Induced Pluripotent Stem Cells in Birds: Opportunities and Challenges for Science and Agriculture
Simple Summary
Abstract
1. Introduction
- One such advantage of using the chicken embryo as a model organism is that it allows visualization of and measurement tracking in life growth.
- Their embryos are easily accessible and quite big, both of which aid in the ability to manipulate and watch developmental activation—reasons they have been frequently used for decades as a “model organism” by biologists studying embryonic development [14].
- Stern (2005) found that the chicken embryo, a traditional model system for morphogenesis and organogenesis research, has yielded important insights into vertebrate development [15].
- Also, chickens are among the most completely sequenced vertebrate genomes and represent a pharmacogenomic model species that is particularly conducive to effective transgenesis or CRISPR/Cas9 gene editing protocols, which could assist in both forward genetic screening for functional annotation of genes involved in instructing lineage-specific mammalian cellular functions [16].
- Chickens (to study pathways of cellular reprogramming in iPSC research).
- Chickens are used as a reprogramming paradigm by Rossello and Torres-Padilla (2011), who have also demonstrated the importance of species-specific factors that could contribute to differences in yet uncharacterized cellular responses [17].
- Liu et al. (2017) generated chicken iPSCs from somatic cells that could differentiate into all three germ layers, such as human and non-human primate (NHP)-derived iPSCs [18].
- Li et al. showed that chicken iPSCs are utilized for regenerative purposes and highlighted valuable aspects of these cells as tools to decipher tissue development [19].
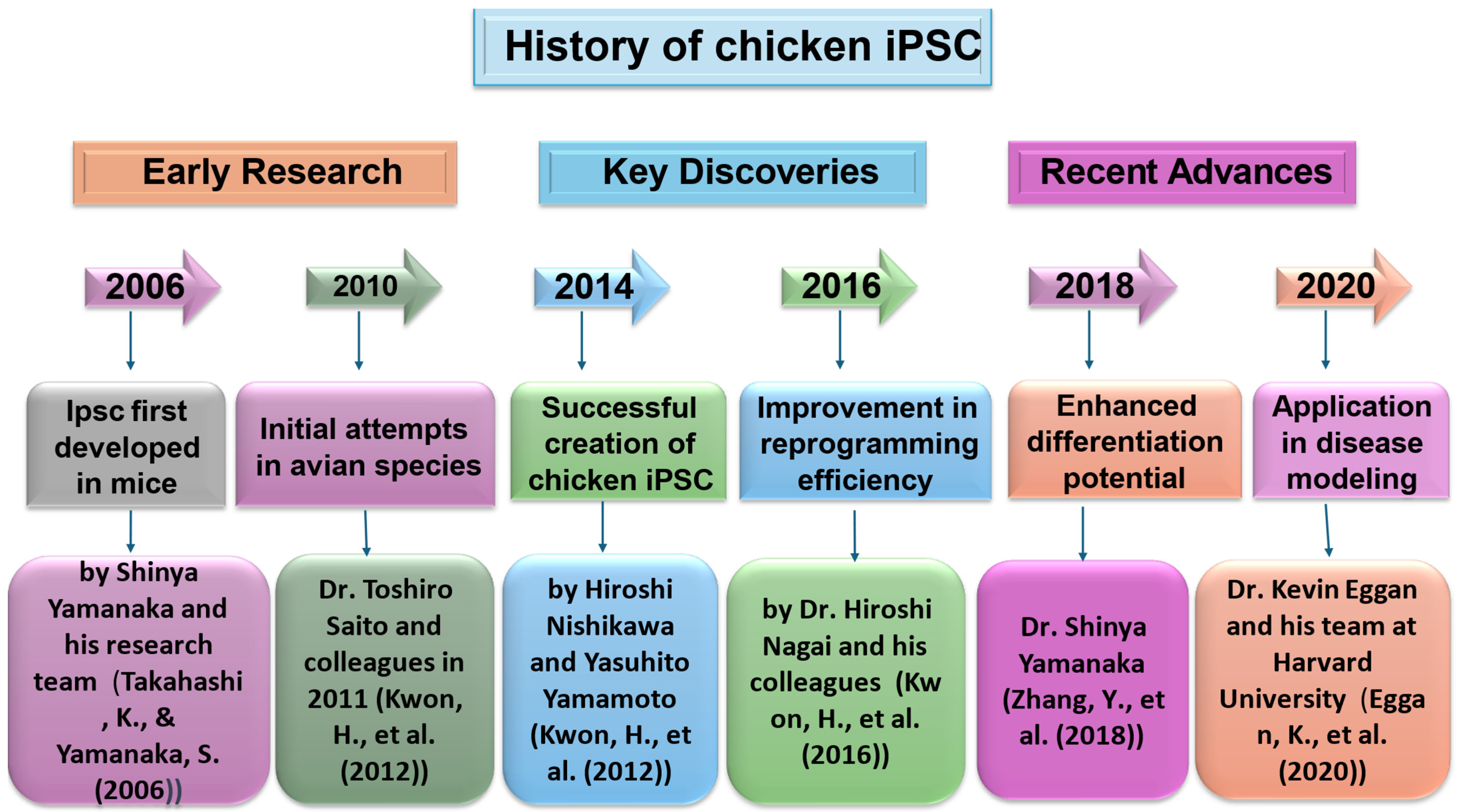
2. Historical Development of iPSCs in Chickens
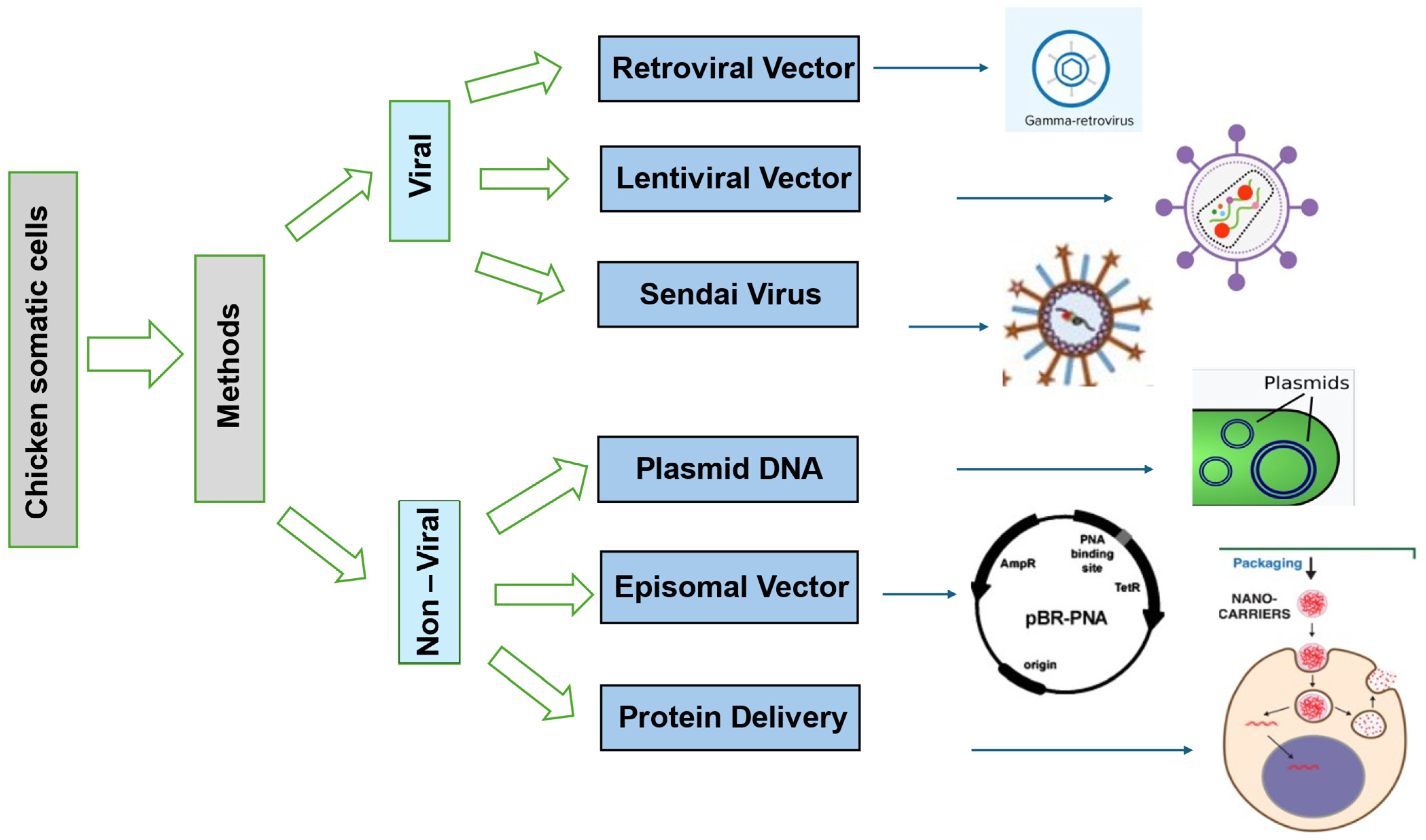
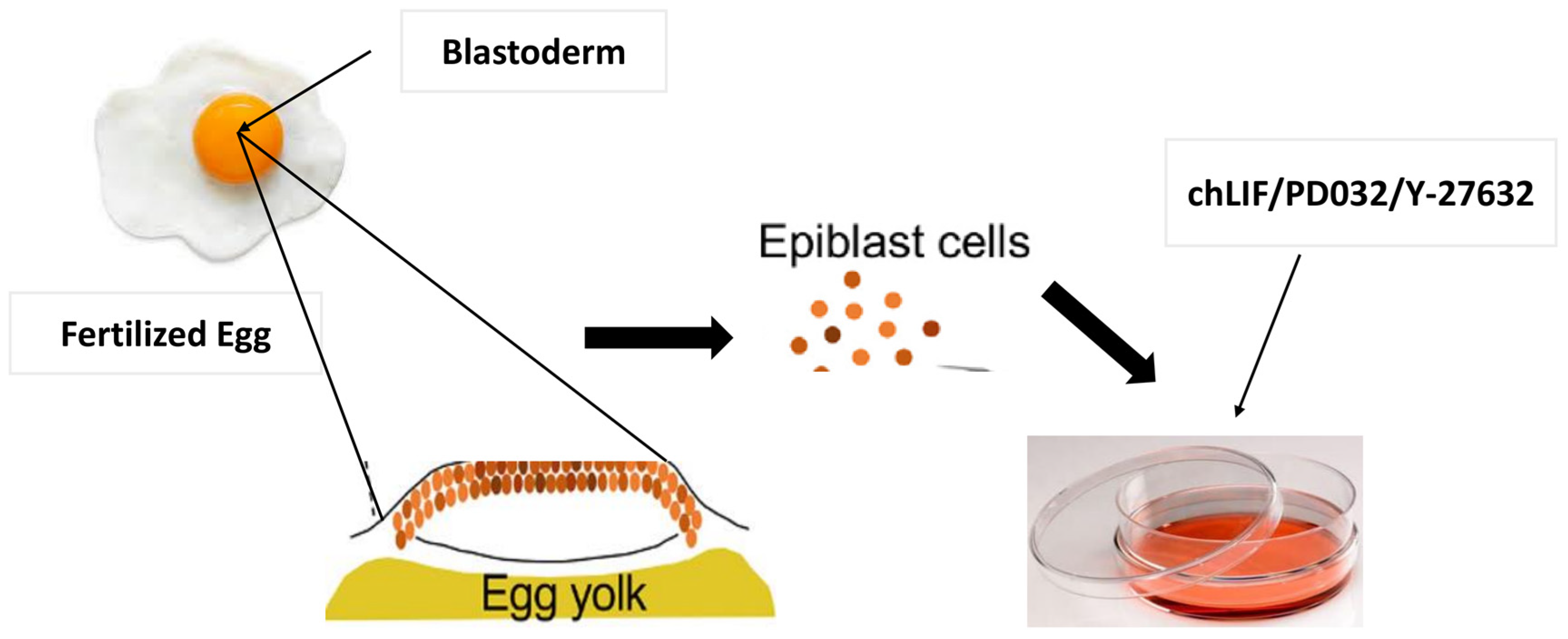
3. Techniques for iPSC Induction in Chickens
4. Molecular Mechanisms of iPSC Induction in Chickens
4.1. Key Factors Involved in Reprogramming
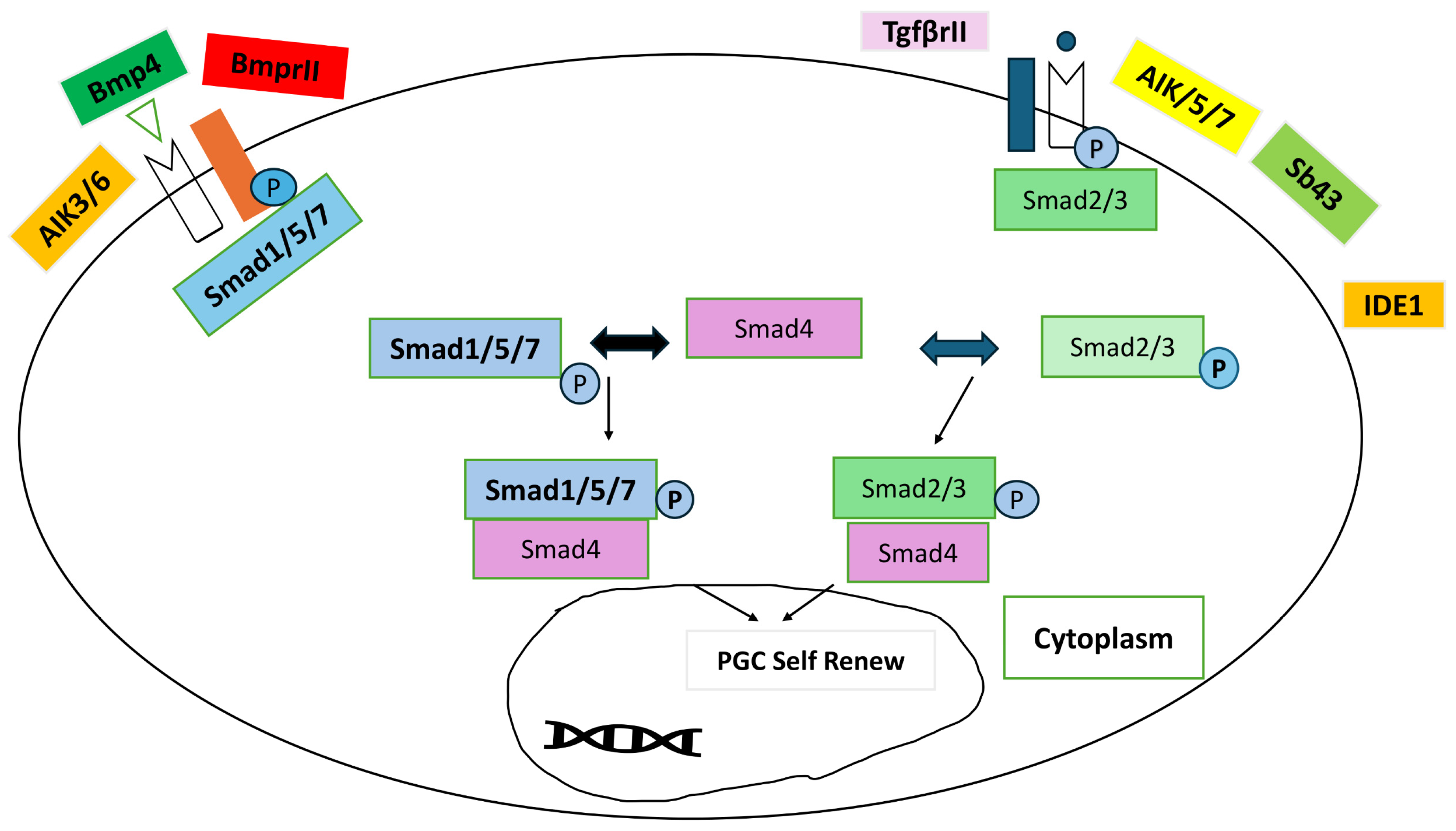
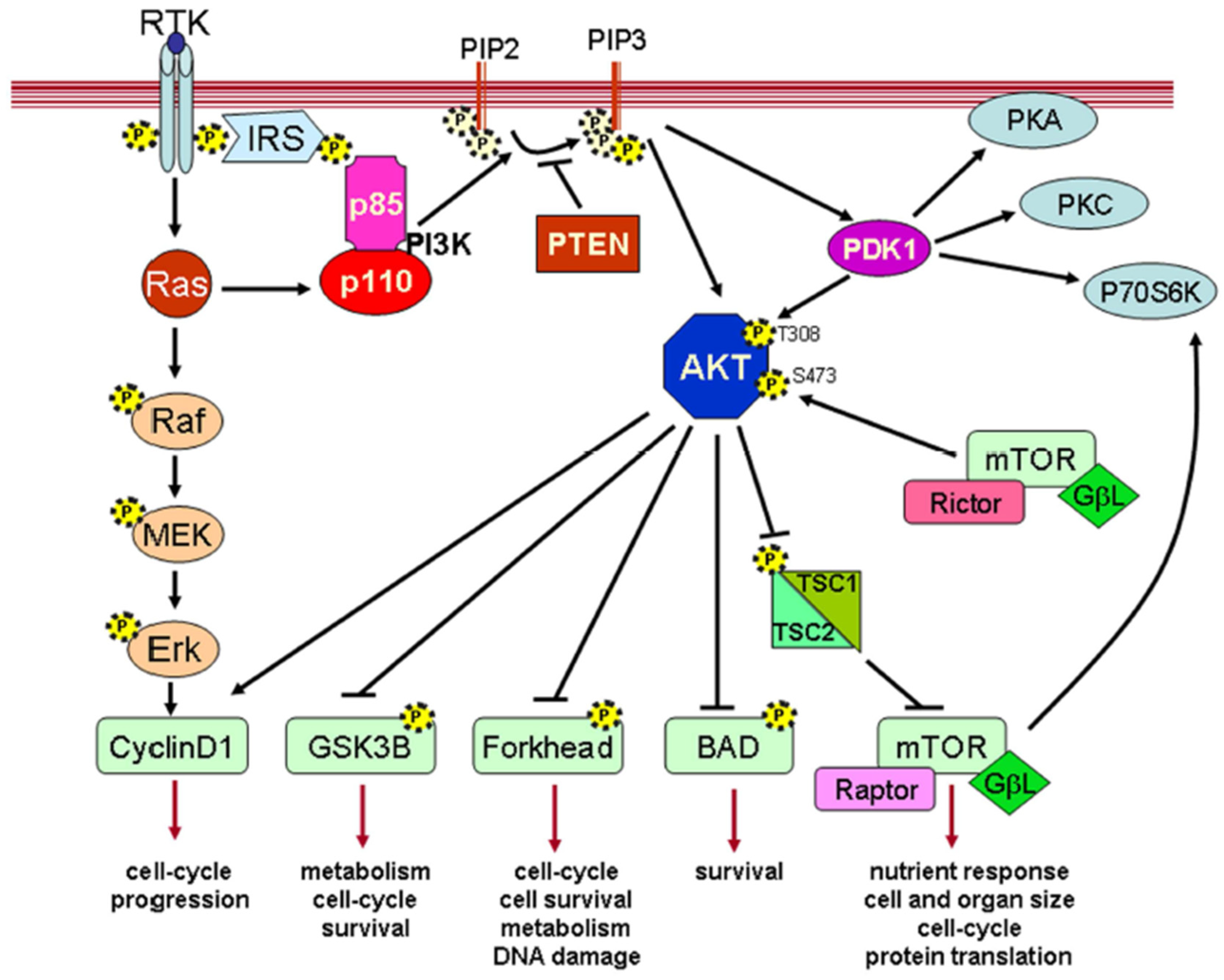
4.2. Signaling Pathways and Gene Expression Profiles
5. Efficiency and Challenges of Different Techniques
6. Genetic and Epigenetic Considerations
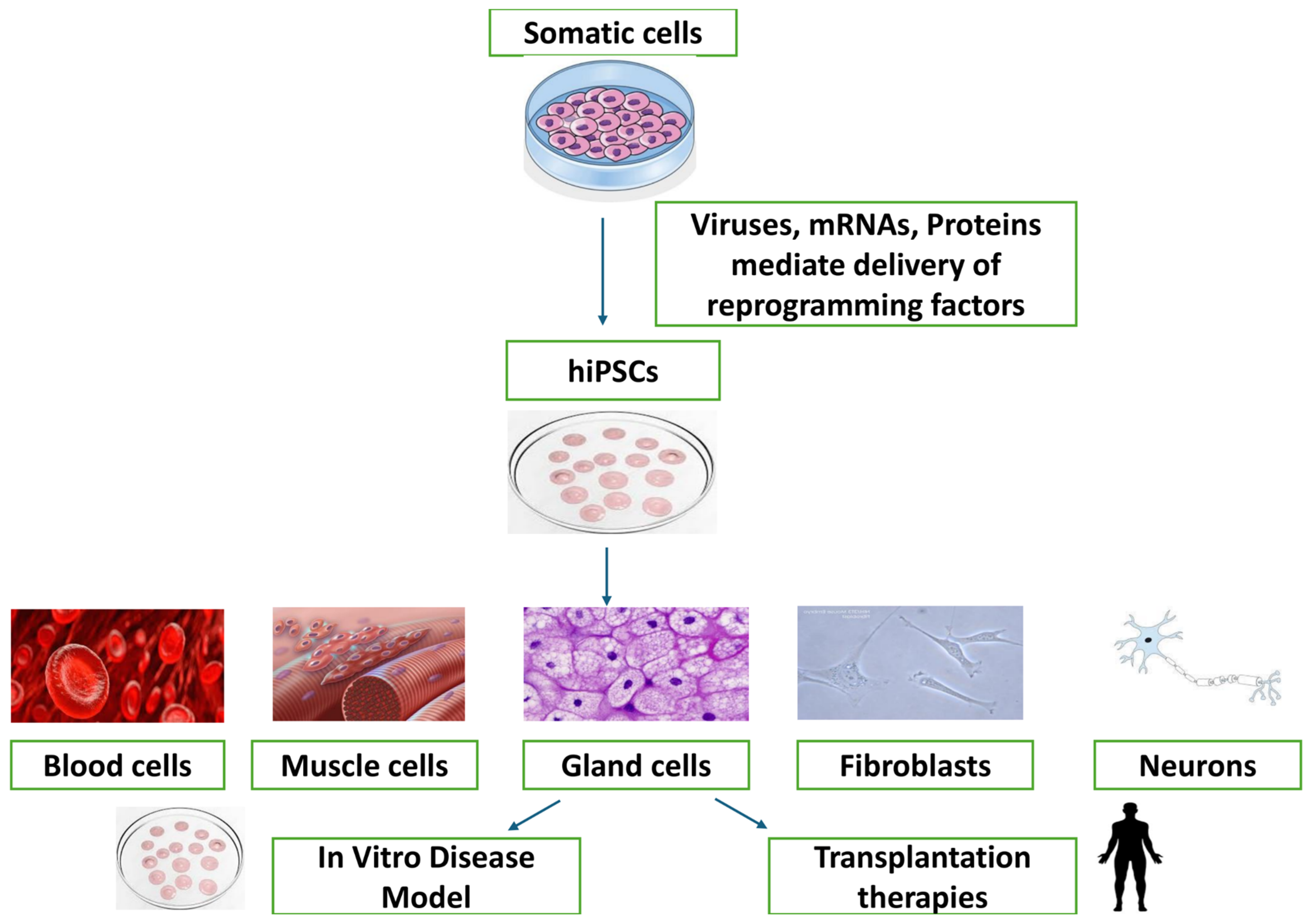
7. Applications of Chicken iPSCs
7.1. Regenerative Medicine and Tissue Engineering
7.2. Genetic Engineering and Transgenics
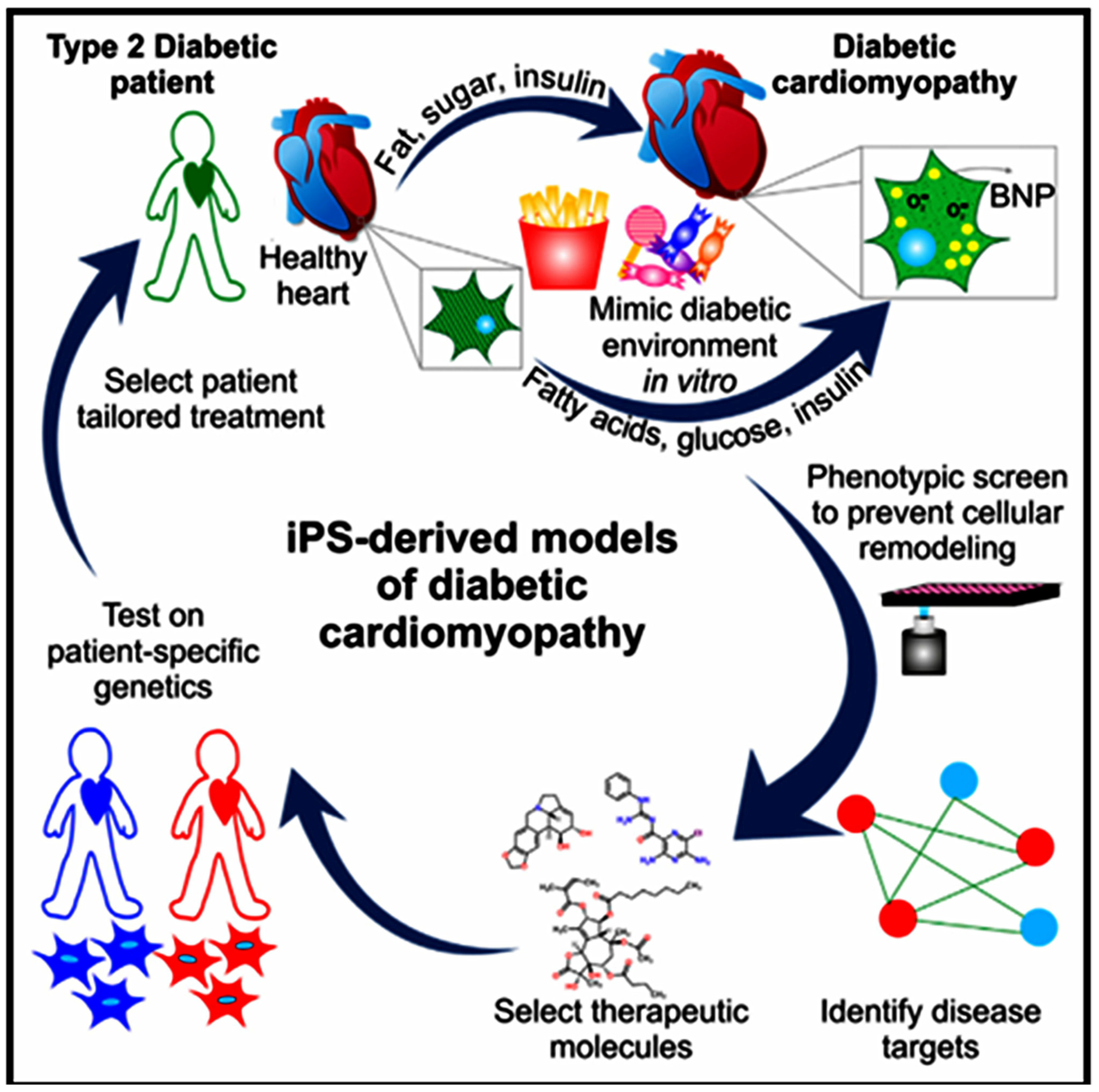
7.3. Disease Modeling and Drug Testing
7.4. Agricultural and Animal Production
7.5. Treatment of Virus-Related Pandemics
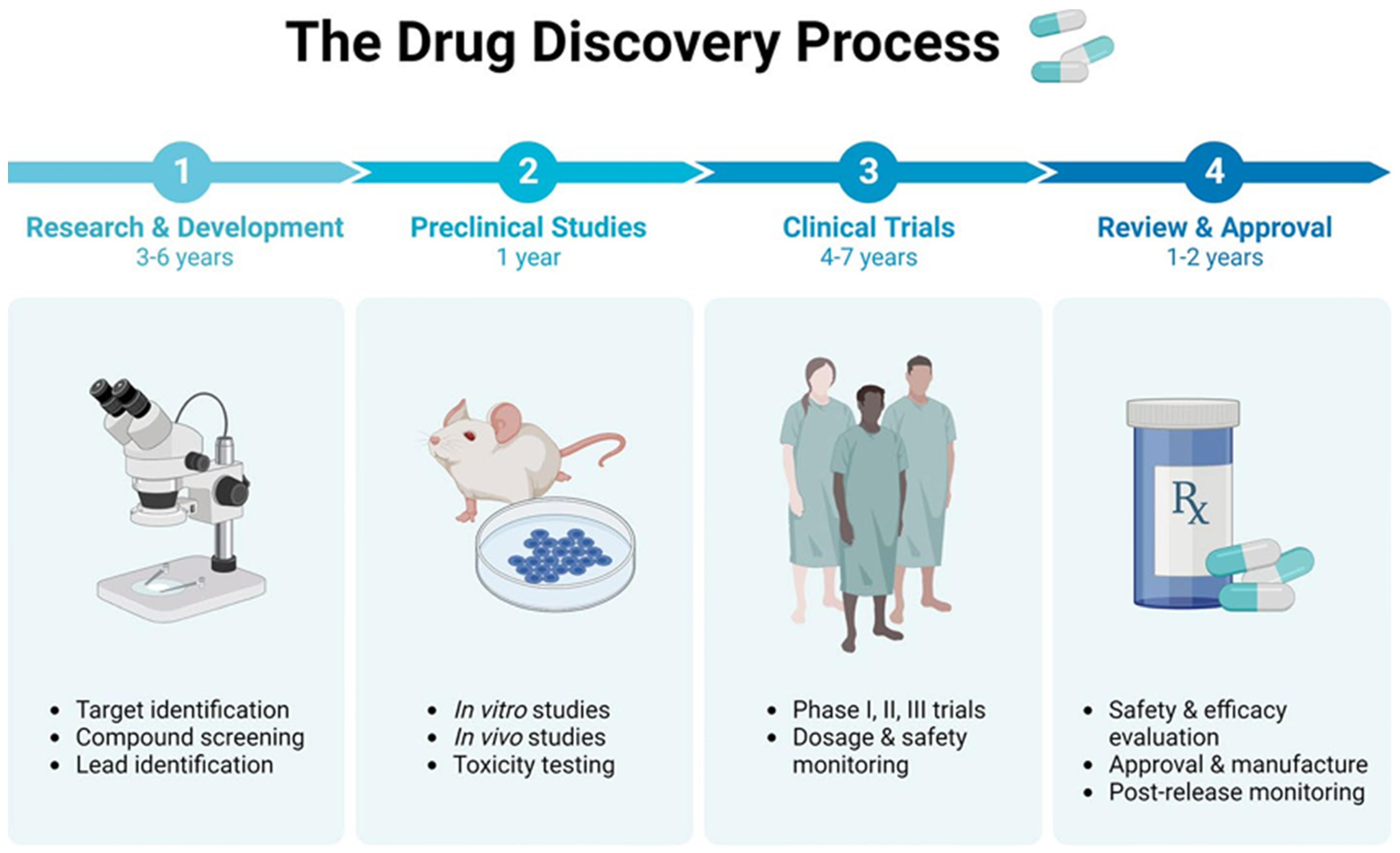
8. Challenges and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- De Souza, N. Naive human pluripotent stem cells. Nat. Methods 2013, 10, 1144. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar]
- Le Douarin, N.M. Embryonic neural chimaeras in the study of brain development. Trends Neurosci. 1993, 16, 64–72. [Google Scholar] [CrossRef]
- Alvarado-Mallart, R.-M. The chick/quail transplantation model to study central nervous system development. Prog. Brain Res. 2000, 127, 67–98. [Google Scholar]
- Zak, P.; Zykova, A.; Trofimova, N.; Khamidakh, A.A.; Fokin, A.; Eskina, E.; Ostrovsky, M. The experimental model for studying of human age retinal degeneration (Japanese quail C. Japonica). In Doklady Biological Sciences; Springer Nature BV: Berlin/Heidelberg, Germany, 2010; p. 297. [Google Scholar]
- Sheykholeslami, K.; Kaga, K.; Mizutani, M. Auditory nerve fiber differences in the normal and neurofilament deficient Japanese quail. Hear. Res. 2001, 159, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kinutani, M.; Coltey, M.; Le Douarin, N.M. Postnatal development of a demyelinating disease in avian spinal cord chimeras. Cell 1986, 45, 307–314. [Google Scholar] [CrossRef]
- Kulesa, P.M.; Bailey, C.M.; Cooper, C.; Fraser, S.E. In ovo live imaging of avian embryos. Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5446. [Google Scholar] [CrossRef]
- Le Douarin, N. Ontogeny of the peripheral nervous system from the neural crest and the placodes. A developmental model studied on the basis of the quail-chick chimaera system. Harvey Lect. 1984, 80, 137–186. [Google Scholar] [PubMed]
