Expression of Mutated BRAFV595E Kinase in Canine Carcinomas—An Immunohistochemical Study
Simple Summary
Abstract
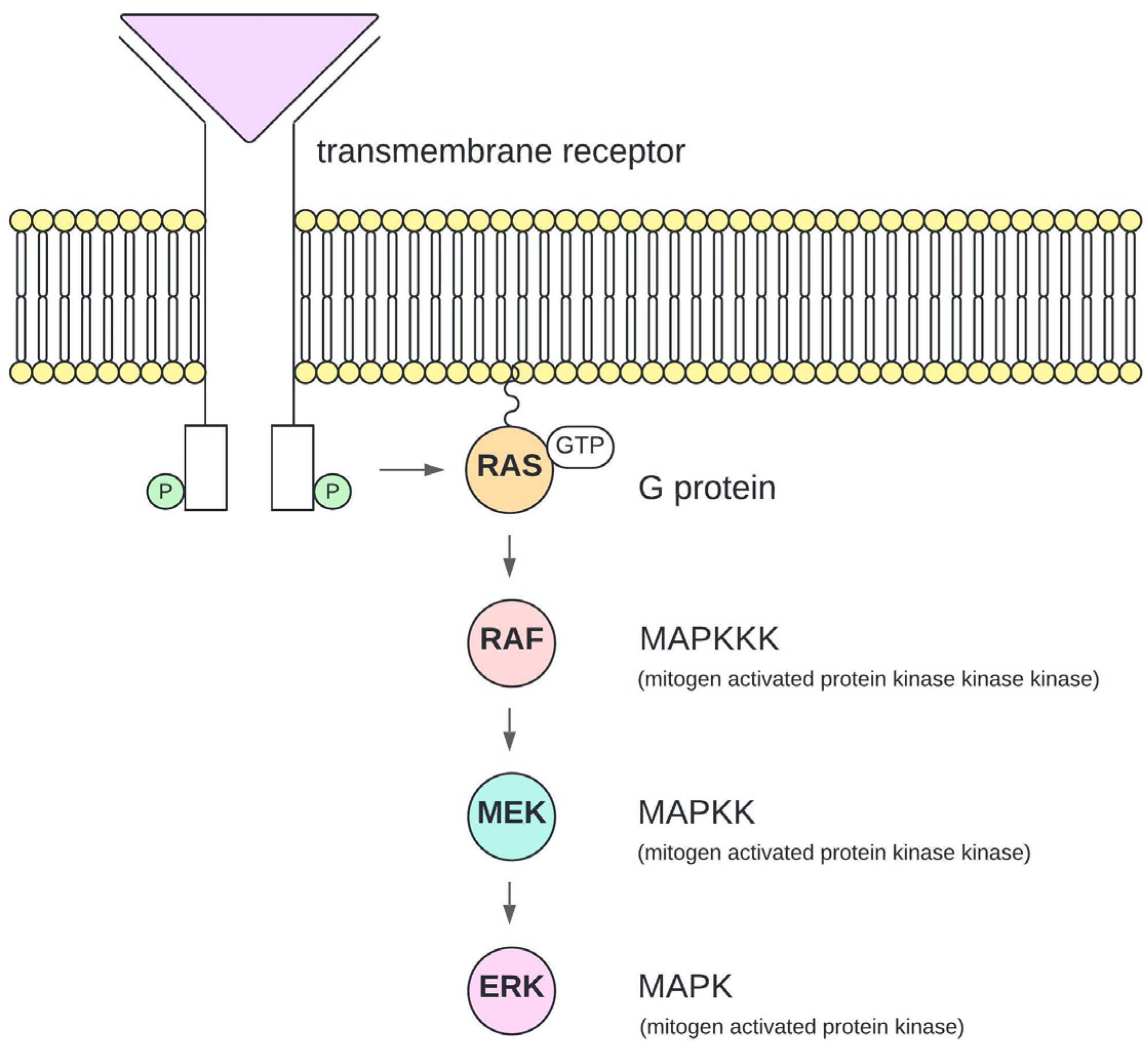
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Histology and Tissue Microarray (TMA)
- -
- Unambiguous diagnosis: A precise diagnosis of a carcinoma was possible.
