Porcine Epidemic Diarrhea Virus Infection of Porcine Intestinal Epithelial Cells Causes Mitochondrial DNA Release and the Activation of the NLRP3 Inflammasome to Mediate Interleukin-1β Secretion
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Virus Strains
2.2. Quantitative PCR
2.3. siRNA
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Caspase-1 Activity Detection
2.6. Western Blot Analysis
2.7. Immunoprecipitation Assay
2.8. Inhibitor Treatment of IPEC-J2 Cells
2.9. Detection of Mitochondrial Membrane Potential, ATP, and Mitochondrial Reactive Oxygen Species (mtROS)
2.10. mtDNA Localization
2.11. DNase I Protein Transfection
2.12. Statistical Analyses
3. Results
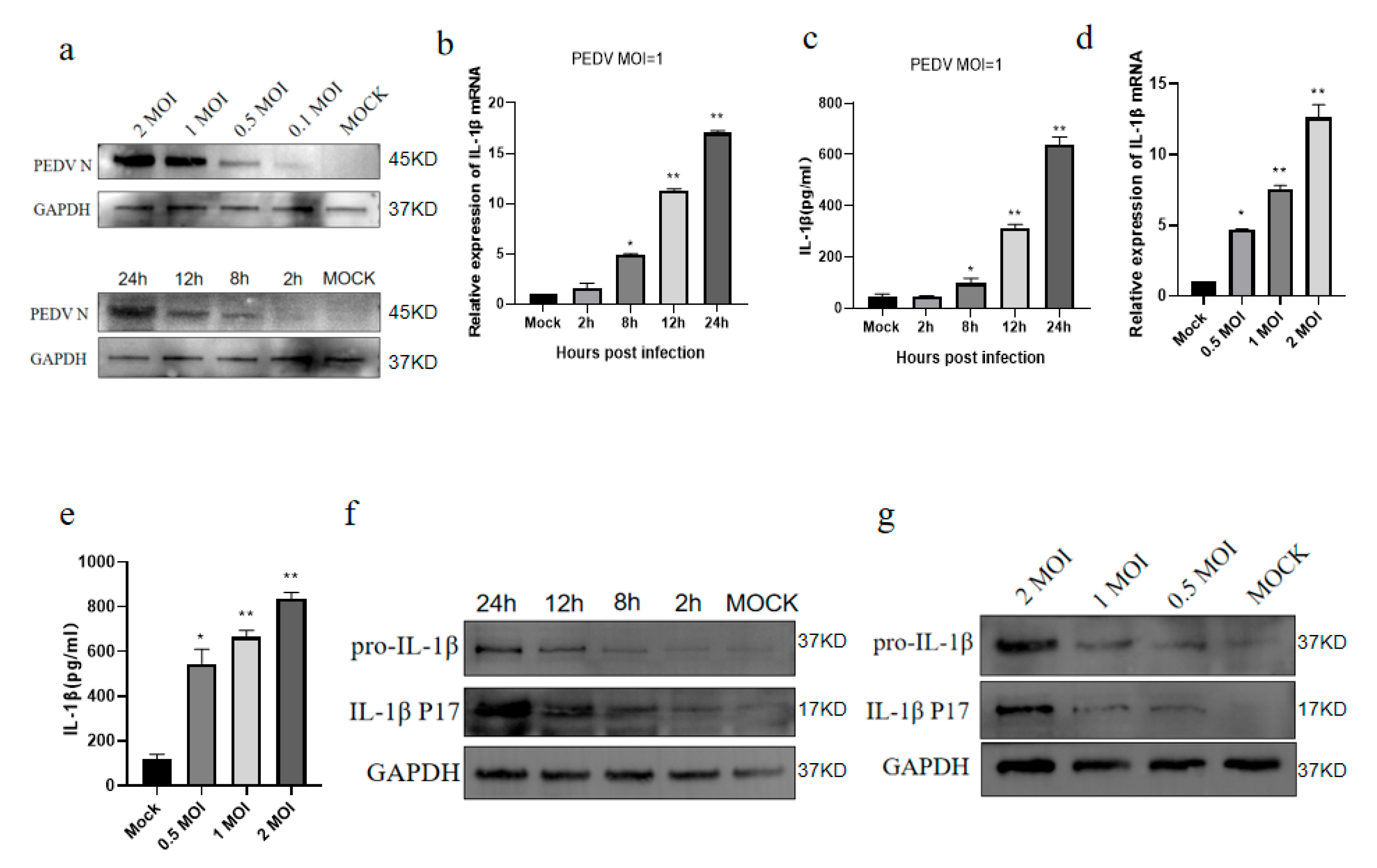
3.1. PEDV Infects IPEC-J2 Cells to Induce IL-1β Production
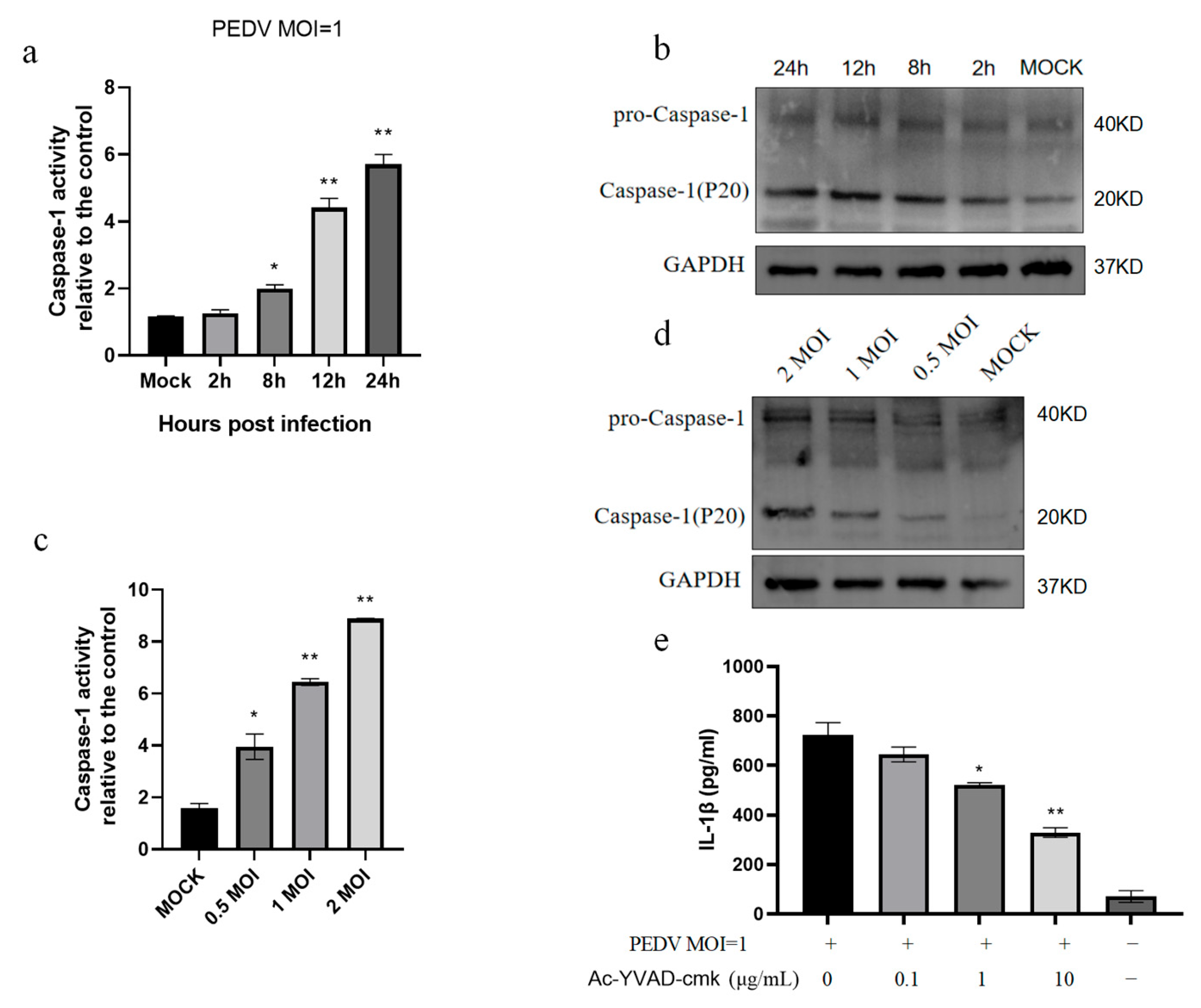
3.2. PEDV Infection of IPEC-J2 Cells Induces Increased Caspase-1 Enzyme Activity
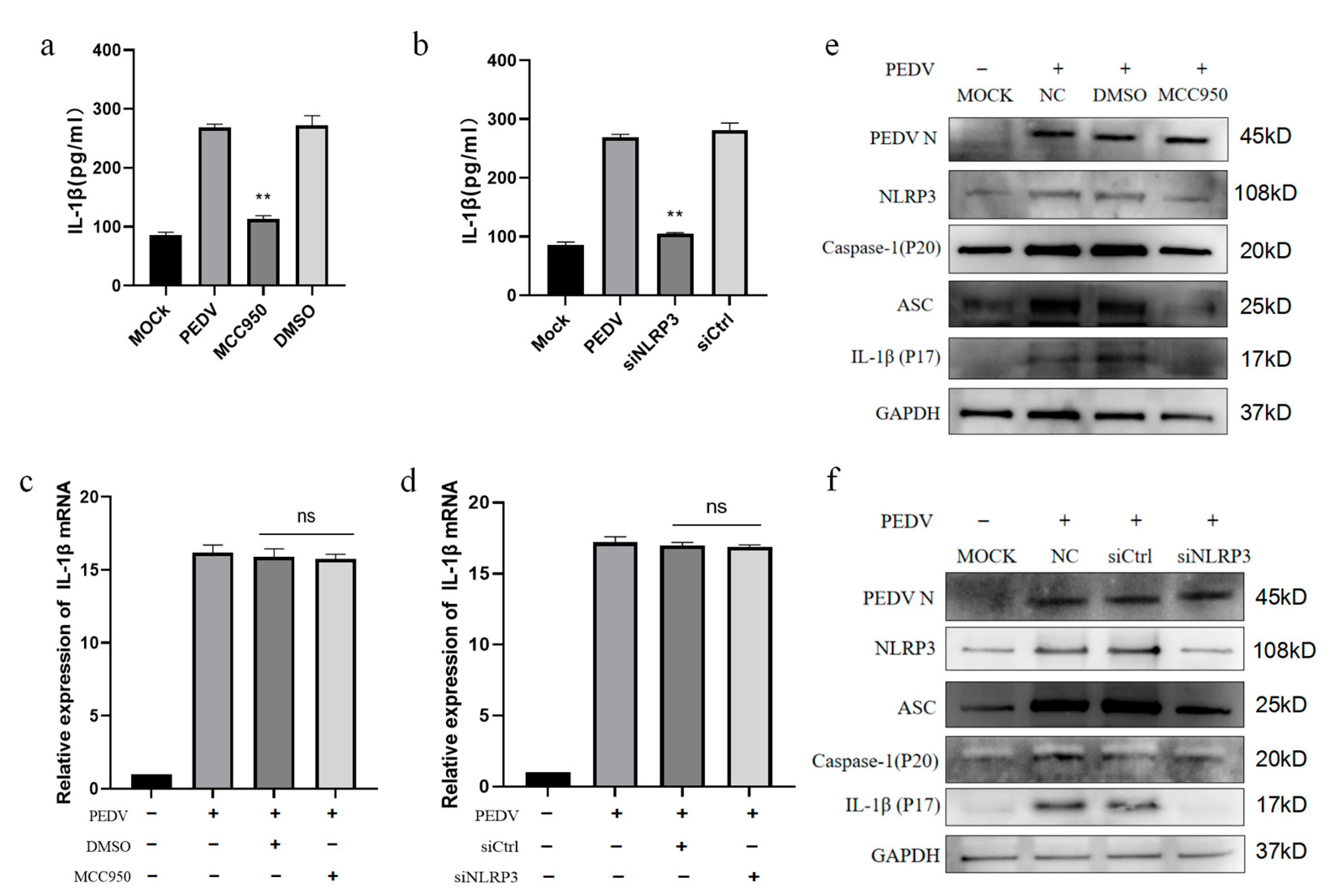
3.3. PEDV-Infected IPEC-J2 Cells Activate the NLRP3 Inflammasome
3.4. PEDV-Infected IPEC-J2 Cells Induce IL-1β Production by Activating the NLRP3 Inflammasome
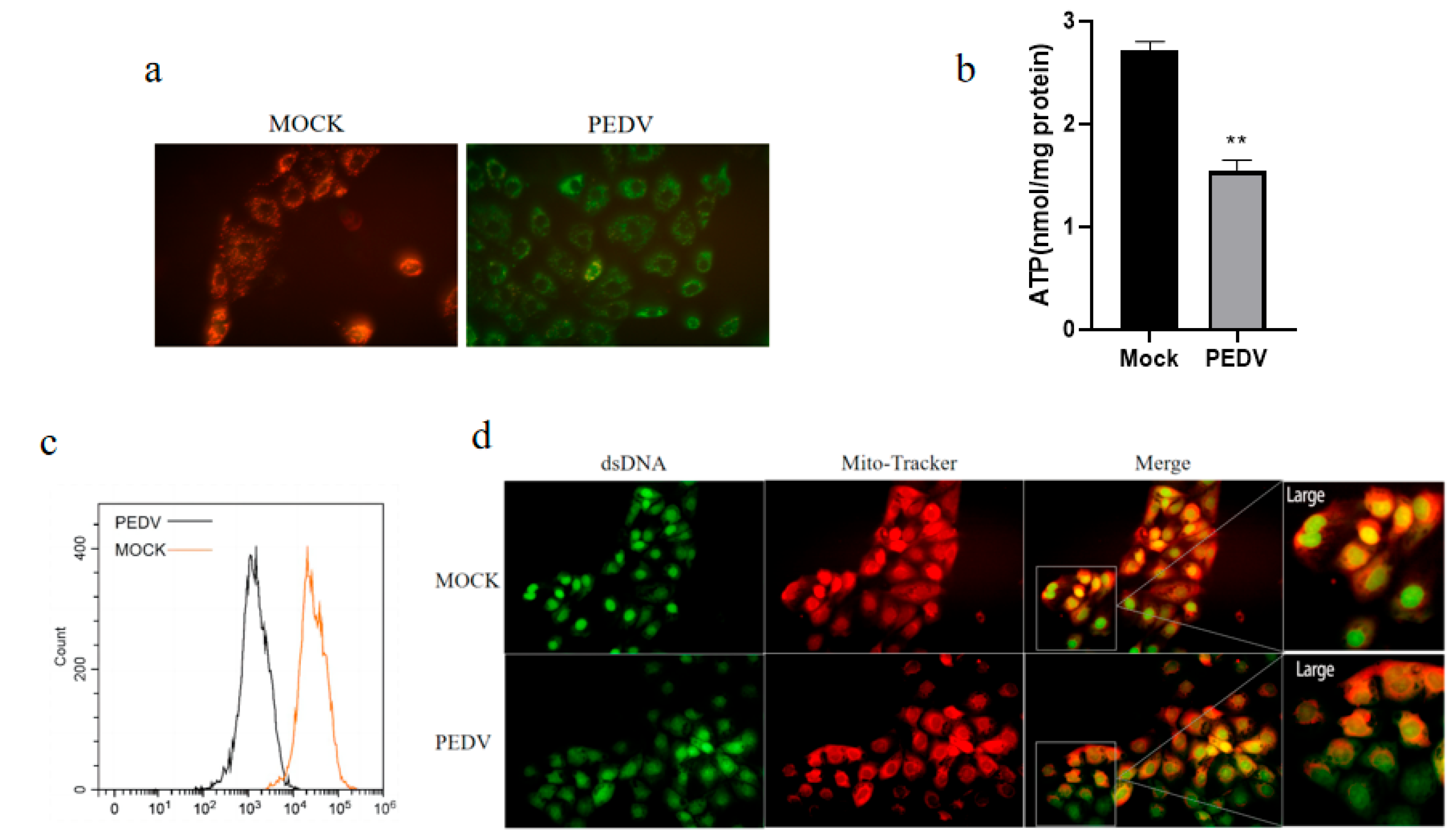
3.5. PEDV Infection of IPEC-J2 Cells Causes Mitochondrial Dysfunction, Leading to mtROS Production and mtDNA Release
3.6. mtROS Activates NF-κB Signaling After PEDV Infection of IPEC-J2 Cells
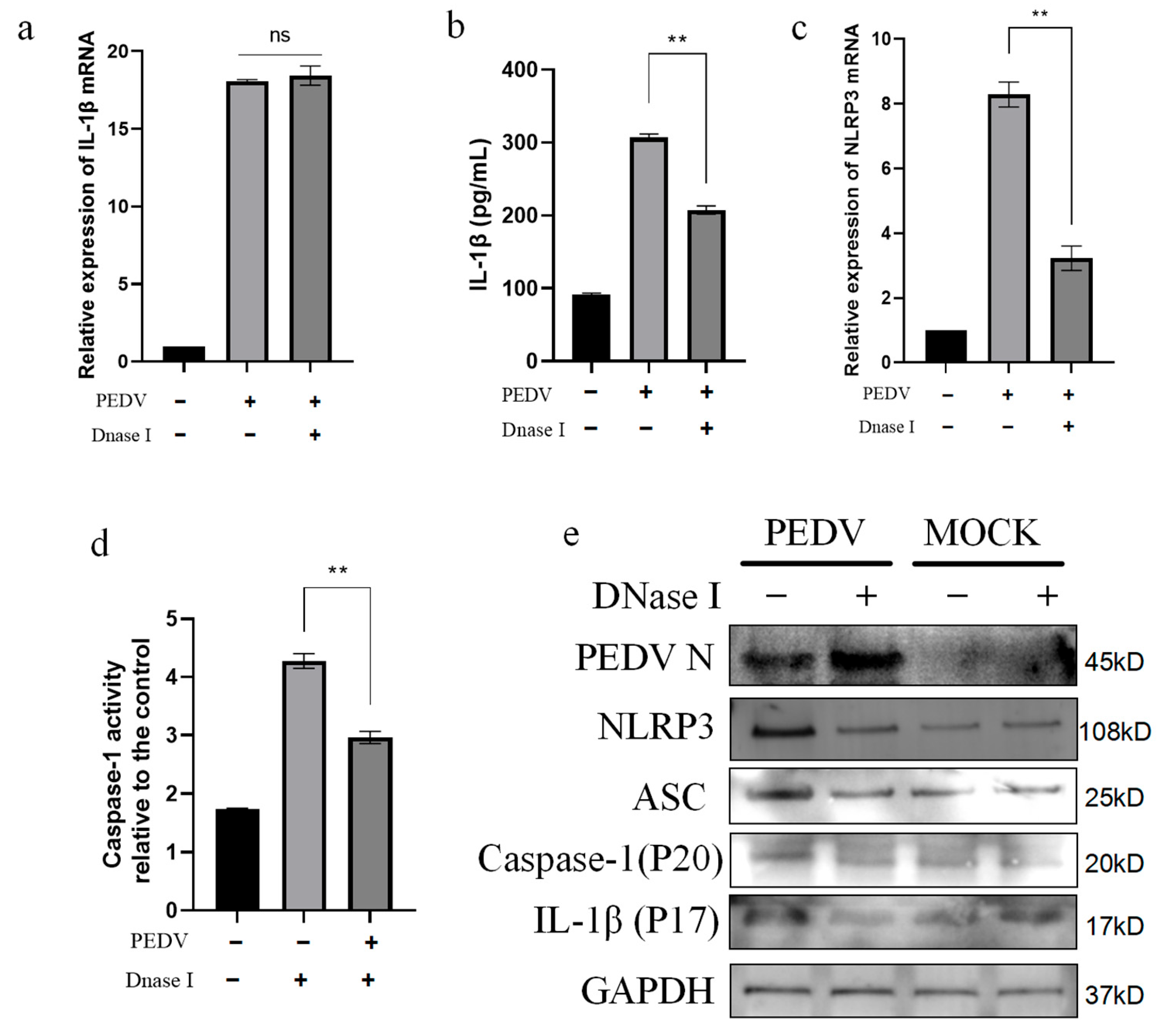
3.7. PEDV-Infected IPEC-J2 Cells Activate the NLRP3 Inflammasome Through mtDNA Release
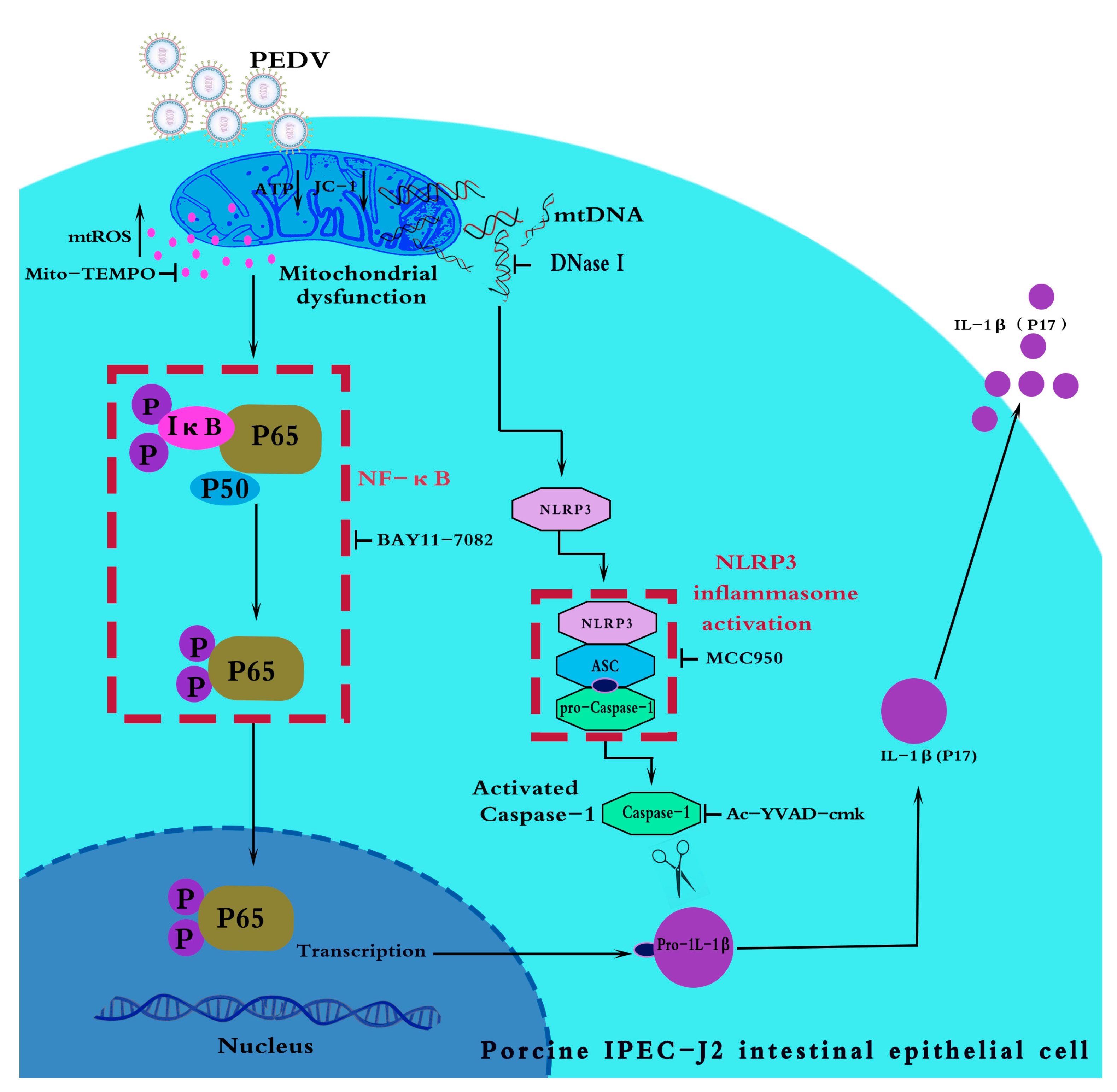
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, L.; Dong, J.; Wang, Y.; Zhang, P.; Liu, Y.; Zhang, L.; Liang, P.; Wang, L.; Song, C. Porcine epidemic diarrhea virus nsp4 induces pro-inflammatory cytokine and chemokine expression inhibiting viral replication in vitro. Arch. Virol. 