- Sheng, G. Defining epithelial-mesenchymal transitions in animal development. Development 2021, 148, dev198036. [Google Scholar] [CrossRef]
- Vilches-Moure, J.G. Embryonic chicken (Gallus gallus domesticus) as a model of cardiac biology and development. Comp. Med. 2019, 69, 184–203. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Scaal, M. Embryogenesis and development. In Reproductive Biology and Phylogeny of Birds, Part B: Sexual Selection, Behavior, Conservation, Embryology and Genetics; CRC Press: Boca Raton, FL, USA, 2007; pp. 411–488. [Google Scholar]
- Telugu, B.P.; Park, K.-E.; Park, C.-H. Genome editing and genetic engineering in livestock for advancing agricultural and biomedical applications. Mamm. Genome 2017, 28, 338–347. [Google Scholar] [CrossRef]
- Aguirre Maclennan, I.O. Generation of Induced Pluripotent Stem (iPS) Cells from the Endangered Tasmanian Devil. Ph.D. Thesis, La Trobe, Bundoora, VIC, Australia, 2018. [Google Scholar]
- Lau, E.; Paik, D.T.; Wu, J.C. Systems-wide approaches in induced pluripotent stem cell models. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 395–419. [Google Scholar] [CrossRef]
- Li, J.; Hua, Y.; Miyagawa, S.; Zhang, J.; Li, L.; Liu, L.; Sawa, Y. hiPSC-derived cardiac tissue for disease modeling and drug discovery. Int. J. Mol. Sci. 2020, 21, 8893. [Google Scholar] [CrossRef]
- Hockemeyer, D.; Jaenisch, R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell 2016, 18, 573–586. [Google Scholar] [CrossRef] [PubMed]
- MJ, B. Adult mice generated from induced pluripotent stem cells. Nature 2009, 461, 91–94. [Google Scholar]
- Promtan, P.; Pattanawong, W. The Develop Cultured Meat from Black-Boned Chicken Embryonic Stem Cell. Ph.D. Thesis, Maejo University, Chiang Mai, Thailand, 2023. [Google Scholar]
- Young, H.; Black, A. Pluripotent Stem Cells, Endogenous versus Reprogrammed, a Review. MOJ Orthop. Rheumatol. 2014, 1, 00019. [Google Scholar] [CrossRef]
- Nogueira, I.P.M.; Costa, G.M.J.; Lacerda, S.M.d.S.N. Avian iPSC Derivation to Recover Threatened Wild Species: A Comprehensive Review in Light of Well-Established Protocols. Animals 2024, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Pessôa, L.V.; Bressan, F.F.; Freude, K.K. Induced pluripotent stem cells throughout the animal kingdom: Availability and applications. World J. Stem Cells 2019, 11, 491. [Google Scholar] [CrossRef]
- Katayama, M.; Fukuda, T.; Kaneko, T. Induced pluripotent stem cells of endangered avian species. Commun. Biol. 2022, 5, 1049. [Google Scholar] [CrossRef] [PubMed]
- Hochedlinger, K.; Plath, K. Epigenetic reprogramming and induced pluripotency. Development 2009, 136, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Beevers, J.E.; Caffrey, T.M.; Wade-Martins, R. Induced pluripotent stem cell (iPSC)-derived dopaminergic models of Parkinson’s disease. Biochem. Soc. Trans. 2013, 41, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. Current reprogramming systems in regenerative medicine: From somatic cells to induced pluripotent stem cells. Regen. Med. 2016, 11, 105–132. [Google Scholar] [CrossRef]
- Spunde, K.; Korotkaja, K.; Zajakina, A. Recombinant viral vectors for therapeutic programming of tumour microenvironment: Advantages and limitations. Biomedicines 2022, 10, 2142. [Google Scholar] [CrossRef]
- Sandoval-Villegas, N.; Nurieva, W.; Amberger, M.; Ivics, Z. Contemporary transposon tools: A review and guide through mechanisms and applications of sleeping beauty, piggyBac and Tol2 for genome engineering. Int. J. Mol. Sci. 2021, 22, 5084. [Google Scholar] [CrossRef]
- Omole, A.E.; Fakoya, A.O.J. Ten years of progress and promise of induced pluripotent stem cells: Historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 2018, 6, e4370. [Google Scholar] [CrossRef]
- Yu, P. Chimeric Chickens Produced from Induced Pluripotent Stem Cells Utilizing A Non-Viral Approach. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2012. [Google Scholar]
- Barquinero, J.; Eixarch, H.; Perez-Melgosa, M. Retroviral vectors: New applications for an old tool. Gene Ther. 2004, 11, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Barry, M.A.; Ikeda, Y. Lentiviral vectors: Basic to translational. Biochem. J. 2012, 443, 603–618. [Google Scholar] [CrossRef]