- -
- -
- Minimal inflammatory cells: The carcinoma showed not more than mild infiltration of inflammatory cells.
2.3. Immunohistochemistry
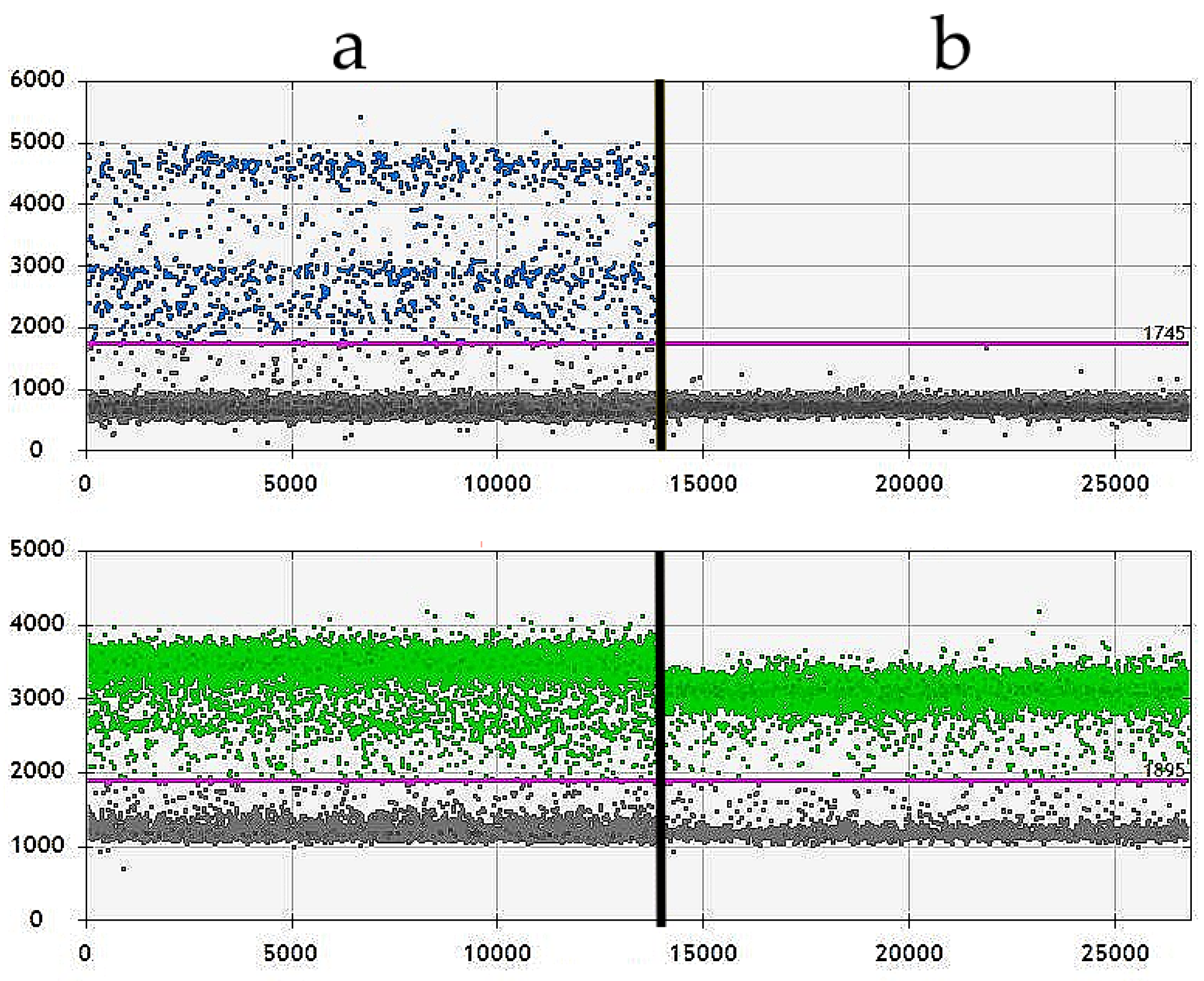
2.4. Droplet Digital PCR (ddPCR)
3. Results
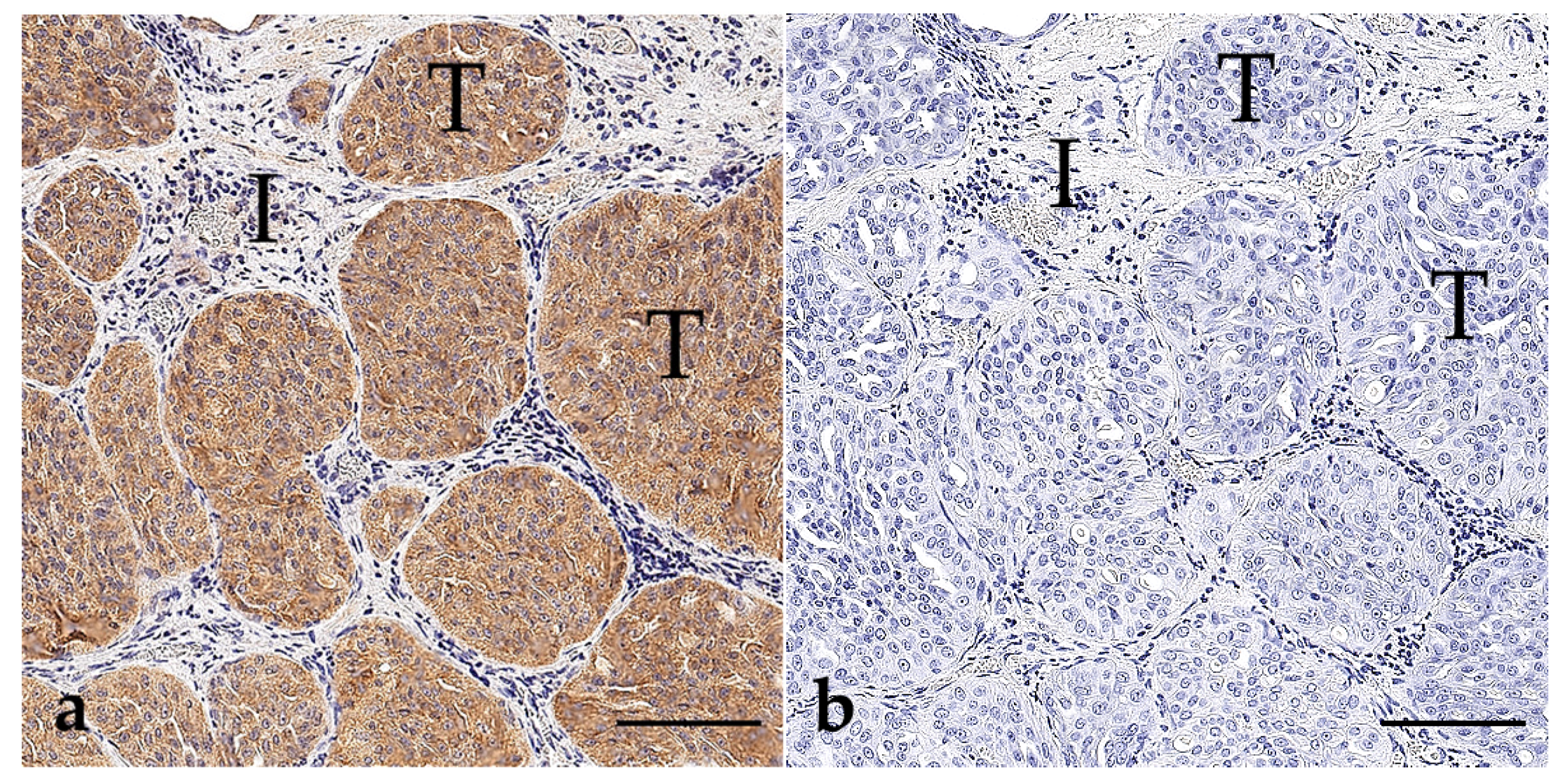
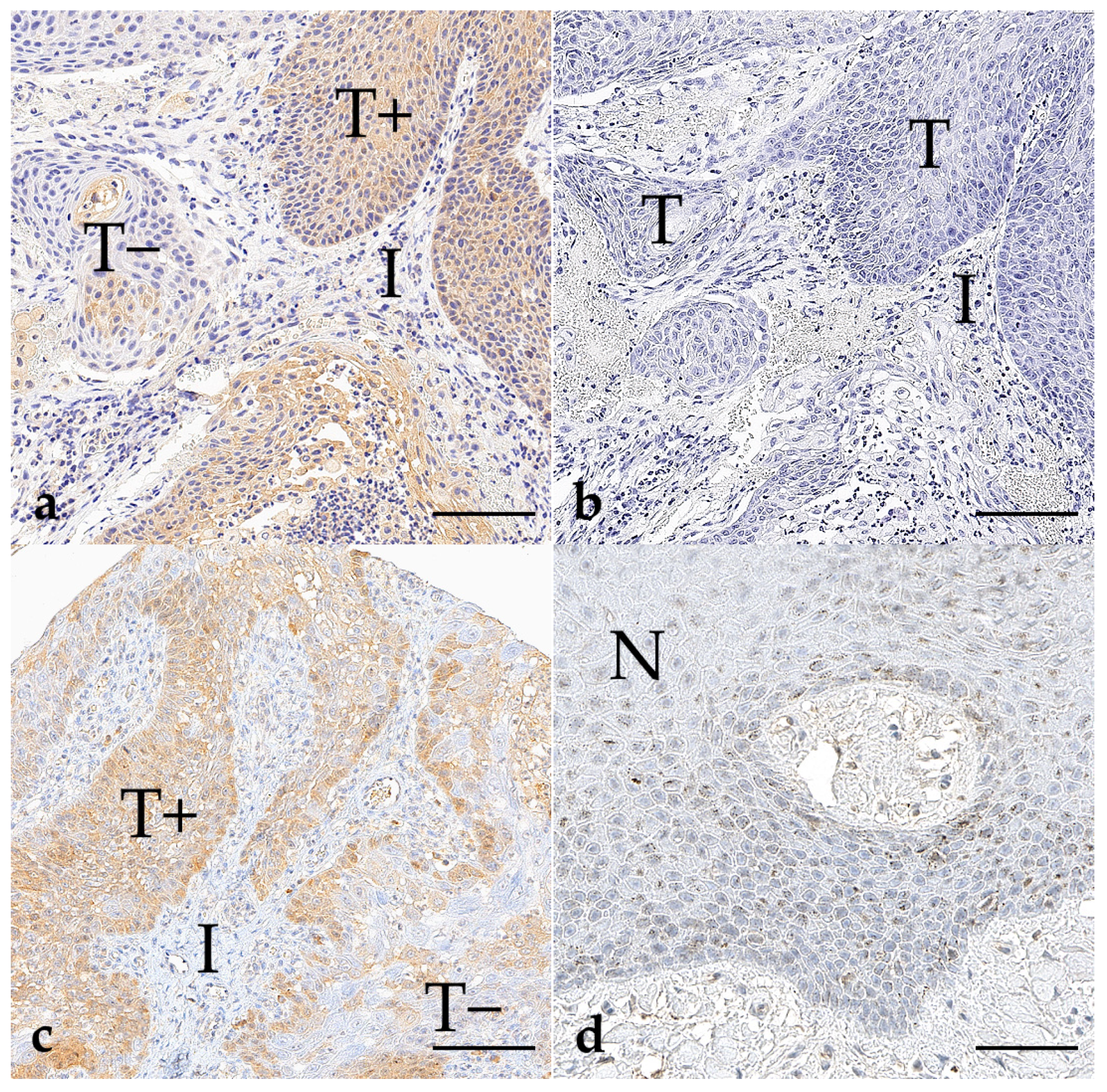
3.1. BRAF V595E Immunohistochemistry
3.2. Droplet Digital PCR (ddPCR)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhein, E.S.; Heikkilä, U.; Oevermann, A.; Blatter, S.; Meier, D.; Hartnack, S.; Guscetti, F. Incidence rates of the most common canine tumors based on data from the Swiss Canine Cancer Registry (2008 to 2020). PLoS ONE 2024, 19, e0302231. [Google Scholar] [CrossRef]
- Aupperle-Lellbach, H.; Grassinger, J.M.; Floren, A.; Törner, K.; Beitzinger, C.; Loesenbeck, G.; Müller, T. Tumour Incidence in Dogs in Germany: A Retrospective Analysis of 109,616 Histopathological Diagnoses (2014–2019). J. Comp. Pathol. 2022, 198, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Aupperle-Lellbach, H.; Kehl, A.; de Brot, S.; van der Weyden, L. Clinical Use of Molecular Biomarkers in Canine and Feline Oncology: Current and Future. Vet. Sci. 2024, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Sholl, L.M. A narrative review of BRAF alterations in human tumors: Diagnostic and predictive implications. Precis. Cancer Med. 2020, 3, 26. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. RAF protein-serine/threonine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2010, 399, 313–317. [Google Scholar] [CrossRef]
