Thiaucourt, F.; Bölske, G.; Leneguersh, B.; Smith, D.; Wesonga, H. Diagnosis and Control of Contagious Caprine Pleuropneumonia. Rev. Sci. Tech. 1996, 15, 1415–1429. [Google Scholar] [CrossRef]
- Nicholas, R.; Ayling, R.; McAuliffe, L. Mycoplasma Diseases of Ruminants, 1st ed.; CABI Publishing: Oxford, UK, 2008. [Google Scholar]
- Sachse, K.; Salam, H.S.H.; Diller, R.; Schubert, E.; Hoffmann, B.; Hotzel, H. Use of a Novel Real-Time PCR Technique to Monitor and Quantitate Mycoplasma Bovis Infection in Cattle Herds with Mastitis and Respiratory Disease. Vet. J. 2010, 186, 299–303. [Google Scholar] [CrossRef]
- Fox, L.K. Mycoplasma Mastitis. Causes, Transmission, and Control. Vet. Clin. N. Am.—Food Anim. Pract. 2012, 28, 225–237. [Google Scholar] [CrossRef]
- Dudek, K.; Nicholas, R.A.J.; Szacawa, E.; Bednarek, D. Mycoplasma Bovis Infections—Occurrence, Diagnosis and Control. Pathogens 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- González, R.N.; Wilson, D.J. Mycoplasmal Mastitis in Dairy Herds. Vet. Clin. N. Am.—Food Anim. Pract. 2003, 19, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Haapala, V.; Vähänikkilä, N.; Kulkas, L.; Tuunainen, E.; Pohjanvirta, T.; Autio, T.; Pelkonen, S.; Soveri, T.; Simojoki, H. Mycoplasma Bovis Infection in Dairy Herds—Risk Factors and Effect of Control Measures. J. Dairy Sci. 2021, 104, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Deeney, A.S.; Collins, R.; Ridley, A.M. Identification of Mycoplasma Species and Related Organisms from Ruminants in England and Wales during 2005–2019. BMC Vet. Res. 2021, 17, 325. [Google Scholar] [CrossRef] [PubMed]
- Timonen, A.A.E.; Autio, T.; Pohjanvirta, T.; Häkkinen, L.; Katholm, J.; Petersen, A.; Mõtus, K.; Kalmus, P. Dynamics of the Within-Herd Prevalence of Mycoplasma Bovis Intramammary Infection in Endemically Infected Dairy Herds. Vet. Microbiol. 2020, 242, 108608. [Google Scholar] [CrossRef] [PubMed]
- Ghadersohi, A.; Hirst, R.G.; Forbes-Faulkener, J.; Coelen, R.J. Preliminary Studies on the Prevalence of Mycoplasma Bovis Mastitis in Dairy Cattle in Australia. Vet. Microbiol. 1999, 65, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ministry for Primary Industries (MPI) New Zealand Mycoplasma Bovis in New Zealand. Available online: https://www.mpi.govt.nz/ (accessed on 10 April 2023).
