Evaluation of Serum Lipids, Biochemical Parameters, Selected Antioxidant Elements and Oxidative Stress Profiles in Late Pregnant Jennies with Hyperlipemia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Welfare Statement
2.2. Animals
2.3. Blood Sampling
2.4. Serum Parameter Analysis
2.5. Statistical Analysis
3. Results
3.1. Analysis of Serum Lipids
3.2. Analysis of Serum Biochemical Parameters Related to Liver Function
3.3. Analysis of Serum Selenium, Zinc and Vitamin E
3.4. Analysis of Serum Oxidative Stress Parameters
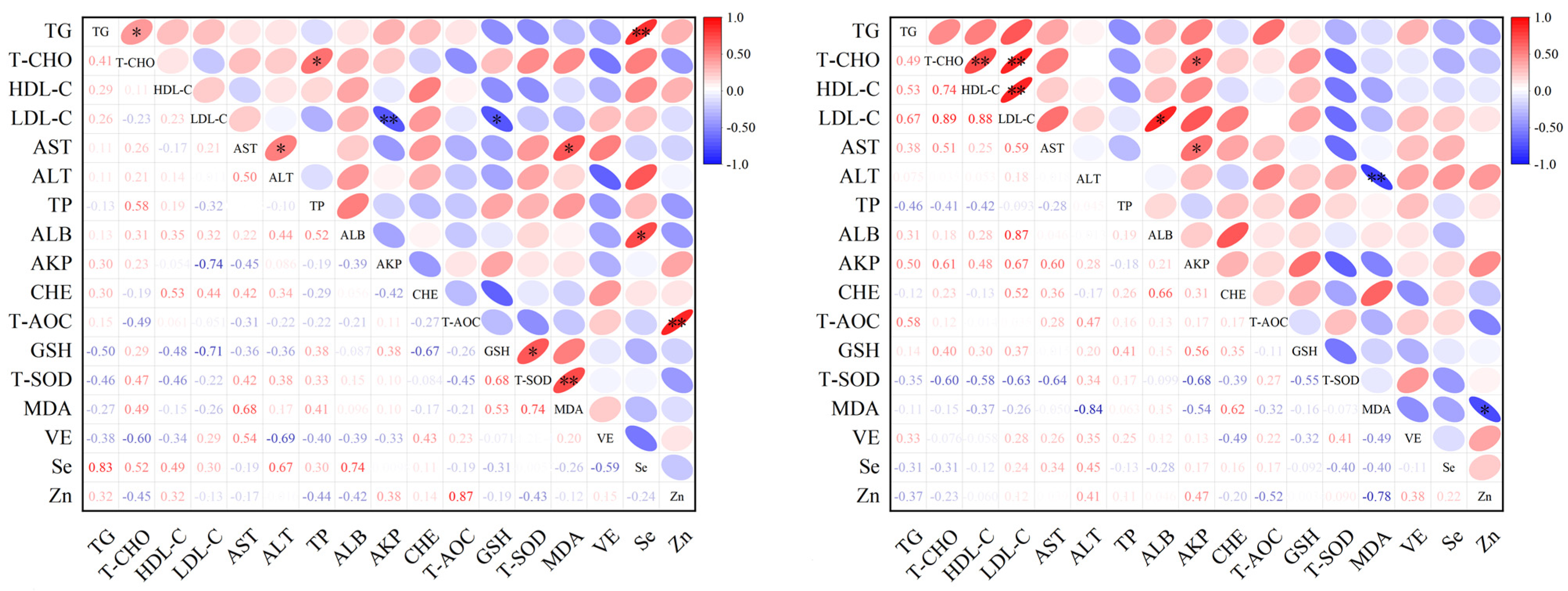
3.5. Analysis of Correlation Among Serum Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.; Baber, M.; Hussain, T.; Awan, F.; Nadeem, A. The contribution of donkeys to human health. Equine Vet. J. 2014, 46, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Fielding, D. Reproductive characteristics of the jenny donkey—Equus asinus: A review. Trop. Anim. Health Prod. 1988, 20, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Frape, D. Equine Nutrition and Feeding, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Martin-Rosset, W. Donkey nutrition and feeding: Nutrient requirements and recommended allowances—A review and prospect. J. Equine Vet. Sci. 2018, 65, 75–85. [Google Scholar] [CrossRef]
- Watson, T. Equine hyperlipemia. In Metabolic and Endocrine Problems of the Horse; WB Saunders: London, UK; Britain, UK, 1998; pp. 23–40. [Google Scholar]
- Mendoza, F.J.; Toribio, R.E.; Perez-Ecija, A. Metabolic and endocrine disorders in donkeys. Vet. Clin. N. Am. Equine Pract. 2019, 35, 399–417. [Google Scholar] [CrossRef] [PubMed]
- De Lima, B.; Da Silva, G.B.; Da Silva, C.J.F.L.; Ferreira, L.M.C.; Da Costa, C.M.H.E.C.; Filho, H.C.M. Blood, metabolic and endocrine biomarkers in donkeys (Equus africanus asinus) supplemented with different energy sources. Acta Vet. Bras. 2016, 10, 135–143. [Google Scholar]
- Agina, O.A. Haematology and clinical biochemistry findings associated with equine diseases—A review. Not. Sci. Biol. 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Harrison, A.; Rickards, K. Hyperlipaemia in donkeys. UK-Vet Equine 2018, 2, 154–157. [Google Scholar] [CrossRef]
- Burden, F.A.; Du Toit, N.; Hazell-Smith, E.; Trawford, A.F. Hyperlipemia in a population of aged donkeys: Description, prevalence, and potential risk factors. J. Vet. Intern. Med. 2011, 25, 1420–1425. [Google Scholar] [CrossRef]