- Esteban-Villarrubia, J.; Soto-Castillo, J.J.; Pozas, J.; San Román-Gil, M.; Orejana-Martín, I.; Torres-Jiménez, J.; Carrato, A.; Alonso-Gordoa, T.; Molina-Cerrillo, J. Tyrosine Kinase Receptors in Oncology. Int. J. Mol. Sci. 2020, 21, 8529. [Google Scholar] [CrossRef]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Wan, P.T.C.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. BRAF Mutations in Canine Cancers. PLoS ONE 2015, 10, e0129534. [Google Scholar] [CrossRef]
- Decker, B.; Parker, H.G.; Dhawan, D.; Kwon, E.M.; Karlins, E.; Davis, B.W.; Ramos-Vara, J.A.; Bonney, P.L.; McNiel, E.A.; Knapp, D.W.; et al. Homologous Mutation to Human BRAF V600E Is Common in Naturally Occurring Canine Bladder Cancer—Evidence for a Relevant Model System and Urine-Based Diagnostic Test. Mol. Cancer Res. 2015, 13, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Breen, M. Comparative Aspects of BRAF Mutations in Canine Cancers. Vet. Sci. 2015, 2, 231–245. [Google Scholar] [CrossRef]
- Mochizuki, H.; Breen, M. Sequence analysis of RAS and RAF mutation hot spots in canine carcinoma. Vet. Comp. Oncol. 2017, 15, 1598–1605. [Google Scholar] [CrossRef]
- Fulkerson, C.M.; Knapp, D.W. Management of transitional cell carcinoma of the urinary bladder in dogs: A review. Vet. J. 2015, 205, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Peralta, S.; Webb, S.M.; Katt, W.P.; Grenier, J.K.; Duhamel, G.E. Highly recurrent BRAF p.V595E mutation in canine papillary oral squamous cell carcinoma. Vet. Comp. Oncol. 