Effect of Different Thawing Regimes on Cell Kinematics and Organelle Integrity of Nitrogen-Stored Wallachian Ram Spermatozoa
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Rams and Sperm Collection
2.3. Sperm Samples Analyses
2.4. Sperm Freezing
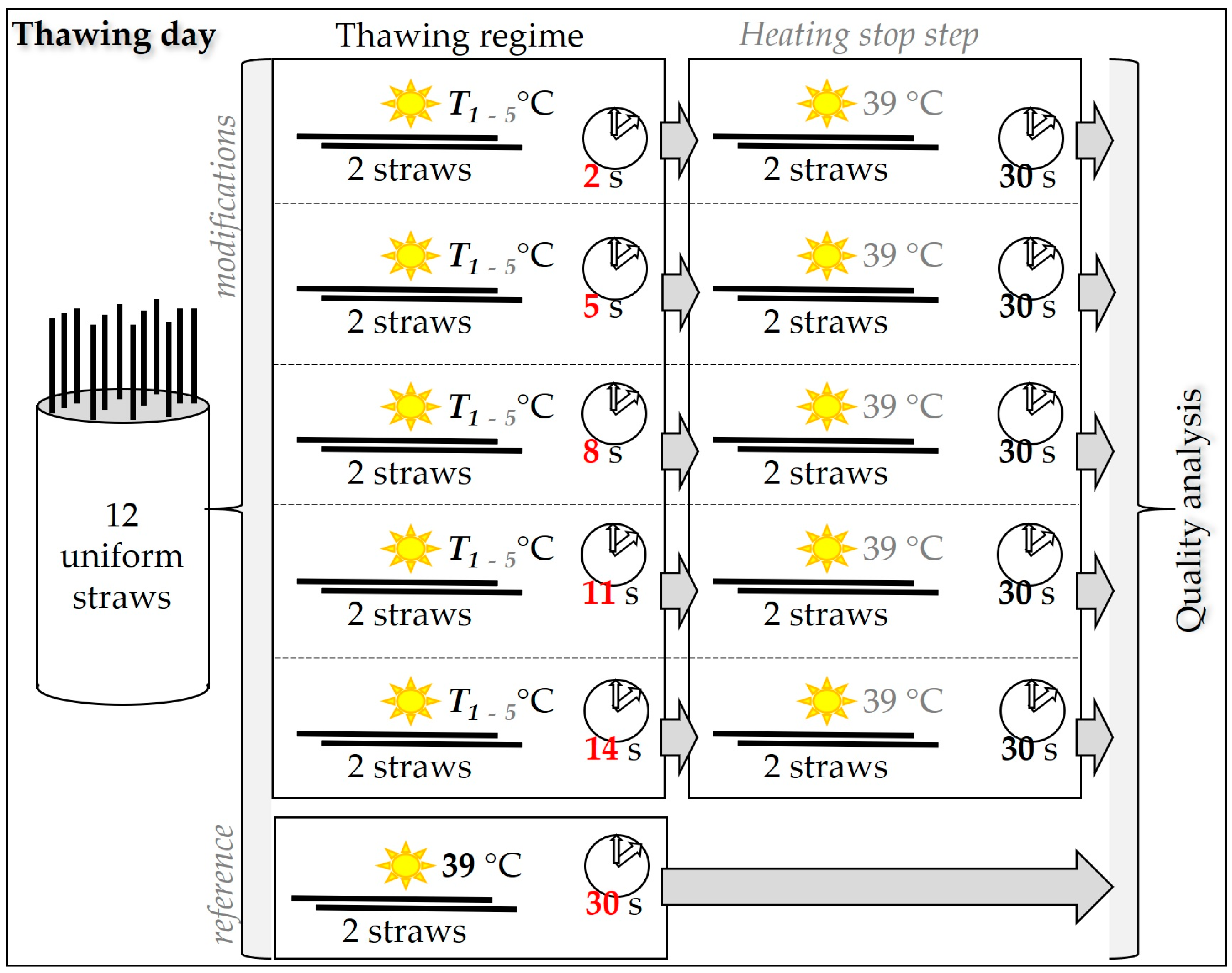
2.5. Semen Thawing
2.6. Sperm Kinematic Analysis
2.7. Flow Cytometric Assay
2.8. Internal Thermal Gradient and Thermal Energy Absorption of Straws During Thawing
2.9. Statistical Analysis
3. Results
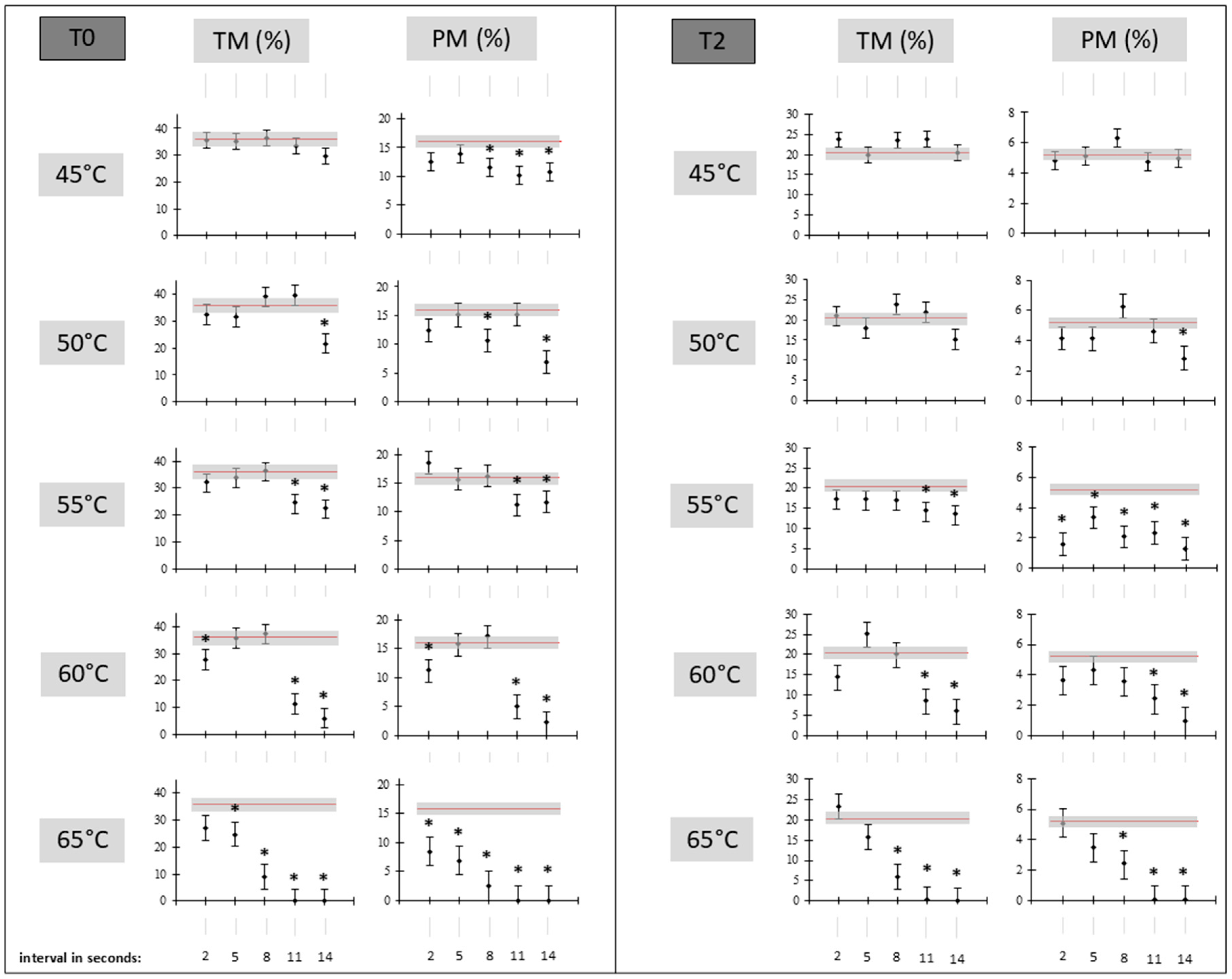
3.1. Sperm Kinematic Analysis
3.2. Flow Cytometry Assay
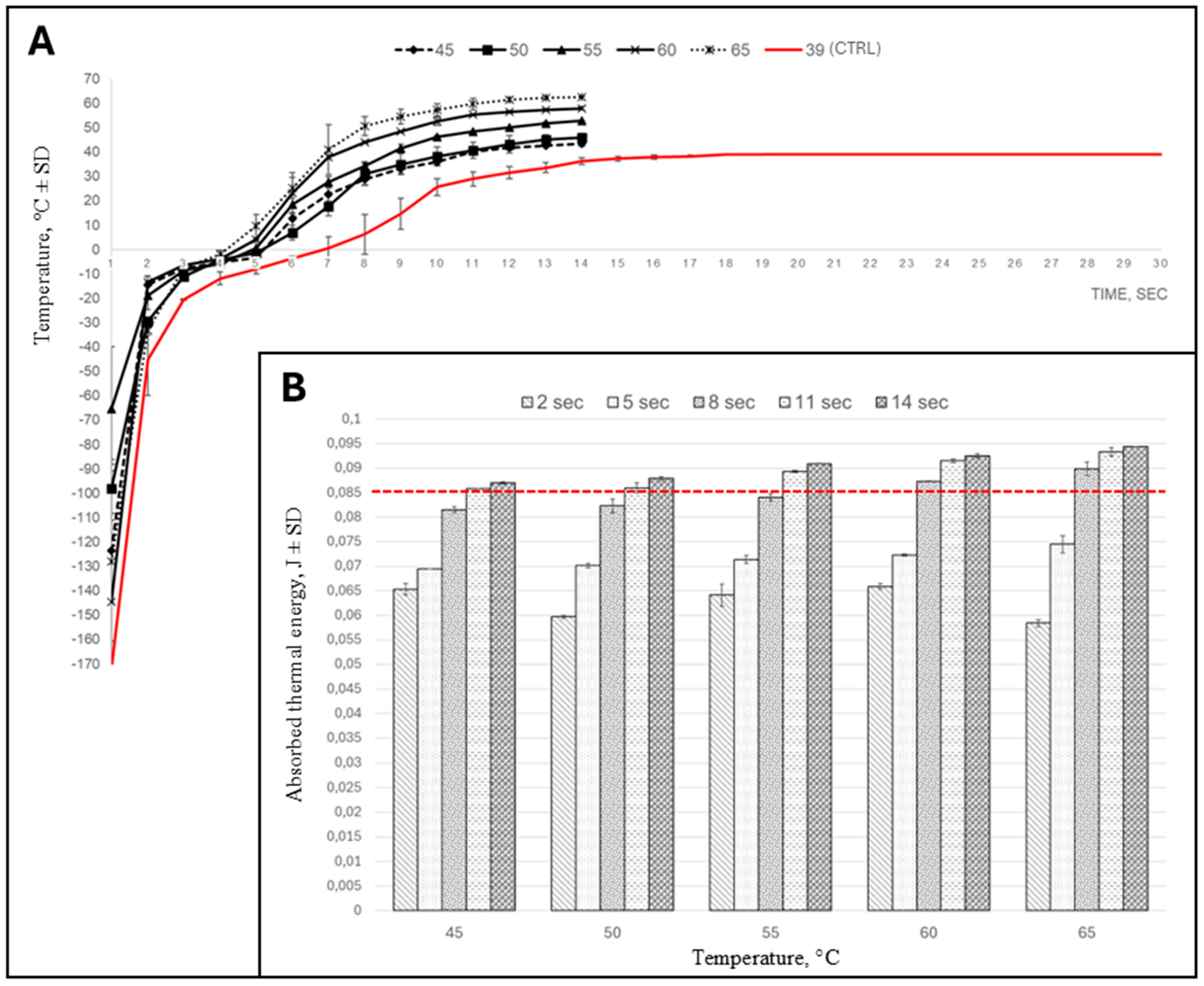
3.3. Internal Thermal Gradient and Thermal Energy Absorption of Insemination Doses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spanner, E.A.; de Graaf, S.P.; Rickard, J.P. Factors affecting the success of laparoscopic artificial insemination in sheep. Anim. Reprod. Sci. 2024, 264, 107453. [Google Scholar] [CrossRef] [PubMed]
- Málková, A.; Ptáček, M.; Savvulidi, F.G.; Nagy, S.T.; Stádník, L. Effects of age and litter-of-origin on cryopreserved spermatozoa in Sumava rams. Czech J. Anim. Sci. 2024, 69, 129–138. [Google Scholar] [CrossRef]
- Mujitaba, M.A.; Tokár, A.; Balogh, E.E.; Debnár, V.J.; Javkhlan, A.; Vásárhelyi, P.B.; Egerszegi, I.; Nagy, S.T.; Kútvölgyi, G. In Vitro Gene Conservation Status and the Quality of the Genetic Resources of Native Hungarian Sheep Breeds. Vet. Sci. 2024, 11, 337. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Ba-Awadh, H.; Olarinre, I.; Saadeldin, I.; Alowaimer, A.; Swelum, A. Comparison between rapid and slow cryopreservation protocols for ram semen. Slov. Vet. Res. 2023, 60, 249–257. [Google Scholar] [CrossRef]
- Hai, E.; Li, B.; Zhang, J.; Zhang, J. Sperm freezing damage: The role of regulated cell death. Cell Death Discov. 2024, 10, 239. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Jiang, C.; Sohail, T.; Sun, Y.; Sun, X.; Wang, J.; Li, Y. Effects of Different Diluents and Freezing Methods on Cryopreservation of Hu Ram Semen. Vet. Sci. 2024, 11, 251. [Google Scholar] [CrossRef]