- D’Archivio, M.; Annuzzi, G.; Varì, R.; Filesi, C.; Giacco, R.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Rivellese, A.A.; Masella, R. Predominant role of obesity/insulin resistance in oxidative stress development. Eur. J. Clin. Investig. 2012, 42, 70–78. [Google Scholar] [CrossRef]
- Spickett, C.M.; Wiswedel, I.; Siems, W.; Zarkovic, K.; Zarkovic, N. Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic. Res. 2010, 44, 1172–1202. [Google Scholar] [CrossRef]
- Yang, R.L.; Shi, Y.H.; Hao, G.; Li, W.; Le, G.W. Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Zago, M.P.; Oteiza, P.I. The antioxidant properties of zinc: Interactions with iron and antioxidants. Free Radic. Biol. Med. 2001, 31, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Noguchi, N. Dynamics of antioxidant action of vitamin E. Acc. Chem. Res. 2004, 37, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Song, Y.X.; Lin, Z.J.; Ma, M.L.; He, L.P. Essential trace elements in patients with dyslipidemia: A meta-analysis. Curr. Med. Chem. 2024, 31, 3604–3623. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Vitamin E as a potential interventional treatment for metabolic syndrome: Evidence from animal and human studies. Front. Pharmacol. 2017, 8, 444. [Google Scholar] [CrossRef]
- The Donkey Sanctuary. Hyperlipaemia and the endocrine system. In The Clinical Companion of the Donkey; Evans, L., Crane, M., Eds.; Matador Publishing Inc.: Leicestershire, UK, 2018; p. 92. [Google Scholar]
- The Donkey Sanctuary. The Donkey Sanctuary. The clinical examination. In The Clinical Companion of the Donkey; Evans, L., Crane, M., Eds.; Matador Publishing Inc.: Leicestershire, UK, 2018; p. 256. [Google Scholar]
- Liao, Q.; Li, Z.; Han, Y.; Deng, L. Comparative analysis of serum mineral and biochemical parameter profiles between late pregnant and early lactating jennies. J. Equine Vet. Sci. 2021, 99, 103401. [Google Scholar] [CrossRef]
- National Research Council. Donkeys and other equids. In Nutrient Requirements of Horses, 6th ed.; The National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Bonelli, F.; Rota, A.; Corazza, M.; Serio, D.; Sgorbini, M. Hematological and biochemical findings in pregnant, postfoaling, and lactating jennies. Theriogenology 2016, 85, 1233–1238. [Google Scholar] [CrossRef]
- Durham, A.E.; Thiemann, A.K. Nutritional management of hyperlipaemia. Equine Vet. Educ. 2015, 27, 482–488. [Google Scholar] [CrossRef]
- Watson, T.D.; Burns, L.; Packard, C.J.; Shepherd, J. Effects of pregnancy and lactation on plasma lipid and lipoprotein concentrations, lipoprotein composition and post-heparin lipase activities in Shetland pony mares. J. Reprod. Fertil. 1993, 97, 563–568. [Google Scholar] [CrossRef]