2023, 21, 138–144. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Rossman, P.; Zabka, T.S.; Ruple, A.; Tuerck, D.; Ramos-Vara, J.A.; Liu, L.; Mohallem, R.; Merchant, M.; Franco, J.; Fulkerson, C.M.; et al. Phase I/II Trial of Vemurafenib in Dogs with Naturally Occurring, BRAF-mutated Urothelial Carcinoma. Mol. Cancer Ther. 2021, 20, 2177–2188. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, D.H.; Moon, J.-S.; Han, H.-J.; Bae, K.; Yoon, K.-A. Longitudinal assessment of B-RAF V595E levels in the peripheral cell-free tumor DNA of a 10-year-old spayed female Korean Jindo dog with unresectable metastatic urethral transitional cell carcinoma for monitoring the treatment response to a RAF inhibitor (sorafenib). Vet. Q. 2021, 41, 153–162. [Google Scholar] [CrossRef]
- Feller, J.K.; Yang, S.; Mahalingam, M. Immunohistochemistry with a mutation-specific monoclonal antibody as a screening tool for the BRAFV600E mutational status in primary cutaneous malignant melanoma. Mod. Pathol. 2013, 26, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Adackapara, C.A.; Sholl, L.M.; Barletta, J.A.; Hornick, J.L. Immunohistochemistry using the BRAF V600E mutation-specific monoclonal antibody VE1 is not a useful surrogate for genotyping in colorectal adenocarcinoma. Histopathology 2013, 63, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Sperveslage, J.; Gierke, M.; Capper, D.; Honegger, J.; Sipos, B.; Beschorner, R.; Schittenhelm, J. VE1 immunohistochemistry in pituitary adenomas is not associated with BRAF V600E mutation. Acta Neuropathol. 