2019, 164, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Zhang, Q.; Dong, J.; Liang, Y.; Huang, Y.; Liu, H.J.; Tong, D. Porcine epidemic diarrhea virus E protein causes endoplasmic reticulum stress and up-regulates interleukin-8 expression. Virol. J. 2013, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Miyazaki, A.; Saif, L.J. Immunohistochemical detection of the vomiting-inducing monoamine neurotransmitter serotonin and enterochromaffin cells in the intestines of conventional or gnotobiotic (Gn) pigs infected with porcine epidemic diarrhea virus (PEDV) and serum cytokine responses of Gn pigs to acute PEDV infection. Res. Vet. Sci. 2018, 119, 99–108. [Google Scholar] [PubMed]
- Sundaram, B.; Tweedell, R.E.; Prasanth Kumar, S.P.; Kanneganti, T.-D. The NLR family of innate immune and cell death sensors. Immunity 2024, 57, 674–699. [Google Scholar] [CrossRef]
- Lu, A.; Wu, H. Structural mechanisms of inflammasome assembly. FEBS J. 2015, 282, 435–444. [Google Scholar] [CrossRef]
- Bae, J.Y.; Park, H.H. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J. Biol. Chem. 2011, 286, 39528–39536. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome complexes: Emerging mechanisms and effector functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef]
- Pandey, A.; Shen, C.; Feng, S.; Man, S.M. Cell biology of inflammasome activation. Trends Cell Biol. 2021, 31, 924–939. [Google Scholar] [CrossRef]
- Nakano, R.; Kitanaka, T.; Namba, S.; Kitanaka, N.; Suwabe, Y.; Konno, T.; Yamazaki, J.; Nakayama, T.; Sugiya, H. Non-transcriptional and translational function of canonical NF-kB signaling in activating ERK1/2, in IL-1β-induced COX-2 expression in synovial fibroblasts. Front. Immunol. 2020, 11, 579266. [Google Scholar] [CrossRef]