- Borgohain, M.P.; Haridhasapavalan, K.K.; Dey, C.; Adhikari, P.; Thummer, R.P. An insight into DNA-free reprogramming approaches to generate integration-free induced pluripotent stem cells for prospective biomedical applications. Stem Cell Rev. Rep. 2019, 15, 286–313. [Google Scholar] [CrossRef]
- Hu, K. Vectorology and factor delivery in induced pluripotent stem cell reprogramming. Stem Cells Dev. 2014, 23, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Nehlsen, K.; Broll, S.; Bode, J. Replicating minicircles: Generation of nonviral episomes for the efficient modification of dividing cells. Gene Ther. Mol. Biol. 2006, 10, 233–244. [Google Scholar]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X. Induced pluripotent stem cell (iPSC)–derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Bayart, E.; Cohen-Haguenauer, O. Technological overview of iPS induction from human adult somatic cells. Curr. Gene Ther. 2013, 13, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Stadtfeld, M.; Hochedlinger, K. Induced pluripotency: History, mechanisms, and applications. Genes Dev. 2010, 24, 2239–2263. [Google Scholar] [CrossRef] [PubMed]
- Rapti, K.; Stillitano, F.; Karakikes, I.; Nonnenmacher, M.; Weber, T.; Hulot, J.-S.; Hajjar, R.J. Effectiveness of gene delivery systems for pluripotent and differentiated cells. Mol. Ther. Methods Clin. Dev. 2015, 2, 14067. [Google Scholar] [CrossRef] [PubMed]
- Fontes, A.; Lakshmipathy, U. Advances in genetic modification of pluripotent stem cells. Biotechnol. Adv. 2013, 31, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Tchieu, J.; Mason, M.J.; Yachechko, R.; Kuoy, E.; Horvath, S.; Zhou, Q.; Plath, K. Role of the murine reprogramming factors in the induction of pluripotency. Cell 2009, 136, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Gois Beghini, D.; Iwao Horita, S.; Cascabulho, C.M.; Anastácio Alves, L.; Henriques-Pons, A. Induced pluripotent stem cells: Hope in the treatment of diseases, including muscular dystrophies. Int. J. Mol. Sci. 2020, 21, 5467. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, T.M. Nonintegrating human somatic cell reprogramming methods. In Engineering and Application of Pluripotent Stem Cells; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–21. [Google Scholar]
- Sridhar, A.; Ohlemacher, S.K.; Langer, K.B.; Meyer, J.S. Robust differentiation of mRNA-reprogrammed human induced pluripotent stem cells toward a retinal lineage. Stem Cells Transl. Med. 2016, 5, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H. Disruption of Postnatal Neuronal Specific Gene Ak045681 Interferes with Anxiety Behavior in Mice. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 2011. [Google Scholar]
- Yusa, K. piggyBac transposony. In Mobile DNA III; WILEY: Hoboken, NJ, USA, 2015; pp. 873–890. [Google Scholar]
- Fuet, A.; Montillet, G.; Jean, C.; Aubel, P.; Kress, C.; Rival-Gervier, S.; Pain, B. NANOG is required for the long-term establishment of avian somatic reprogrammed cells. Stem Cell Rep. 2018, 11, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Maupetit-Mehouas, S.; Vaury, C. Transposon reactivation in the germline may be useful for both transposons and their host genomes. Cells 2020, 9, 1172. [Google Scholar] [CrossRef]
- Wagner, J.M.; Williams, E.V.; Alper, H.S. Developing a piggyBac transposon system and compatible selection markers for insertional mutagenesis and genome engineering in Yarrowia lipolytica. Biotechnol. J. 2018, 13, 1800022. [Google Scholar] [CrossRef]
- Woodard, L.E.; Wilson, M.H. piggyBac-ing models and new therapeutic strategies. Trends Biotechnol. 2015, 33, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Haridhasapavalan, K.K.; Borgohain, M.P.; Dey, C.; Saha, B.; Narayan, G.; Kumar, S.; Thummer, R.P. An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene 2019, 686, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Bashor, C.J.; Hilton, I.B.; Bandukwala, H.; Smith, D.M.; Veiseh, O. Engineering the next generation of cell-based therapeutics. Nat. Rev. Drug Discov. 2022, 21, 655–675. [Google Scholar] [CrossRef]