2013, 125, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Aeschlimann, L.; Kehl, A.; Guscetti, F.; Posthaus, C.; Aupperle-Lellbach, H.; Rottenberg, S.; de Brot, S. Effective detection of BRAF(V595E) mutation in canine urothelial and prostate carcinomas using immunohistochemistry. Vet. Comp. Oncol. 2024, 22, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Anti-BRAF (Mutated V600E) Antibody [VE1] (ab228461)|Abcam. Available online: https://www.abcam.com/en-us/products/primary-antibodies/braf-mutated-v600e-antibody-ve1-ab228461# (accessed on 22 September 2024).
- Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons: Ames, IA, USA, 2017; ISBN 9780813821795. [Google Scholar]
- Palmieri, C.; Foster, R.A.; Grieco, V.; Fonseca-Alves, C.E.; Wood, G.A.; Culp, W.T.N.; Murua Escobar, H.; de Marzo, A.M.; Laufer-Amorim, R. Histopathological Terminology Standards for the Reporting of Prostatic Epithelial Lesions in Dogs. J. Comp. Pathol. 2019, 171, 30–37. [Google Scholar] [CrossRef]
- Jawhar, N.M.T. Tissue Microarray: A rapidly evolving diagnostic and research tool. Ann. Saudi Med. 2009, 29, 123–127. [Google Scholar] [CrossRef]
- ROCHE—eLabDoc. Available online: https://elabdoc-prod.roche.com/eLD/web/global/en/products/RTD001191 (accessed on 22 September 2024).