- Newton, R.; Kuitert, L.M.; Bergmann, M.; Adcock, I.M.; Barnes, P.J. Evidence for involvement of NF-κB in the transcriptional control of COX-2 gene expression by IL-1β. Biochem. Biophys. Res. Commun. 1997, 237, 28–32. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-κB as an inducible regulator of inflammation in the central nervous system. Cells 2024, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Schroder, K.; Wu, H. Mechanistic insights from inflammasome structures. Nat. Rev. Immunol. 2024, 24, 518–535. [Google Scholar] [CrossRef] [PubMed]
- Lupfer, C.; Malik, A.; Kanneganti, T.-D. Inflammasome control of viral infection. Curr. Opin. Virol. 2015, 12, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ge, X.; Gao, Y.; Ren, Y.; Ren, X.; Li, G. Porcine epidemic diarrhea virus infection induces NF-κB activation through the TLR2, TLR3 and TLR9 pathways in porcine intestinal epithelial cells. J. Gen. Virol. 2015, 96, 1757–1767. [Google Scholar] [CrossRef]
- Sun, P.; Fahd, Q.; Li, Y.; Sun, Y.; Li, J.; Qaria, M.A.; He, Z.S.; Fan, Y.; Zhang, Q.; Xu, Q.; et al. Transcriptomic analysis of small intestinal mucosa from porcine epidemic diarrhea virus infected piglets. Microb. Pathog. 2019, 132, 73–79. [Google Scholar] [CrossRef]
- Arnoult, D.; Soares, F.; Tattoli, I.; Girardin, S.E. Mitochondria in innate immunity. EMBO Rep. 2011, 12, 901–910. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef]
- Banoth, B.; Cassel, S.L. Mitochondria in innate immune signaling. Transl. Res. 2018, 202, 52–68. [Google Scholar] [CrossRef]
- Lai, J.H.; Wang, M.Y.; Huang, C.Y.; Wu, C.H.; Hung, L.F.; Yang, C.Y.; Ke, P.Y.; Luo, S.F.; Liu, S.J.; Ho, L.J. Infection with the dengue RNA virus activates TLR9 signaling in human dendritic cells. EMBO Rep. 2018, 19, e46182. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Song, Y.; Zhu, Q.; Liao, Z.; Liang, Y.; Guo, J.; Wan, B.; Bao, D. PRRSV infection activates NLRP3 inflammasome through inducing cytosolic mitochondrial DNA stress. Vet. Microbiol. 2023, 279, 109673. [Google Scholar] [CrossRef]
- Moriyama, M.; Koshiba, T.; Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 2019, 10, 4624. [Google Scholar] [CrossRef]