- Burden, F.A.; Hazell-Smith, E.; Mulugeta, G.; Patrick, V.; Trawford, R.; Brownlie, H.W.B. Reference intervals for biochemical and haematological parameters in mature domestic donkeys (Equus asinus) in the UK. Equine Vet. Educ. 2016, 28, 134–139. [Google Scholar] [CrossRef]
- Watson, T.D.; Murphy, D.; Love, S. Equine hyperlipaemia in the United Kingdom: Clinical features and blood biochemistry of 18 cases. Vet. Rec. 1992, 131, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Shiba, T.; Yoshida, M.; Ide, T.; Seri, N.; Kudou, W.; Kinugawa, S.; Tsutsui, H. Fatty acids increase the circulating levels of oxidative stress factors in mice with diet-induced obesity via redox changes of albumin. FEBS J. 2007, 274, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Ghosian Moghaddam, M.H.; Roghani, M.; Maleki, M. Effect of hypericum perforatum aqueous extracts on serum lipids, aminotransferases, and lipid peroxidation in hyperlipidemic rats. Res. Cardiovasc. Med. 2016, 5, e31326. [Google Scholar]
- Ju, W.; Ji, M.; Li, X.; Li, Z.; Wu, G.; Fu, X.; Yang, X.; Gao, X. Relationship between higher serum selenium level and adverse blood lipid profile. Clin. Nutr. 2018, 37, 1512–1517. [Google Scholar] [CrossRef]
- Reddy, K.P.; Sailaja, G.; Krishnaiah, C. Protective effects of selenium on fluoride induced alterations in certain enzymes in brain of mice. J. Environ. Biol. 2009, 30 (Suppl. 5), 859–864. [Google Scholar]
- Bleys, J.; Navas-Acien, A.; Stranges, S.; Menke, A.; Miller, E.R., 3rd; Guallar, E. Serum selenium and serum lipids in US adults. Am. J. Clin. Nutr. 2008, 88, 416–423. [Google Scholar] [CrossRef]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef]
- Stapleton, S.R. Selenium: An insulin mimetic. Cell Mol. Life Sci. 2000, 57, 1874–1879. [Google Scholar] [CrossRef]
- Erbayraktar, Z.; Yılmaz, O.; Artmann, A.T.; Cehreli, R.; Coker, C. Effects of selenium supplementation on antioxidant defense and glucose homeostasis in experimental diabetes mellitus. Biol. Trace Elem. Res. 2007, 118, 217–226. [Google Scholar] [CrossRef]