- Hazelton, M.S.; Morton, J.M.; Parker, A.M.; Sheehy, P.A.; Bosward, K.L.; Malmo, J.; House, J.K. Whole Dairy Herd Sampling to Detect Subclinical Intramammary Mycoplasma Bovis Infection after Clinical Mastitis Outbreaks. Vet. Microbiol. 2020, 244, 108662. [Google Scholar] [CrossRef]
- Timonen, A.A.E.; Katholm, J.; Petersen, A.; Mõtus, K.; Kalmus, P. Within-Herd Prevalence of Intramammary Infection Caused by Mycoplasma Bovis and Associations between Cow Udder Health, Milk Yield, and Composition. J. Dairy Sci. 2017, 100, 6554–6561. [Google Scholar] [CrossRef]
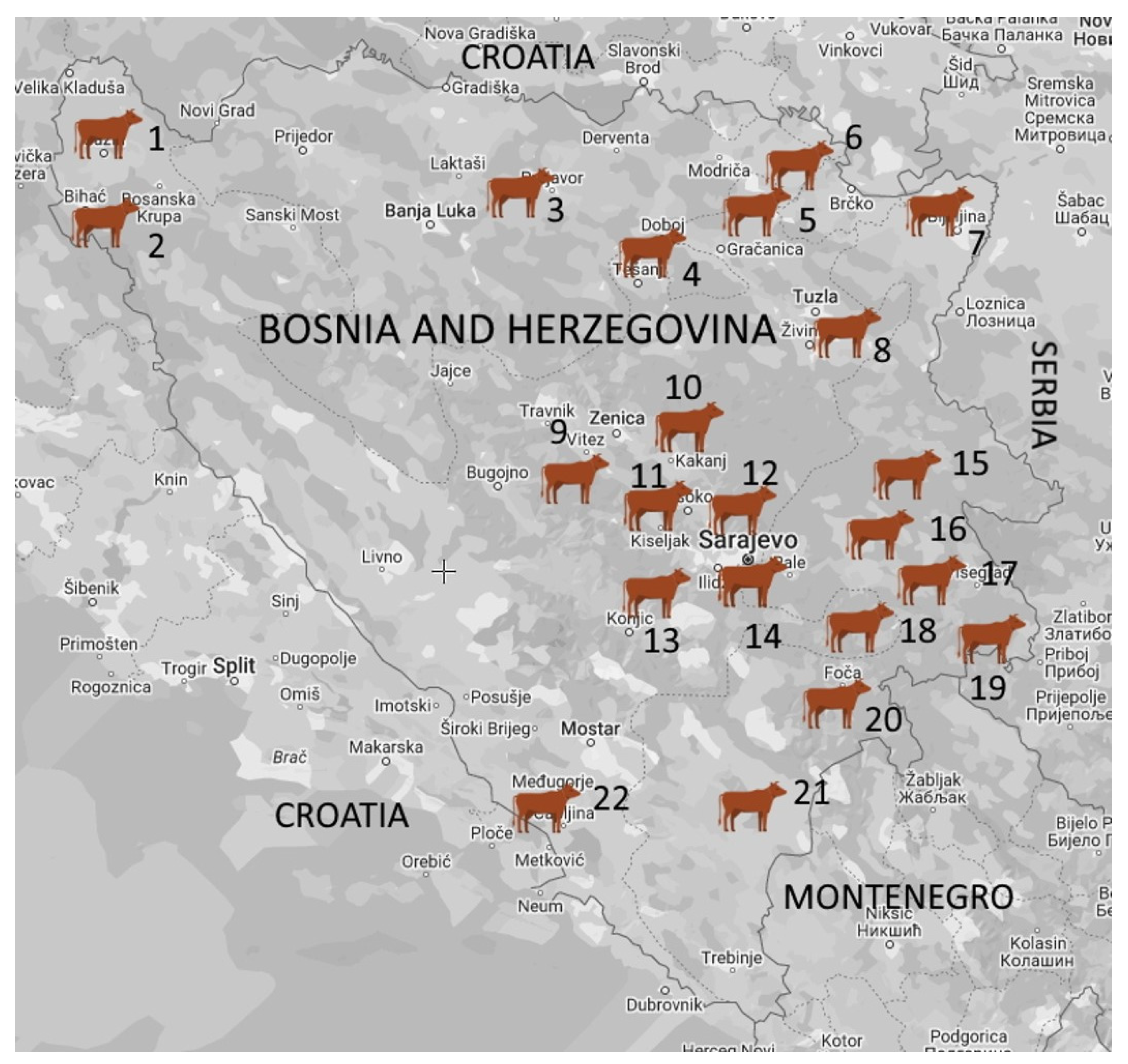
| Farm Size | No. of Positive Farms (%) | Clinical Mastitis | Subclinical Mastitis | Total | |||
|---|---|---|---|---|---|---|---|
| No. of Samples | No. of Positive Samples (%) | No. of Samples | No. of Positive Samples (%) | No. of Samples | No. of Positive Samples (%) | ||
| Individual (≤5 cows) | 14/19 (73.7) | 17 | 12 (70.6) | 7 | 6 (85.7) | 24 | 18 (75) |
| Small (6–20 cows) | 9/17 (52.9) | 10 | 7 (70) | 18 | 6 (33.3) | 28 | 13 (46.4) |
| Medium (21–49 cows) | 2/3 (66.7) | 21 | 18 (85.7) | 9 | 0 | 30 | 18 (60) |
| Large (≥50 cows) | 7/8 (87.5) | 20 | 10 (50) | 77 | 29 (37.7) | 97 | 39 (40.2) |
| Total (%) | 32/47 (68.1) | 68 | 47 (69.1) | 111 | 41 (36.9) | 179 | 88 (49.2) |
| Total (%) | Monomicrobial Samples | Polymicrobial Samples | ||||
|---|---|---|---|---|---|---|
| No. of Samples (%) | No. of Positive Samples (%) | No. of Positive Samples (%) | % of Total Samples | No. of Positive Samples (%) | % of Total Samples | |
| Clinical mastitis | 68 (38) | 47 (69.1) | 35 (74.5) | 51.5 | 12 (25.5) | 17.6 |
| Subclinical mastitis | 111 (62) | 41 (36.9) | 38 (92.7) | 34.2 | 3 (7.3) | 2.7 |
| Total (%) | 179 | 88 (49.2) | 73 (83) | 40.8 | 15 (17) | 8.4 |