- Chen, M.; Qi, L.S. Repurposing CRISPR system for transcriptional activation. In RNA Activation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 147–157. [Google Scholar]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.; Kellner, M.J.; Regev, A. RNA targeting with CRISPR–Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Senís, E.; Mosteiro, L.; Wilkening, S.; Wiedtke, E.; Nowrouzi, A.; Afzal, S.; Fronza, R.; Landerer, H.; Abad, M.; Niopek, D. AAV vector-mediated in vivo reprogramming into pluripotency. Nat. Commun. 2018, 9, 2651. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mücke, M.; Seidman, C.E. Human Induced Pluripotent Stem Cell Production and Expansion from Blood using a Non-Integrating Viral Reprogramming Vector. Curr. Protoc. Mol. Biol. 2018, 122, e58. [Google Scholar] [CrossRef] [PubMed]
- Perez-Campo, F.M.; Costa, G.; Lie-a-Ling, M.; Stifani, S.; Kouskoff, V.; Lacaud, G. MOZ-mediated repression of p16INK4a is critical for the self-renewal of neural and hematopoietic stem cells. Stem Cells 2014, 32, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B 2009, 85, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stewart, R.; Slukvin, I.I.; Thomson, J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009, 324, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Woltjen, K.; Michael, I.P.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hämäläinen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009, 458, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, C.-H.; Moon, J.-I.; Chung, Y.-G.; Chang, M.-Y.; Han, B.-S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef]
- Chavez, A.; Scheiman, J.; Vora, S.; Pruitt, B.W.; Tuttle, M.; PR Iyer, E.; Lin, S.; Kiani, S.; Guzman, C.D.; Wiegand, D.J. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 2015, 12, 326–328. [Google Scholar] [CrossRef]
- Lu, Y.; West, F.D.; Jordan, B.J.; Mumaw, J.L.; Jordan, E.T.; Gallegos-Cardenas, A.; Beckstead, R.B.; Stice, S.L. Avian-induced pluripotent stem cells derived using human reprogramming factors. Stem Cells Dev. 2012, 21, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yuan, X.; Zou, Y.; Gao, J.; Xu, X.; Sun, H.; Zuo, Q.; Zhang, Y.; Li, B. OCT4, SOX2 and NANOG co-regulate glycolysis and participate in somatic induced reprogramming. Cytotechnology 2022, 74, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Park, J.H.; Jung, K.H.; Levine, H.; Kaipparettu, B.A. Elucidating the metabolic plasticity of cancer: Mitochondrial reprogramming and hybrid metabolic states. Cells 2018, 7, 21. [Google Scholar] [CrossRef]
- Bukowiecki, R.; Adjaye, J.; Prigione, A. Mitochondrial function in pluripotent stem cells and cellular reprogramming. Gerontology 2014, 60, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Duan, X.; Yang, L.; Nilsson-Payant, B.E.; Wang, P.; Duan, F.; Tang, X.; Yaron, T.M.; Zhang, T.; Uhl, S. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 2021, 589, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Raya, Á.; Kawakami, Y.; Morita, M.; Matsui, T.; Nakashima, K.; Gage, F.H.; Rodríguez-Esteban, C.; Izpisúa Belmonte, J.C. Nanog binds to Smad1 and blocks bone morphogenetic protein-induced differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10294–10299. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Caboni, L.; Das, D.; Yumoto, F.; Clayton, T.; Deller, M.C.; Nguyen, P.; Farr, C.L.; Chiu, H.-J.; Miller, M.D. Structure-based discovery of NANOG variant with enhanced properties to promote self-renewal and reprogramming of pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4666–4671. [Google Scholar] [CrossRef]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, R.; Ezaki, R.; Ichikawa, K.; Watanabe, T.; Terada, T.; Matsuzaki, M.; Horiuchi, H. Wnt signaling blockade is essential for maintaining the pluripotency of chicken embryonic stem cells. Poult. Sci. 2024, 103, 103361. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Miyazaki, J.-i.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Al Abbar, A.; Ngai, S.C.; Nograles, N.; Alhaji, S.Y.; Abdullah, S. Induced pluripotent stem cells: Reprogramming platforms and applications in cell replacement therapy. BioRes. Open Access 2020, 9, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Moon, R.T. Wnt/β-catenin pathway. Sci. STKE 2005, 2005, cm1. [Google Scholar] [CrossRef]