- Liang, R.; Song, H.; Wang, K.; Ding, F.; Xuan, D.; Miao, J.; Fei, R.; Zhang, J. Porcine epidemic diarrhea virus 3CLpro causes apoptosis and collapse of mitochondrial membrane potential requiring its protease activity and signaling through MAVS. Vet. Microbiol. 2022, 275, 109596. [Google Scholar] [CrossRef] [PubMed]
- Caucheteux, S.M.; Hu-Li, J.; Guo, L.; Bhattacharyya, N.; Crank, M.; Collins, M.T.; Paul, W.E. IL-1β enhances inflammatory TH2 differentiation. J. Allergy Clin. Immunol. 2016, 138, 898–901.e4. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Epate, A.; Celsi, F.; Crovella, S. Alendronate treatment induces IL-1B expression and apoptosis in glioblastoma cell line. Inflammopharmacology 2018, 26, 285–290. [Google Scholar] [CrossRef]
- Chen, J.; Cui, Y.; Wang, Z.; Liu, G. Identification and characterization of PEDV infection in rat crypt epithelial cells. Vet. Microbiol. 2020, 249, 108848. [Google Scholar] [CrossRef]
- Yuan, J.; Najafov, A.; Py, B.F. Roles of caspases in necrotic cell death. Cell 2016, 167, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Luo, S.; Wu, W.; Hu, J.; Zhou, R. Activation of interleukin-1β release and pyroptosis by transmissible gastroenteritis virus is dependent on the NOD-like receptor Protein 3 inflammasome in porcine intestinal epithelial cell line. Viral Immunol. 2021, 34, 401–409. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, L.; Chen, H.; Hu, X.; Wang, W.; Zhang, H.; Wei, R.; Zhang, X.; Chen, Y.; Liu, X. PRRSV infection induces gasdermin D-driven pyroptosis of porcine alveolar macrophages through NLRP3 inflammasome activation. J. Virol. 2022, 96, e0212721. [Google Scholar] [CrossRef]
- Ji, S.; Dai, M.-Y.; Huang, Y.; Ren, X.-C.; Jiang, M.-L.; Qiao, J.-P.; Zhang, W.-Y.; Xu, Y.-H.; Shen, J.-L.; Zhang, R.-Q.; et al. Influenza A virus triggers acute exacerbation of chronic obstructive pulmonary disease by increasing proinflammatory cytokines secretion via NLRP3 inflammasome activation. J. Inflamm. 2022, 19, 8. [Google Scholar] [CrossRef]