- Salamon, S.; Maxwell, W.M.C. Storage of ram semen. Anim. Reprod. Sci. 2000, 62, 77–111. [Google Scholar] [CrossRef] [PubMed]
- Pezo, F.; Contreras, M.J.; Zambrano, F.; Uribe, P.; Risopatron, J.; de Andrade, A.F.C.; Yeste, M.; Sánchez, R. Thawing of cryopreserved sperm from domestic animals: Impact of temperature, time, and addition of molecules to thawing/insemination medium. Anim. Reprod. Sci. 2024, 268, 107572. [Google Scholar] [CrossRef]
- Paulenz, H.; Söderquist, L.; Ådnøy, T.; Nordstoga, A.B.; Andersen Berg, K. Effect of vaginal and cervical deposition of semen on the fertility of sheep inseminated with frozen-thawed semen. Vet. Rec. 2005, 156, 372–375. [Google Scholar] [CrossRef]
- Söderquist, L.; Madrid-Bury, N.; Rodriguez-Martinez, H. Assessment of ram sperm membrane integrity following different thawing procedures. Theriogenology 1997, 48, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Anel, L.; De Paz, P.; Alvarez, M.; Chamorro, C.A.; Boixo, J.C.; Manso, A.; González, M.; Kaabi, M.; Anel, E. Field and in vitro assay of three methods for freezing ram semen. Theriogenology 2003, 60, 1293–1308. [Google Scholar] [CrossRef]
- Palacin-Martinez, C.; Alvarez, M.; Montes-Garrido, R.; Neila-Montero, M.; Anel-Lopez, L.; de Paz, P.; Anel, L.; Riesco, M.F. Frequency of semen collection affects ram sperm cryoresistance. Animals 2022, 12, 1492. [Google Scholar] [CrossRef]
- Wu, C.; Dai, J.; Zhang, S.; Sun, L.; Liu, Y.; Zhang, D. Effect of Thawing Rates and Antioxidants on Semen Cryopreservation in Hu Sheep. Biopreserv. Biobanking 2021, 19, 204–209. [Google Scholar] [CrossRef]
- Richardson, L.; Hanrahan, J.P.; Donovan, A.; Martí, J.I.; Fair, S.; Evans, A.C.; Lonergan, P. Effect of site of deposition on the fertility of sheep inseminated with frozen-thawed semen. Anim. Reprod. Sci. 2012, 131, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Maxwell, M.C. Salamon’s Artificial Insemination of Sheep and Goats; Butterworths: Sydney, Australia, 1987; p. 194. [Google Scholar]