- Torlakovic, E.E.; Francis, G.; Garratt, J.; Gilks, B.; Hyjek, E.; Ibrahim, M.; Miller, R.; Nielsen, S.; Petcu, E.B.; Swanson, P.E.; et al. Standardization of negative controls in diagnostic immunohistochemistry: Recommendations from the international ad hoc expert panel. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 241–252. [Google Scholar] [CrossRef]
- Mochizuki, H.; Shapiro, S.G.; Breen, M. Detection of BRAF Mutation in Urine DNA as a Molecular Diagnostic for Canine Urothelial and Prostatic Carcinoma. PLoS ONE 2015, 10, e0144170. [Google Scholar] [CrossRef]
- Podolski, A.; Castellucci, E.; Halmos, B. Precision medicine: BRAF mutations in thyroid cancer. Precis. Cancer Med. 2019, 2, 29. [Google Scholar] [CrossRef]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef]
- Djanani, A.; Eller, S.; Öfner, D.; Troppmair, J.; Maglione, M. The Role of BRAF in Metastatic Colorectal Carcinoma–Past, Present, and Future. Int. J. Mol. Sci. 2020, 21, 9001. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Licchetta, A.; Memeo, R.; Argentiero, A.; Solimando, A.G.; Longo, V.; Delcuratolo, S.; Brunetti, O. Role of BRAF in Hepatocellular Carcinoma: A Rationale for Future Targeted Cancer Therapies. Medicina 2019, 55, 754. [Google Scholar] [CrossRef] [PubMed]
- Jafarian, A.H.; Mirshekar Nasirabadi, K.; Etemad, S.; Jafaripour, M.; Darijani, M.; Sheikhi, M.; Ayatollahi, H.; Shakeri, S.; Shams, S.F.; Davari, S. Molecular Status of BRAF Mutation in Prostate Adenocarcinoma: The Analysis of 100 Cases in North-East of IRAN. Iran. J. Pathol. 2018, 13, 415–421. [Google Scholar] [PubMed]