- Angajala, A.; Lim, S.; Phillips, J.B.; Kim, J.-H.; Yates, C.; You, Z.; Tan, M. Diverse roles of mitochondria in immune responses: Novel insights into immuno-metabolism. Front. Immunol. 2018, 9, 1605. [Google Scholar] [CrossRef]
- Sho, T.; Xu, J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol. Appl. Biochem. 2019, 66, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Chen, L.; Song, Z.; He, H. The fate of damaged mitochondrial DNA in the cell. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119233. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Huang, Y.; Chen, M.; Yang, Y.; Li, X.; Zhang, W. Mitochondrial DNA in NLRP3 inflammasome activation. Int. Immunopharmacol. 2022, 108, 108719. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial oxidative stress and ‘mito-inflammation’: Actors in the diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Kang, D.; Hamasaki, N. Mitochondrial oxidative stress and mitochondrial DNA. Clin. Chem. Lab. Med. 2003, 41, 1281–1288. [Google Scholar] [CrossRef]
- Newman, L.E.; Shadel, G.S. Mitochondrial DNA release in innate immune signaling. Annu. Rev. Biochem. 2023, 92, 299–332. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y.; et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef]
- Cheng, J.; Tao, J.; Li, B.; Shi, Y.; Liu, H. Coinfection with PEDV and BVDV induces inflammatory bowel disease pathway highly enriched in PK-15 cells. Virol. J. 2022, 19, 119. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Wang, F.; Wang, H.; Wu, Z.; Wu, S.; Bao, W. Expression pattern analysis of antiviral genes and inflammatory cytokines in PEDV-infected porcine intestinal epithelial cells. Front. Vet. Sci. 2020, 7, 75. [Google Scholar] [CrossRef]