- Thoen, R.U.; Barther, N.N.; Schemitt, E.; Bona, S.; Fernandes, S.; Coral, G.; Marroni, N.P.; Tovo, C.; Guedes, R.P.; Porawski, M. Zinc supplementation reduces diet-induced obesity and improves insulin sensitivity in rats. Appl. Physiol. Nutr. Metab. 2019, 44, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Rios-Lugo, M.J.; Madrigal-Arellano, C.; Gaytán-Hernández, D.; Hernández-Mendoza, H.; Romero-Guzmán, E.T. Association of serum zinc levels in overweight and obesity. Biol. Trace Elem. Res. 2020, 198, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F. Use of antioxidants in the prevention and treatment of disease. J. Int. Fed. Clin. Chem. 1998, 10, 21–23. [Google Scholar] [PubMed]
- Ranasinghe, P.; Wathurapatha, W.S.; Ishara, M.H.; Jayawardana, R.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Effects of Zinc supplementation on serum lipids: A systematic review and meta-analysis. Nutr. Metab. 2015, 12, 26. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhang, J.; Wang, X.; Liu, M.; Zhang, C.; Jia, L. Hepatoprotective and in vitro antioxidant effects of native depolymerised-exopolysaccharides derived from Termitomyces albuminosus. Sci. Rep. 2017, 7, 3910. [Google Scholar] [CrossRef]
- Wolin, M.S. Interactions of oxidants with vascular signaling systems. Arterioscl. Throm. Vas. 2000, 20, 1430–1442. [Google Scholar] [CrossRef]
- Godin, D.V.; Dahlman, D.M. Effects of hypercholesterolemia on tissue antioxidant status in two species differing in susceptibility to atherosclerosis. Res. Commun. Chem. Pathol. Pharmacol. 1993, 79, 151–166. [Google Scholar]
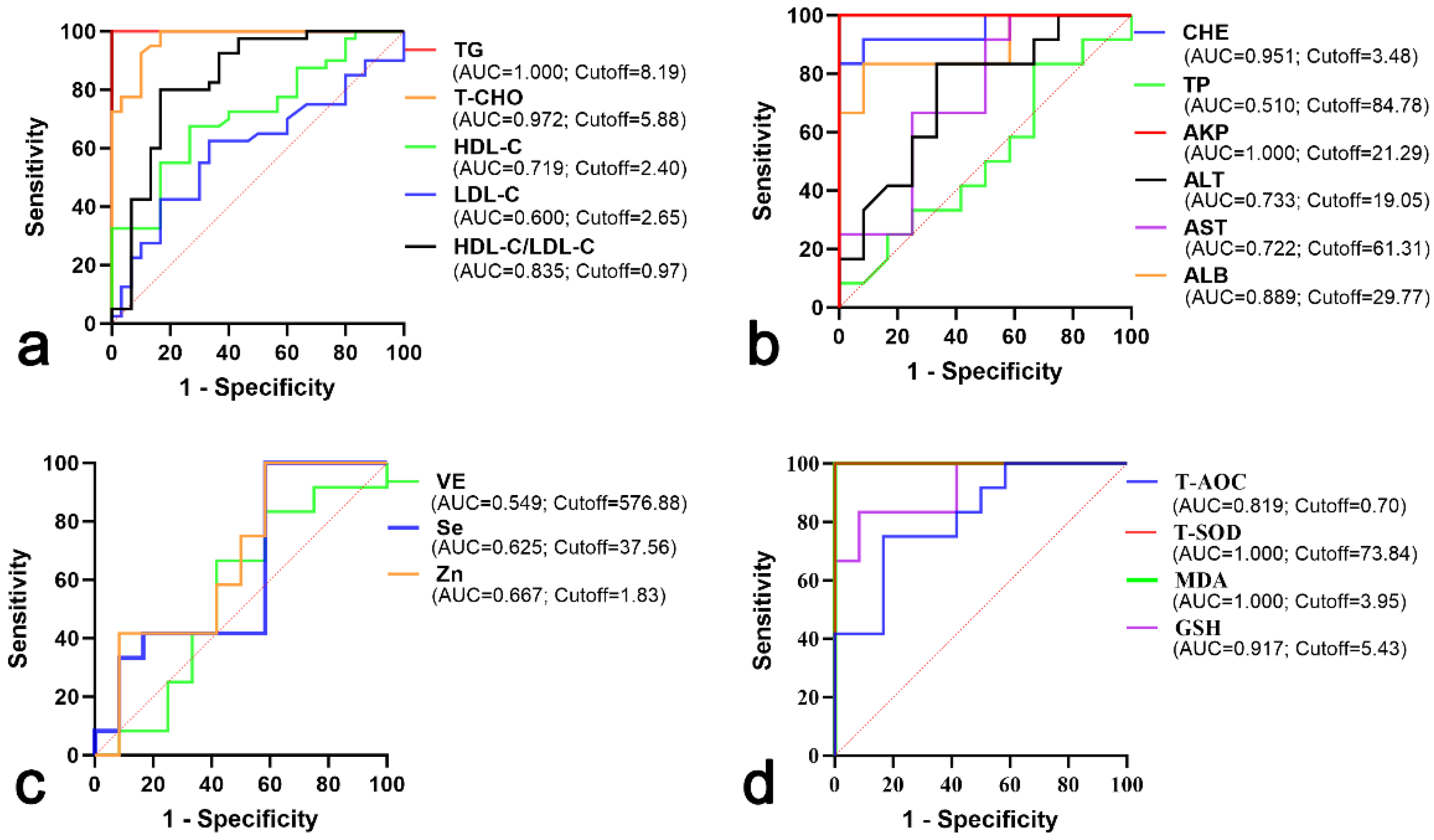
| Parameters | Hyperlipemia | Healthy | p Value |
|---|---|---|---|
| Age | 6.1± 0.2 | 5.8 ± 0.2 | 0.403 |
| Parity | 1.9 ± 0.1 | 1.8 ± 0.2 | 0.687 |
| Body weight (kg) | 209.4 ± 4.1 b | 247.5 ± 3.8 a | 0.000 |
| BCS | 1.9 ± 0.1 b | 3.1 ± 0.1 a | 0.000 |
| Gestation length (days) | 367.2 ± 1.6 | 362.7 ± 1.5 | 0.392 |
| Parameters | Hyperlipemia | Healthy | p Value | |
|---|---|---|---|---|
| TG (mmol/L) | Mean ± SE | 21.35 ± 1.44 a | 1.84 ± 0.14 b | 0.000 |
| 95% CI | 18.31~24.39 | 1.55~2.12 | ||
| T-CHO (mmol/L) | Mean ± SE | 10.75 ± 1.14 a | 3.50 ± 0.15 b | 0.000 |
| 95% CI | 8.36~13.15 | 3.20~3.79 | ||
| HDL-C (mmol/L) | Mean ± SE | 1.85 ± 0.22 b | 2.86 ± 0.16 a | 0.002 |
| 95% CI | 1.37~2.32 | 2.53~3.18 | ||
| LDL-C (mmol/L) | Mean ± SE | 3.46 ± 0.37 a | 1.96 ± 0.15 b | 0.001 |
| 95% CI | 2.69~4.23 | 1.66~2.26 |
| Parameters | Hyperlipemia | Healthy | p Value | |
|---|---|---|---|---|
| TP (g/L) | Mean ± SE | 77.80 ± 2.79 | 75.55 ± 1.61 | 0.490 |
| 95% CI | 71.97~83.64 | 72.16~78.94 | ||
| ALB (g/L) | Mean ± SE | 25.84 ± 0.96 b | 31.98 ± 1.22 a | 0.001 |
| 95% CI | 23.72~27.96 | 29.29~34.68 | ||
| AST (U/L) | Mean ± SE | 68.51 ± 20.41 a | 37.33 ± 3.87 b | 0.019 |