- Purdy, P.H.; Spiller, S.F.; McGuire, E.; McGuire, K.; Koepke, K.; Lake, S.; Blackburn, H.D. Critical factors for non-surgical artificial insemination in sheep. Small Rumin. Res. 2020, 191, 106179. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, H.; Cui, H.; Adetunji, A.O.; Min, L. Resveratrol Improves the Frozen-Thawed Ram Sperm Quality. Animals 2023, 13, 3887. [Google Scholar] [CrossRef]
- Crilly, J.P.; Söderquist, L.; Holmström, A.; Sargison, N.D. Proof of concept of ovine artificial insemination by vaginal deposition of frozen-thawed semen under UK sheep-farming conditions. Vet. Rec. 2016, 178, 532. [Google Scholar] [CrossRef]
- Nur, Z.; Dogan, I.; Soylu, M.K.; Ak, K. Effect of different thawing procedures on the quality of bull semen. Rev. Med. Vet. 2003, 154, 487–490. [Google Scholar]
- Pau, S.; Falchi, L.; Ledda, M.; Pivato, I.; Valentino, M.; Bogliolo, L.; Ariu, F.; Zedda, M.T. Reproductive performance following transcervical insemination with frozen thawed semen in ewes submitted to surgical incision of cervical folds (SICF): Comparison with Laparoscopic Artificial Insemination. Animals 2020, 10, 108. [Google Scholar] [CrossRef]
- Bucak, M.N.; Keskin, N.; Ili, P.; Bodu, M.; Akalın, P.P.; Öztürk, A.E.; Özkan, H.; Topraggaleh, T.R.; Sari, F.; Başpınar, N.; et al. Decreasing glycerol content by co-supplementation of trehalose and taxifolin hydrate in ram semen extender: Microscopic, oxidative stress, and gene expression analyses. Cryobiology 2020, 96, 19–29. [Google Scholar] [CrossRef]
- Tuli, R.K.; Schmidt-Baulain, R.; Holtz, W. Influence of thawing temperature on viability and release of glutamic oxaloacetic transaminase in frozen semen from Boer goats. Anim. Reprod. Sci. 1991, 25, 125–131. [Google Scholar] [CrossRef]
- Daghigh, K.H.; Vatankhah, S.; Ebrahimi, M.; Moghaddam, G. Effect of different thawing procedures on frozen semen quality of Ghezel rams. J. Ruminant Res. 2018, 6, 117–132. [Google Scholar]