- Zare, M.; Mirhoseini, S.; Hassani, S.; Ghovvati, S. Signaling Roadmap Modulating Chicken Primordial Germ Cells Proliferation and Self-Renewal. Iran. J. Appl. Anim. Sci. 2023, 13, 1–10. [Google Scholar]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.-S.; Li, X. FGF signaling pathway: A key regulator of stem cell pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Ramana, C.V.; Grammatikakis, N.; Chernov, M.; Nguyen, H.; Goh, K.C.; Williams, B.R.; Stark, G.R. Regulation of c-myc expression by IFN-γ through Stat1-dependent and-independent pathways. EMBO J. 2000, 19, 263–272. [Google Scholar] [CrossRef]
- Jirmanova, L.; Afanassieff, M.; Gobert-Gosse, S.; Markossian, S.; Savatier, P. Differential contributions of ERK and PI3-kinase to the regulation of cyclin D1 expression and to the control of the G1/S transition in mouse embryonic stem cells. Oncogene 2002, 21, 5515–5528. [Google Scholar] [CrossRef]
- Gross, V.S.; Hess, M.; Cooper, G.M. Mouse embryonic stem cells and preimplantation embryos require signaling through the phosphatidylinositol 3-kinase pathway to suppress apoptosis. Mol. Reprod. Dev. Inc. Gamete Res. 2005, 70, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Valdimarsdottir, G.; Mummery, C. Functions of the TGFβ superfamily in human embryonic stem cells. Apmis 2005, 113, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Carnero, A.; Paramio, J.M. The PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Front. Oncol. 2014, 4, 252. [Google Scholar] [CrossRef]
- Yakhkeshi, S.; Rahimi, S.; Sharafi, M.; Hassani, S.N.; Taleahmad, S.; Shahverdi, A.; Baharvand, H. In vitro improvement of quail primordial germ cell expansion through activation of TGF-beta signaling pathway. J. Cell. Biochem. 2018, 119, 4309–4319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bjørbæk, C.; Moller, D.E. Regulation and interaction of pp90rsk isoforms with mitogen-activated protein kinases. J. Biol. Chem. 1996, 271, 29773–29779. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.C.; Patel, N.N.; Eccardt, A.M.; Fisher, J.S. Glucose-dependent trans-plasma membrane electron transport and p70S6k phosphorylation in skeletal muscle cells. Redox Biol. 2019, 27, 101075. [Google Scholar] [CrossRef]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef]
- Alateeq, S.; RJ Fortuna, P.; Wolvetang, E. Advances in reprogramming to pluripotency. Curr. Stem Cell Res. Ther. 2015, 10, 193–207. [Google Scholar] [CrossRef]
- Ishida, T.; Nakao, S.; Ueyama, T.; Harada, Y.; Kawamura, T. Metabolic remodeling during somatic cell reprogramming to induced pluripotent stem cells: Involvement of hypoxia-inducible factor 1. Inflamm. Regen. 2020, 40, 8. [Google Scholar] [CrossRef] [PubMed]
- Papp, B.; Plath, K. Reprogramming to pluripotency: Stepwise resetting of the epigenetic landscape. Cell Res. 2011, 21, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Till, J.E.; McCulloch, E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961, 14, 213–222. [Google Scholar] [CrossRef]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.-M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef]
- Steinestel, K.; Eder, S.; Schrader, A.J.; Steinestel, J. Clinical significance of epithelial-mesenchymal transition. Clin. Transl. Med. 2014, 3, 17. [Google Scholar] [CrossRef]
- Islam, M.A.; Rony, S.A.; Rahman, M.B.; Cinar, M.U.; Villena, J.; Uddin, M.J.; Kitazawa, H. Improvement of disease resistance in livestock: Application of immunogenomics and CRISPR/Cas9 technology. Animals 2020, 10, 2236. [Google Scholar] [CrossRef] [PubMed]
- Burnight, E.R.; Gupta, M.; Wiley, L.A.; Anfinson, K.R.; Tran, A.; Triboulet, R.; Hoffmann, J.M.; Klaahsen, D.L.; Andorf, J.L.; Jiao, C. Using CRISPR-Cas9 to generate gene-corrected autologous iPSCs for the treatment of inherited retinal degeneration. Mol. Ther. 2017, 25, 1999–2013. [Google Scholar] [CrossRef]
- Inui, M.; Miyado, M.; Igarashi, M.; Tamano, M.; Kubo, A.; Yamashita, S.; Asahara, H.; Fukami, M.; Takada, S. Rapid generation of mouse models with defined point mutations by the CRISPR/Cas9 system. Sci. Rep. 2014, 4, 5396. [Google Scholar] [CrossRef]
- Paolini Sguazzi, G.; Muto, V.; Tartaglia, M.; Bertini, E.; Compagnucci, C. Induced pluripotent stem cells (iPSCs) and gene therapy: A new era for the treatment of neurological diseases. Int. J. Mol. Sci. 2021, 22, 13674. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Parker, N.; Ylä-Herttuala, S. History of gene therapy. Gene 2013, 525, 162–169. [Google Scholar] [CrossRef]