- Kim, N.-E.; Kim, D.-K.; Song, Y.-J. SARS-CoV-2 nonstructural proteins 1 and 13 suppress caspase-1 and the NLRP3 inflammasome activation. Microorganisms 2021, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.Y.; Komarasamy, T.V.; Rmt Balasubramaniam, V. Hyperinflammatory Immune Response and COVID-19: A Double Edged Sword. Front. Immunol. 2021, 12, 742941. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Zhao, T.-Q.; Xu, D.-P.; Zhang, X.; Ji, C.-J.; Zhang, D.-L. The influence of porcine epidemic diarrhea virus on pig small intestine mucosal epithelial cell function. Arch. Virol. 2019, 164, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet. Microbiol. 2012, 156, 229–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yang, S.; Yin, B.; Zhao, Z.; Huang, Z.; Wu, J.; Lin, S.; Wang, X. Water extract of Portulaca oleracea inhibits PEDV infection-induced pyrolysis by caspase-1/GSDMD. Curr. Issues Mol. Biol. 2023, 45, 10211–10224. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; He, W.; Ge, L.; Huang, K. Deoxynivalenol aggravates the immunosuppression in piglets and PAMs under the condition of PEDV infection through inhibiting TLR4/NLRP3 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 231, 113209. [Google Scholar] [CrossRef]
- Rubartelli, A.; Bajetto, A.; Allavena, G.; Cozzolino, F.; Sitia, R. Post-translational regulation of interleukin 1β secretion. Cytokine 1993, 5, 117–124. [Google Scholar] [CrossRef]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef]
- Zhou, D.; Yu, T.; Chen, G.; Brown, S.A.; Yu, Z.; Mattson, M.P.; Thompson, J.S. Effects of NF-κB1. Int. J. Radiat. Biol. 2001, 77, 763–772. [Google Scholar] [CrossRef]
- O’Neill, L.A. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr. Top. Microbiol. Immunol. 2002, 270, 47–61. [Google Scholar]