- Russel, A.J.F.; Doney, J.M.; Gunn, R.G. Subjective assessment of body fat in live sheep. J. Agric. Sci. 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Jurado-Campos, A.; Soria-Meneses, P.J.; Arenas-Moreira, M.; Alonso-Moreno, C.; Rodríguez-Robledo, V.; Soler, A.J.; Garde, J.J.; del Rocío Fernández-Santos, M. Minimizing sperm oxidative stress using nanotechnology for breeding programs in rams. J. Animal Sci. Biotechnol. 2023, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- David, I.; Kohnke, P.; Lagriffoul, G.; Praud, O.; Plouarboué, F.; Degond, P.; Druart, X. Mass sperm motility is associated with fertility in sheep. Anim. Reprod. Sci. 2015, 161, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ptáček, M.; Stádníková, M.; Savvulidi, F.; Stádník, L. Ram semen cryopreservation using egg yolk or egg yolk-free extenders: Preliminary results. Sci. Agric. Bohem. 2019, 50, 96–103. [Google Scholar] [CrossRef]
- Savvulidi, F.G.; Ptáček, M.; Málková, A.; Beránek, J.; Stádník, L. Optimizing the conventional method of sperm freezing in liquid nitrogen vapour for Wallachian sheep conservation program. Czech J. Anim. Sci. 2021, 66, 55–64. [Google Scholar] [CrossRef]
- Mendoza, N.; Casao, A.; Domingo, J.; Quintín, F.; Laviña, A.; Fantova, E.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T.; Pérez-Pe, R. Influence of Non-conventional Sperm Quality Parameters on Field Fertility in Ovine. Front. Vet. Sci. 2021, 8, 650572. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Mata-Campuzano, M.; Alvarez-Rodríguez, M.; Alvarez, M.; Anel, L.; de Paz, P. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. 2010, 45, 67–78. [Google Scholar] [CrossRef]
- Málková, A.; Savvulidi, F.G.; Ptáček, M.; Machová, K.; Janošíková, M.; Nagy, S.; Stádník, L. Glycerol-free equilibration with the addition of glycerol shortly before the freezing procedure: A perspective strategy for cryopreservation of Wallachian ram sperm. Animals 2023, 13, 1200. [Google Scholar] [CrossRef] [PubMed]
- Pizzutto, C.S.; Colbachini, H.; Jorge-Neto, P.N. One Conservation: The integrated view of biodiversity conservation. Anim. Reprod. 2021, 18, e20210024. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Almenar, C.; de Mercado, E. Optimization of the thawing protocol for Iberian boar sperm. Animals 2022, 12, 2600. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, M.; Tomás-Almenar, C.; Nieto-Cristóbal, H.; de Mercado, E. Evaluation of Different Thawing Protocols on Iberian Boar Sperm Preserved for 10 Years at Different Liquid Nitrogen Levels. Animals 2024, 14, 914. [Google Scholar] [CrossRef]