- Pearson, S.; Jia, H.; Kandachi, K. China approves first gene therapy. Nat. Biotechnol. 2004, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S. Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef]
- Giotis, E.S.; Montillet, G.; Pain, B.; Skinner, M.A. Chicken embryonic-stem cells are permissive to poxvirus recombinant vaccine vectors. Genes 2019, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gong, W.; Yao, Z.; Jin, K.; Niu, Y.; Li, B.; Zuo, Q. Mechanisms of Embryonic Stem Cell Pluripotency Maintenance and Their Application in Livestock and Poultry Breeding. Animals 2024, 14, 1742. [Google Scholar] [CrossRef]
- Shittu, I.; Zhu, Z.; Lu, Y.; Hutcheson, J.M.; Stice, S.L.; West, F.D.; Donadeu, M.; Dungu, B.; Fadly, A.M.; Zavala, G. Development, characterization and optimization of a new suspension chicken-induced pluripotent cell line for the production of Newcastle disease vaccine. Biologicals 2016, 44, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Doran, T.J.; Challagulla, A.; Tizard, M.L.; Jenkins, K.A. Innovative approaches to genome editing in avian species. J. Anim. Sci. Biotechnol. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Takahashi, S.; Fukushima, K.; Saya, H.; Suzuki, N.; Aoki, M.; Okano, H.; Nakahara, J. Ropinirole hydrochloride remedy for amyotrophic lateral sclerosis–protocol for a randomized, double-blind, placebo-controlled, single-center, and open-label continuation phase I/IIa clinical trial (ROPALS trial). Regen. Ther. 2019, 11, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Horigome, K.; Nishio, M.; Komura, S.; Nagata, S.; Zhao, C.; Jin, Y.; Kawakami, K.; Yamada, Y.; Ohta, A. Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J. Clin. Investig. 2017, 127, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Durán-Alonso, M.B.; Petković, H. Induced pluripotent stem cells, a stepping stone to in vitro human models of hearing loss. Cells 2022, 11, 3331. [Google Scholar] [CrossRef] [PubMed]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Cantone, I.; Fisher, A.G. Epigenetic programming and reprogramming during development. Nat. Struct. Mol. Biol. 2013, 20, 282–289. [Google Scholar] [CrossRef]
- Saito-Diaz, K.; Zeltner, N. Induced pluripotent stem cells for disease modeling, cell therapy and drug discovery in genetic autonomic disorders: A review. Clin. Auton. Res. 2019, 29, 367–384. [Google Scholar] [CrossRef]
- Dunislawska, A.; Slawinska, A.; Siwek, M.; Bednarczyk, M. Epigenetic changes in poultry due to reprogramming of the gut microbiota. Anim. Front. 2021, 11, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.S.; Dhanjal, J.K.; Das, M.; Singh, B.; Naithani, R. Cell transdifferentiation and reprogramming in disease modeling: Insights into the neuronal and cardiac disease models and current translational strategies. Cells 2021, 10, 2558. [Google Scholar] [CrossRef]
- Takeda, Y.; Harada, Y.; Yoshikawa, T.; Dai, P. Chemical compound-based direct reprogramming for future clinical applications. Biosci. Rep. 2018, 38, BSR20171650. [Google Scholar] [CrossRef]
- Beghini, D.G.; Kasai-Brunswick, T.H.; Henriques-Pons, A. Induced Pluripotent Stem Cells in Drug Discovery and Neurodegenerative Disease Modelling. Int. J. Mol. Sci. 2024, 25, 2392. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, H.; Yao, X.; Yu, Y.; Tian, T.; Xu, W.; Zhou, Y.; Ouyang, H. Pharmaceutical therapeutics for articular regeneration and restoration: State-of-the-art technology for screening small molecular drugs. Cell. Mol. Life Sci. 2021, 78, 8127–8155. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J. Genomic selection for poultry breeding. Anim. Front. 2012, 2, 30–36. [Google Scholar] [CrossRef]
- Scarfone, R.A.; Pena, S.M.; Russell, K.A.; Betts, D.H.; Koch, T.G. The use of induced pluripotent stem cells in domestic animals: A narrative review. BMC Vet. Res. 2020, 16, 477. [Google Scholar] [CrossRef] [PubMed]
- Kafarnik, C. Deriving an In Vitro Source of Canine Corneal Stromal Cells for Future Studies of Corneal Disease and Therapeutic Applications. Ph.D. Thesis, UCL (University College London), London, UK, 2022. [Google Scholar]