| Pathogen | Monomicrobial | Polymicrobial | Clinical Mastitis | Subclinical Mastitis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Isolates | % of Total Samples | % of Positive Samples | % of Isolates | No. of Herds (%) | No. of Isolates (%) | % of Positive Samples | No. of Isolates (%) | % of Positive Samples | No. of Isolates (%) | % of Positive Samples | No. of Isolates (%) | % of Positive Samples | |
| M. bovis 1 | 23 | 12.8 | 26.1 | 21.9 | 12 (25.5) | 13 (12.4) | 17.8 | 10 (9.5) | 66.7 | 23 (21.9) | 48.9 | 0 | 0 |
| E. coli 2 | 20 | 11.2 | 22.7 | 19 | 10 (21.3) | 13 (12.4) | 17.8 | 7 (6.7) | 46.7 | 16 (15.2) | 34 | 4 (3.8) | 9.8 |
| S. aureus | 10 | 5.6 | 11.4 | 9.5 | 6 (12.8) | 8 (7.6) | 11 | 2 (1.9) | 13.3 | 3 (2.9) | 6.4 | 7 (6.7) | 17.1 |
| Streptococcus spp. | 8 | 4.5 | 9.1 | 7.6 | 6 (12.8) | 5 (4.8) | 6.8 | 3 (2.9) | 20 | 3 (2.9) | 6.4 | 5 (4.8) | 12.2 |
| S. uberis | 7 | 3.9 | 8 | 6.7 | 3 (6.4) | 7 (6.7) | 9.6 | 0 | 0 | 2 (1.9) | 4.3 | 5 (4.8) | 12.2 |
| CoNS 3 | 7 | 3.9 | 8 | 6.7 | 6 (12.8) | 7 (6.7) | 9.6 | 0 | 0 | 0 | 0 | 7 (6.7) | 17.1 |
| S. agalactiae | 7 | 3.9 | 8 | 6.7 | 3 (6.4) | 4 (3.8) | 5.5 | 3 (2.9) | 20 | 5 (4.8) | 10.6 | 2 (1.9) | 4.9 |
| Trueperella pyogenes | 5 | 2.8 | 5.7 | 4.8 | 3 (6.4) | 3 (2.9) | 4.1 | 2 (1.9) | 13.3 | 1 | 2.1 | 4 (3.8) | 9.8 |
| Pasteurella multocida | 3 | 1.7 | 3.4 | 2.9 | 2 (4.3) | 3 (2.9) | 4.1 | 0 | 0 | 0 | 0 | 3 (2.9) | 7.3 |
| Enterococcus spp. | 3 | 1.7 | 3.4 | 2.9 | 2 (4.3) | 2 (1.9) | 2.7 | 1 | 6.7 | 0 | 0 | 3 (2.9) | 7.3 |
| Candida spp. | 3 | 1.7 | 3.4 | 2.9 | 3 (6.4) | 1 | 1.4 | 2 (1.9) | 13.3 | 1 | 2.1 | 2 (1.9) | 4.9 |
| P. zopfii | 2 | 1.1 | 2.3 | 1.9 | 2 (4.3) | 2 (1.9) | 2.7 | 0 | 0 | 1 | 2.1 | 1 | 2.4 |
| Klebsiella spp. | 2 | 1.1 | 2.3 | 1.9 | 2 (4.3) | 1 | 1.4 | 1 | 6.7 | 1 | 2.1 | 1 | 2.4 |
| Pasteurella spp. | 1 | 0.6 | 1.1 | 1 | 1 (2.1) | 0 | 0 | 1 | 6.7 | 0 | 0 | 1 | 2.4 |
| SDSD 4 | 1 | 0.6 | 1.1 | 1 | 1 (2.1) | 1 | 1.4 | 0 | 0 | 1 | 2.1 | 0 | 0 |
| Enterobacter cloacae | 1 | 0.6 | 1.1 | 1 | 1 (2.1) | 1 | 1.4 | 0 | 0 | 1 | 2.1 | 0 | 0 |