- Eriksson, B.M.; Rodriguez-Martinez, H. Effect of freezing and thawing rates on the post-thaw viability of boar spermatozoa frozen in FlatPacks and Maxi-straws. Anim. Reprod. Sci 2000, 63, 205–220. [Google Scholar] [CrossRef]
- Nur, Z.; Zik, B.; Ustuner, B.; Sagirkaya, H.; Ozguden, C.G. Effects of different cryoprotective agents on ram sperm morphology and DNAintegrity. Theriogenology 2010, 73, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Doležalová, M.; Ptáček, M.; Stádník, L.; Ducháček, J. Effect of different thawing methods on bull’s semen characteristics. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 815–822. [Google Scholar] [CrossRef]
- Paulenz, H.; Ådnøy, T.; Söderquist, L. Comparison of fertility results after vaginal insemination using different thawing procedures and packages for frozen ram semen. Acta Vet. Scan. 2007, 49, 26. [Google Scholar] [CrossRef]
- Nordstoga, A.B.; Söderquist, L.; Ådnøy, T.; Paulenz, H. Effect of different packages and freezing/thawing protocols on fertility of ram semen. Reprod. Domest. Anim. 2009, 44, 527–531. [Google Scholar] [CrossRef]
- Fukui, Y.; Kohno, H.; Togari, T.; Hiwasa, M.; Okabe, K. Fertility after artificial insemination using a soybean-based semen extender in sheep. J Reprod Dev. 2008, 54, 286–289. [Google Scholar] [CrossRef]
- Ardebili, R.; Towhidi, A.; Zeinodini, S.; Bahreini, M.; Dirandeh, E. 85 The comparison of sperm freezability using two egg yolk-free diluents in zandi ram. Reprod. Fertil. Dev. 2009, 22, 201. [Google Scholar] [CrossRef]
- Akçay, E.; Kulaksiz, R.; Daşkin, A.; Cebi, C.; Tekin, K. The effect of different dilution rates on post-thaw quality of ram semen frozen in two different egg-yolk free extenders. Slov. Vet. Res. 2012, 49, 97–102. [Google Scholar]