- Ichida, J.K.; Blanchard, J.; Lam, K.; Son, E.Y.; Chung, J.E.; Egli, D.; Loh, K.M.; Carter, A.C.; Di Giorgio, F.P.; Koszka, K. A small-molecule inhibitor of Tgf-β signaling replaces Sox2 in reprogramming by inducing Nanog. Cell Stem Cell 2009, 5, 491–503. [Google Scholar] [CrossRef]
- Moradi, S.; Sharifi-Zarchi, A.; Ahmadi, A.; Mollamohammadi, S.; Stubenvoll, A.; Günther, S.; Salekdeh, G.H.; Asgari, S.; Braun, T.; Baharvand, H. Small RNA sequencing reveals Dlk1-Dio3 locus-embedded microRNAs as major drivers of ground-state pluripotency. Stem Cell Rep. 2017, 9, 2081–2096. [Google Scholar] [CrossRef]
- Zhu, S.; Li, W.; Zhou, H.; Wei, W.; Ambasudhan, R.; Lin, T.; Kim, J.; Zhang, K.; Ding, S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 2010, 7, 651–655. [Google Scholar] [CrossRef]
- Ma, X.; Kong, L.; Zhu, S. Reprogramming cell fates by small molecules. Protein Cell 2017, 8, 328–348. [Google Scholar] [CrossRef]
- Hu, K. All roads lead to induced pluripotent stem cells: The technologies of iPSC generation. Stem Cells Dev. 2014, 23, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Si-Tayeb, K.; Noto, F.K.; Sepac, A.; Sedlic, F.; Bosnjak, Z.J.; Lough, J.W.; Duncan, S.A. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev. Biol. 2010, 10, 81. [Google Scholar] [CrossRef]
- Sugimoto, N.; Eto, K. Generation and manipulation of human iPSC-derived platelets. Cell. Mol. Life Sci. 2021, 78, 3385–3401. [Google Scholar] [CrossRef] [PubMed]
- Ivics, Z.; Garrels, W.; Mátés, L.; Yau, T.Y.; Bashir, S.; Zidek, V.; Landa, V.; Geurts, A.; Pravenec, M.; Rülicke, T. Germline transgenesis in pigs by cytoplasmic microinjection of Sleeping Beauty transposons. Nat. Protoc. 2014, 9, 810–827. [Google Scholar] [CrossRef]
- Drawnel, F.M.; Boccardo, S.; Prummer, M.; Delobel, F.; Graff, A.; Weber, M.; Gerard, R.; Badi, L.; Kam-Thong, T.; Bu, L. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014, 9, 810–820. [Google Scholar] [CrossRef]
- Bennett, P.M. Genome plasticity: Insertion sequence elements, transposons and integrons, and DNA rearrangement. In Genomics, Proteomics, and Clinical Bacteriology: Methods and Reviews; Springer: Berlin/Heidelberg, 2004; pp. 71–113. [Google Scholar]
- Moghaddam, B. Assessment of Cell Penetrating Peptides as a Vehicle for Delivering Transcription Factors for Stem Cell Reprogramming and Controlling Fate Decisions. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2011. [Google Scholar]
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Nanocarrier fabrication and macromolecule drug delivery: Challenges and opportunities. Ther. Deliv. 2016, 7, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.A.; Lim, W.A.; Qi, L.S. Beyond editing: Repurposing CRISPR–Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 2016, 17, 5–15. [Google Scholar] [CrossRef]
- Wilbie, D.; Walther, J.; Mastrobattista, E. Delivery aspects of CRISPR/Cas for in vivo genome editing. Acc. Chem. Res. 2019, 52, 1555–1564. [Google Scholar] [CrossRef]
- Ebert, A.D.; Liang, P.; Wu, J.C. Induced pluripotent stem cells as a disease modeling and drug screening platform. J. Cardiovasc. Pharmacol. 2012, 60, 408–416. [Google Scholar] [CrossRef]
- Marei, H.E.; Khan, M.U.A.; Hasan, A. Potential use of iPSCs for disease modeling, drug screening, and cell-based therapy for Alzheimer’s disease. Cell. Mol. Biol. Lett. 2023, 28, 98. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Gros, E.; Li, H.-R.; Kumar, S.; Deacon, D.C.; Maron, C.; Muotri, A.R.; Chi, N.C.; Fu, X.-D.; Benjamin, D.Y. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell 2013, 13, 246–254. [Google Scholar] [CrossRef]
- Stanton, M.M.; Tzatzalos, E.; Donne, M.; Kolundzic, N.; Helgason, I.; Ilic, D. Prospects for the use of induced pluripotent stem cells in animal conservation and environmental protection. Stem Cells Transl. Med. 2019, 8, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Lee, Y.; Salamone, D.F. iPSC technology: An innovative tool for developing clean meat, livestock, and frozen Ark. Animals 2022, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.; Li, Z.; Fung, H.-L.; Young, J.E.; Agarwal, S.; Antosiewicz-Bourget, J.; Canto, I.; Giorgetti, A.; Israel, M.A.; Kiskinis, E. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011, 471, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, J.D.; Hall, C.; Chen, V.C.-y.; Li, A.X.; Wu, X.; Hsu, D.; Couture, L.A.; Riggs, A.D. Epigenetic stability, adaptability, and reversibility in human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 12544–12549. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.; Sinigaglia, A.; Desole, G.; Berto, A.; Pacenti, M.; Palù, G.; Barzon, L. Modeling viral infectious diseases and development of antiviral therapies using human induced pluripotent stem cell-derived systems. Viruses 2015, 7, 3835–3856. [Google Scholar] [CrossRef] [PubMed]