- Verstrepen, L.; Bekaert, T.; Chau, T.L.; Tavernier, J.; Chariot, A.; Beyaert, R. TLR4, IL-1R and TNF-R signaling to NF-κB: Variations on a common theme. Cell. Mol. Life Sci. 2008, 65, 2964–2978. [Google Scholar] [CrossRef]
- Ma, T.Y.; Boivin, M.A.; Ye, D.; Pedram, A.; Said, H.M. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G422–G430. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Ye, D.; Dokladny, K.; Ma, T.Y. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008, 180, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A mitochondrial love–hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, M.-J.; Park, D.Y.; Chung, H.J.; Kim, C.-H.; Yoon, J.-H.; Kim, H.J. Mitochondrial reactive oxygen species modulate innate immune response to influenza A virus in human nasal epithelium. Antiviral Res. 2015, 119, 78–83. [Google Scholar] [CrossRef]
- Tal, M.C.; Sasai, M.; Lee, H.K.; Yordy, B.; Shadel, G.S.; Iwasaki, A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 2770–2775. [Google Scholar] [CrossRef]
- Silwal, P.; Kim, J.K.; Kim, Y.J.; Jo, E.-K. Mitochondrial reactive oxygen species: Double-edged weapon in host defense and pathological inflammation during infection. Front. Immunol. 2020, 11, 1649. [Google Scholar] [CrossRef]
- Bhowal, C.; Ghosh, S.; Ghatak, D.; De, R. Pathophysiological involvement of host mitochondria in SARS-CoV-2 infection that causes COVID-19: A comprehensive evidential insight. Mol. Cell. Biochem. 2023, 478, 1325–1343. [Google Scholar] [CrossRef]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef]
- Cossarizza, A.; Pinti, M.; Nasi, M.; Gibellini, L.; Manzini, S.; Roat, E.; De Biasi, S.; Bertoncelli, L.; Montagna, J.P.; Bisi, L.; et al. Increased plasma levels of extracellular mitochondrial DNA during HIV infection: A new role for mitochondrial damage-associated molecular patterns during inflammation. Mitochondrion 2011, 11, 750–755. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017, 17, 363–375. [Google Scholar] [CrossRef]


| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| IL-1β | AAGAGGGACATGGAGAAGCGATTTG | TTGTTCTGCTTGAGAGGTGCTGATG |
| NLRP3 | TGTATTGAGAACTGTCGCCATGTGG | CTCCTCTTCCTCCTCCTCCTCTTTG |
| GAPDH | GATTCCACCCACGGCAAGTTCC | AGCACCAGCATCACCCCATTTG |
| ASC | GAAGGTGCTGACGGAAGAGC | TCCTTGCAGGTCAGGTTCCA |
| siRNA | Sense (5′–3′) | Anti-Sense (5′–3′) |
|---|---|---|
| siNLRP3 | CGGUUAAGUUGUCUCAAAUTT | AUUUCACAGTTCUUAAGGCTT |
| siCtrl | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, D.; Yi, S.; Zhao, L.; Zhao, H.; Liu, J.; Wei, Y.; Hu, G.; Liu, X. Porcine Epidemic Diarrhea Virus Infection of Porcine Intestinal Epithelial Cells Causes Mitochondrial DNA Release and the Activation of the NLRP3 Inflammasome to Mediate Interleukin-1β Secretion. Vet. Sci. 2024, 11, 643. https://doi.org/10.3390/vetsci11120643
Bao D, Yi S, Zhao L, Zhao H, Liu J, Wei Y, Hu G, Liu X. Porcine Epidemic Diarrhea Virus Infection of Porcine Intestinal Epithelial Cells Causes Mitochondrial DNA Release and the Activation of the NLRP3 Inflammasome to Mediate Interleukin-1β Secretion. Veterinary Sciences. 2024; 11(12):643. https://doi.org/10.3390/vetsci11120643
Chicago/Turabian StyleBao, Di, Shushuai Yi, Luobing Zhao, Han Zhao, Jiuyuan Liu, Yiming Wei, Guixue Hu, and Xinxin Liu. 2024. "Porcine Epidemic Diarrhea Virus Infection of Porcine Intestinal Epithelial Cells Causes Mitochondrial DNA Release and the Activation of the NLRP3 Inflammasome to Mediate Interleukin-1β Secretion" Veterinary Sciences 11, no. 12: 643. https://doi.org/10.3390/vetsci11120643
APA StyleBao, D., Yi, S., Zhao, L., Zhao, H., Liu, J., Wei, Y., Hu, G., & Liu, X. (2024). Porcine Epidemic Diarrhea Virus Infection of Porcine Intestinal Epithelial Cells Causes Mitochondrial DNA Release and the Activation of the NLRP3 Inflammasome to Mediate Interleukin-1β Secretion. Veterinary Sciences, 11(12), 643. https://doi.org/10.3390/vetsci11120643