- Kuroki, K.; Hoang, C.T.; Rogic, A.M.; Rindt, H.; Simenson, A.; Noall, L.G.; Bryan, J.N.; Johnson, G.C.; Chu, S. Hotspot Exon 15 Mutations in BRAF Are Uncommon in Feline Tumours. Vet. Comp. Oncol. 2024, 22, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.K.; Takahashi, N.; Kato, S.; Hashimoto, Y.; Goto-Koshino, Y.; Uchida, K. Diagnostic challenge in veterinary pathology: Detection of BRAFV595E mutation in a dog with follicular cystitis and flat urothelial lesion with atypia. Vet. Pathol. 2024, 61, 335–338. [Google Scholar] [CrossRef]
- Engel, K.B.; Moore, H.M. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch. Pathol. Lab. Med. 2011, 135, 537–543. [Google Scholar] [CrossRef]
- Gohar, F.; Gohar, A.; Hülskamp, G.; Debus, O. The Translational Medicine Professional: A Bridge Between Bench and Bedside? Front. Med. 2018, 5, 294. [Google Scholar] [CrossRef]
- Gentilini, F.; Palgrave, C.J.; Neta, M.; Tornago, R.; Furlanello, T.; McKay, J.S.; Sacchini, F.; Turba, M.E. Validation of a Liquid Biopsy Protocol for Canine BRAFV595E Variant Detection in Dog Urine and Its Evaluation as a Diagnostic Test Complementary to Cytology. Front. Vet. Sci. 2022, 9, 909934. [Google Scholar] [CrossRef]
- Gibson, E.A.; Culp, W.T.N. Canine Prostate Cancer: Current Treatments and the Role of Interventional Oncology. Vet. Sci. 2024, 11, 169. [Google Scholar] [CrossRef]
- Gouda, M.A.; Subbiah, V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: From melanoma to tissue-agnostic therapy. ESMO Open 2023, 8, 100788. [Google Scholar] [CrossRef]