- Carro, M.; Ramírez-Vasquez, R.R.A.; Peñalva, D.A.; Buschiazzo, J.; Hozbor, F.A. Desmosterol Incorporation Into Ram Sperm Membrane Before Cryopreservation Improves in vitro and in vivo Fertility. Front. Cell Dev. Biol. 2021, 9, 660165. [Google Scholar] [CrossRef] [PubMed]
- Zacarías, C.A.C.; Ugalde, J.P.R.; Concha, H.J.L.; Bustillos, R.Z.; López, L.L.P.; Gutiérrez, G.I.R.; Rebolledo, Á.E.D. Effect of two animal protein-free extenders on cryopreservation of Pelibuey and Blackbelly ram semen. Vet. México OA 2023, 10, 1–18. [Google Scholar]
- Fleisch, A.; Malama, E.; Witschi, U.; Leiding, C.; Siuda, M.; Janett, F.; Bollwein, H. Effects of an extension of the equilibration period up to 96 hours on the characteristics of cryopreserved bull semen. Theriogenology 2017, 89, 255–262. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptáček, M.; Savvulidi, F.G.; LeBrun, C.; Janošíková, M.; Kenzhebaev, T.; Omashev, K.; Kulataev, B.; Malmakov, N. Effect of Different Thawing Regimes on Cell Kinematics and Organelle Integrity of Nitrogen-Stored Wallachian Ram Spermatozoa. Vet. Sci. 2024, 11, 602. https://doi.org/10.3390/vetsci11120602
Ptáček M, Savvulidi FG, LeBrun C, Janošíková M, Kenzhebaev T, Omashev K, Kulataev B, Malmakov N. Effect of Different Thawing Regimes on Cell Kinematics and Organelle Integrity of Nitrogen-Stored Wallachian Ram Spermatozoa. Veterinary Sciences. 2024; 11(12):602. https://doi.org/10.3390/vetsci11120602
Chicago/Turabian StylePtáček, Martin, Filipp Georgijevič Savvulidi, Christopher LeBrun, Martina Janošíková, Temirkhan Kenzhebaev, Kairly Omashev, Beybit Kulataev, and Nurlan Malmakov. 2024. "Effect of Different Thawing Regimes on Cell Kinematics and Organelle Integrity of Nitrogen-Stored Wallachian Ram Spermatozoa" Veterinary Sciences 11, no. 12: 602. https://doi.org/10.3390/vetsci11120602
APA StylePtáček, M., Savvulidi, F. G., LeBrun, C., Janošíková, M., Kenzhebaev, T., Omashev, K., Kulataev, B., & Malmakov, N. (2024). Effect of Different Thawing Regimes on Cell Kinematics and Organelle Integrity of Nitrogen-Stored Wallachian Ram Spermatozoa. Veterinary Sciences, 11(12), 602. https://doi.org/10.3390/vetsci11120602