- Esmail, S.; Danter, W. Viral pandemic preparedness: A pluripotent stem cell-based machine-learning platform for simulating SARS-CoV-2 infection to enable drug discovery and repurposing. Stem Cells Transl. Med. 2021, 10, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Yoon, K.-J. Modeling host-virus interactions in viral infectious diseases using stem-cell-derived systems and CRISPR/Cas9 technology. Viruses 2019, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, R.R.; Li, Y.-S.; Hsu, C.-W.; Jenny, L.A.; Kong, Y.; Ruan, M.Z.; Sparrow, J.R.; Tsang, S.H. Evaluating precision medicine approaches for gene therapy in patient-specific cellular models of Bietti crystalline dystrophy. JCI Insight 2024, 9, e177231. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Allen, A.; Luo, C.; Finn, J.D. Unlocking the potential of iPSC-derived immune cells: Engineering iNK and iT cells for cutting-edge immunotherapy. Front. Immunol. 2024, 15, 1457629. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Lee, S.B.; Kim, K.; Lim, K.M.; Jeon, T.-i.; Seok, J.; Cho, S.-G. Production of mesenchymal stem cells through stem cell reprogramming. Int. J. Mol. Sci. 2019, 20, 1922. [Google Scholar] [CrossRef]
- Giallongo, S.; Rehakova, D.; Raffaele, M.; Lo Re, O.; Koutna, I.; Vinciguerra, M. Redox and epigenetics in human pluripotent stem cells differentiation. Antioxid. Redox Signal. 2021, 34, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, O. Patenting Bioprinting: An Ethical Dilemma in the Provision of Accessible Health Technologies. Ph.D. Thesis, University of Tasmania, Hobart, TAS, Australia, 2021. [Google Scholar]


| Vectors | Genetic Considerations | Epigenetic Considerations |
|---|---|---|
| Viral Vectors | Integration into host genome; risk of insertional mutagenesis | May cause aberrant epigenetic changes due to random integration |
| Sendai Virus | Does not integrate into host genome; transient expression | Minimal epigenetic impact; requires repeated transduction for efficiency |
| Episomal Plasmids | No integration; extrachromosomal; may be lost over time | Reduced risk of epigenetic alterations; may require repeated transfection |
| PiggyBac Transposon System | Integration into specific sites; excision complexity | Potential for precise epigenetic reprogramming; requires careful monitoring for complete removal |
| CRISPR-Based Activation | Precision targeting; potential off-target effects | Can induce specific epigenetic changes; delivery optimization required |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahoor, N.; Arif, A.; Shuaib, M.; Jin, K.; Li, B.; Li, Z.; Pei, X.; Zhu, X.; Zuo, Q.; Niu, Y.; et al. Induced Pluripotent Stem Cells in Birds: Opportunities and Challenges for Science and Agriculture. Vet. Sci. 2024, 11, 666. https://doi.org/10.3390/vetsci11120666
Zahoor N, Arif A, Shuaib M, Jin K, Li B, Li Z, Pei X, Zhu X, Zuo Q, Niu Y, et al. Induced Pluripotent Stem Cells in Birds: Opportunities and Challenges for Science and Agriculture. Veterinary Sciences. 2024; 11(12):666. https://doi.org/10.3390/vetsci11120666
Chicago/Turabian StyleZahoor, Nousheen, Areej Arif, Muhammad Shuaib, Kai Jin, Bichun Li, Zeyu Li, Xiaomeng Pei, Xilin Zhu, Qisheng Zuo, Yingjie Niu, and et al. 2024. "Induced Pluripotent Stem Cells in Birds: Opportunities and Challenges for Science and Agriculture" Veterinary Sciences 11, no. 12: 666. https://doi.org/10.3390/vetsci11120666
APA StyleZahoor, N., Arif, A., Shuaib, M., Jin, K., Li, B., Li, Z., Pei, X., Zhu, X., Zuo, Q., Niu, Y., Song, J., & Chen, G. (2024). Induced Pluripotent Stem Cells in Birds: Opportunities and Challenges for Science and Agriculture. Veterinary Sciences, 11(12), 666. https://doi.org/10.3390/vetsci11120666