| Enterobacter spp. | 1 | 0.6 | 1.1 | 1 | 1 (2.1) | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | 2.4 |
| Pseudomonas aeruginosa | 1 | 0.6 | 1.1 | 1 | 1 (2.1) | 1 | 1.4 | 0 | 0 | 1 | 2.1 | 0 | 0 |
| Total (%) | 105 | 58.7 | - | 100 | - | 73 (69.5) | 32 (30.5) | - | 59 (56.2) | - | 46 (43.8) | - | |
| Pathogens | No. of Positive Samples |
|---|---|
| Mycoplasma bovis/Escherichia coli | 3 |
| Mycoplasma bovis/Streptococcus agalactiae | 3 |
| Escherichia coli/Staphylococcus aureus 1 | 2 |
| Mycoplasma bovis/Streptococcus spp. | 2 |
| Mycoplasma bovis/Candida spp. | 1 |
| Mycoplasma bovis/Trueperella pyogenes | 1 |
| Escherichia coli/Streptococcus spp. | 1 |
| Trueperella pyogenes/Pasteurella spp. 2 | 1 |
| Escherichia coli/Klebsiella spp./Enterococcus spp./Candida spp. 2 | 1 |
| Total | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rifatbegović, M.; Nicholas, R.A.J.; Mutevelić, T.; Hadžiomerović, M.; Maksimović, Z. Pathogens Associated with Bovine Mastitis: The Experience of Bosnia and Herzegovina. Vet. Sci. 2024, 11, 63. https://doi.org/10.3390/vetsci11020063
Rifatbegović M, Nicholas RAJ, Mutevelić T, Hadžiomerović M, Maksimović Z. Pathogens Associated with Bovine Mastitis: The Experience of Bosnia and Herzegovina. Veterinary Sciences. 2024; 11(2):63. https://doi.org/10.3390/vetsci11020063
Chicago/Turabian StyleRifatbegović, Maid, Robin A. J. Nicholas, Tarik Mutevelić, Mithat Hadžiomerović, and Zinka Maksimović. 2024. "Pathogens Associated with Bovine Mastitis: The Experience of Bosnia and Herzegovina" Veterinary Sciences 11, no. 2: 63. https://doi.org/10.3390/vetsci11020063
APA StyleRifatbegović, M., Nicholas, R. A. J., Mutevelić, T., Hadžiomerović, M., & Maksimović, Z. (2024). Pathogens Associated with Bovine Mastitis: The Experience of Bosnia and Herzegovina. Veterinary Sciences, 11(2), 63. https://doi.org/10.3390/vetsci11020063