| 95% CI | 21.45~115.56 | 29.44~45.21 | ||
| ALT (U/L) | Mean ± SE | 22.41 ± 3.24 a | 13.08 ± 1.21 b | 0.013 |
| 95% CI | 15.61~29.21 | 10.65~15.51 | ||
| AKP (King unit/100 mL) | Mean ± SE | 66.70 ± 6.45 a | 15.08 ± 0.73 b | 0.000 |
| 95% CI | 53.20~80.20 | 13.55~16.61 | ||
| CHE (U/mL) | Mean ± SE | 4.15 ± 0.19 a | 2.11 ± 0.19 b | 0.000 |
| 95% CI | 3.75~4.55 | 1.71~2.52 |
| Parameters | Hyperlipemia | Healthy | p Value | |
|---|---|---|---|---|
| Se (μg/L) | Mean ± SE | 42.19 ± 4.65 b | 55.51 ± 3.12 a | 0.031 |
| 95% CI | 31.67~52.71 | 48.32~62.71 | ||
| Zn (mg/L) | Mean ± SE | 1.60 ± 0.13 | 1.45 ± 0.09 | 0.345 |
| 95% CI | 1.29~1.90 | 1.24~1.65 | ||
| VE (nmol/L) | Mean ± SE | 560.80 ± 43.07 | 552.58 ± 43.33 | 0.894 |
| 95% CI | 463.36~658.24 | 454.55~650.61 |
| Parameters | Hyperlipemia | Healthy | p Value | |
|---|---|---|---|---|
| T-AOC (mmol/L) | Mean ± SE | 0.67 ± 0.01 b | 0.71 ± 0.01 a | 0.004 |
| 95% CI | 0.65~0.68 | 0.69~0.72 | ||
| GSH (μmol/L) | Mean ± SE | 2.87 ± 0.35 b | 9.92 ± 1.06 a | 0.000 |
| 95% CI | 2.15~3.59 | 7.70~12.13 | ||
| T-SOD (U/mL) | Mean ± SE | 70.80 ± 0.15 b | 76.85 ± 0.18 a | 0.000 |
| 95% CI | 70.49~71.12 | 76.47~77.23 | ||
| MDA (nmol/mL) | Mean ± SE | 6.34 ± 0.47 a | 2.78 ± 0.09 b | 0.000 |
| 95% CI | 5.36~7.32 | 2.59~2.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Shao, Y.; Li, W.; Lu, J.; Wang, X.; Deng, L. Evaluation of Serum Lipids, Biochemical Parameters, Selected Antioxidant Elements and Oxidative Stress Profiles in Late Pregnant Jennies with Hyperlipemia. Vet. Sci. 2024, 11, 664. https://doi.org/10.3390/vetsci11120664
Meng Q, Shao Y, Li W, Lu J, Wang X, Deng L. Evaluation of Serum Lipids, Biochemical Parameters, Selected Antioxidant Elements and Oxidative Stress Profiles in Late Pregnant Jennies with Hyperlipemia. Veterinary Sciences. 2024; 11(12):664. https://doi.org/10.3390/vetsci11120664
Chicago/Turabian StyleMeng, Qingze, Yang Shao, Wei Li, Jia Lu, Xinyue Wang, and Liang Deng. 2024. "Evaluation of Serum Lipids, Biochemical Parameters, Selected Antioxidant Elements and Oxidative Stress Profiles in Late Pregnant Jennies with Hyperlipemia" Veterinary Sciences 11, no. 12: 664. https://doi.org/10.3390/vetsci11120664
APA StyleMeng, Q., Shao, Y., Li, W., Lu, J., Wang, X., & Deng, L. (2024). Evaluation of Serum Lipids, Biochemical Parameters, Selected Antioxidant Elements and Oxidative Stress Profiles in Late Pregnant Jennies with Hyperlipemia. Veterinary Sciences, 11(12), 664. https://doi.org/10.3390/vetsci11120664