- Follows, G.A.; Sims, H.; Bloxham, D.M.; Zenz, T.; Hopper, M.A.; Liu, H.; Bench, A.; Wright, P.; Van’t Veer, M.B.; Scott, M.A. Rapid response of biallelic BRAF V600E mutated hairy cell leukaemia to low dose vemurafenib. Br. J. Haematol. 2013, 161, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Seghers, A.-K.; Cuyle, P.-J.; van Cutsem, E. Molecular Targeting of a BRAF Mutation in Pancreatic Ductal Adenocarcinoma: Case Report and Literature Review. Target. Oncol. 2020, 15, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, C.M.; Boehm, J.S.; Kim, S.Y.; Thomas, S.R.; Wardwell, L.; Johnson, L.A.; Emery, C.M.; Stransky, N.; Cogdill, A.P.; Barretina, J.; et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010, 468, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef]
- Jung, H.; Bae, K.; Lee, J.Y.; Kim, J.-H.; Han, H.-J.; Yoon, H.-Y.; Yoon, K.-A. Establishment of Canine Transitional Cell Carcinoma Cell Lines Harboring BRAF V595E Mutation as a Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 9151. [Google Scholar] [CrossRef]
- Foskett, A.; Manley, C.; Naramore, R.; Gordon, I.K.; Stewart, B.M.; Khanna, C. Tolerability of oral sorafenib in pet dogs with a diagnosis of cancer. Vet. Med. 2017, 8, 97–102. [Google Scholar] [CrossRef]
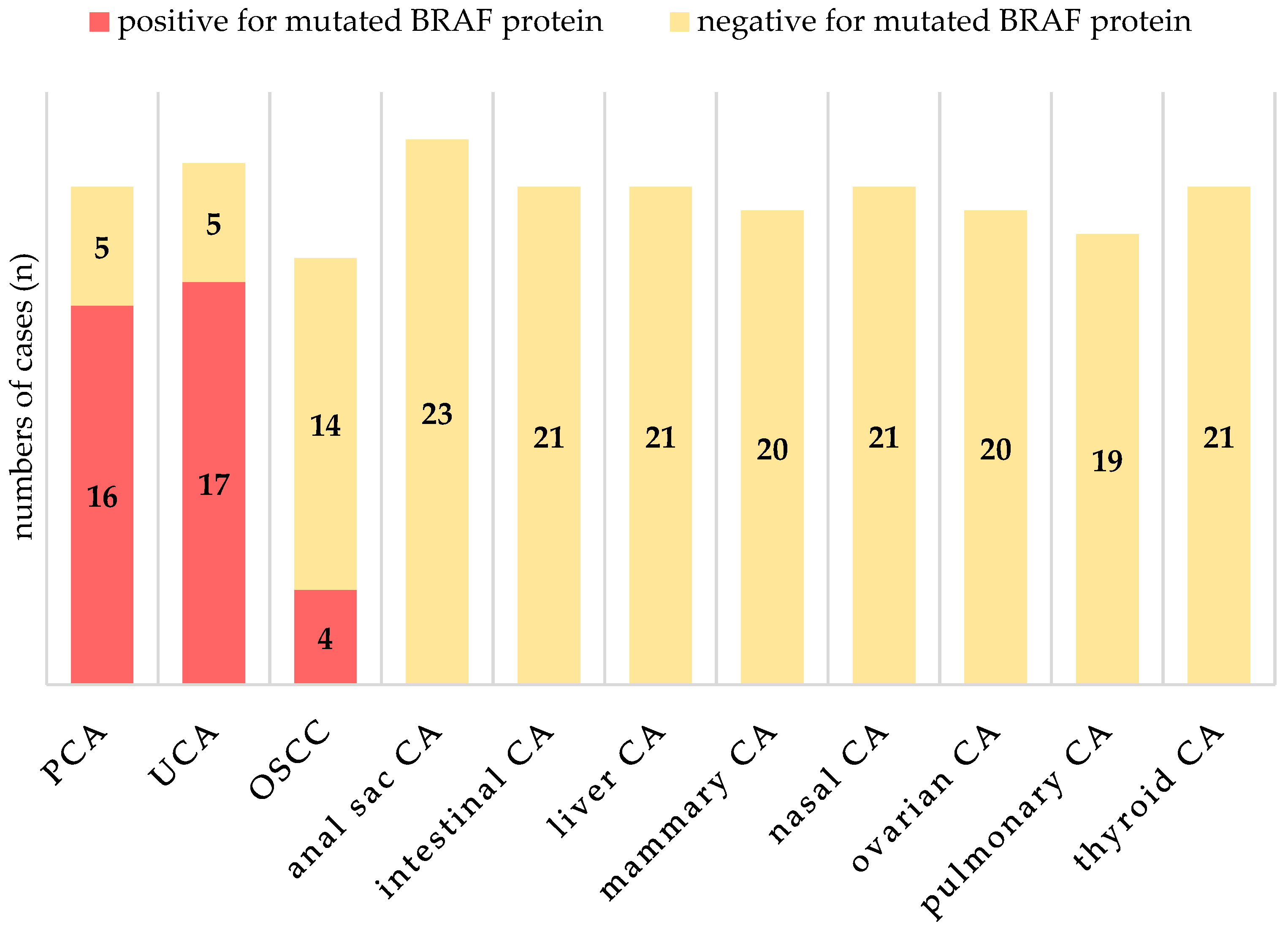


| Origin of Carcinoma | Age Range (Years) | Sex | Growth Patterns |
|---|---|---|---|
| Anal sac (n = 23) | 5–12 | 4 f, 3 fn, 5 m, 11 mn | 13 solid, 3 tubular, 7 mixed solid-tubular |
| Intestine (n = 21; 15 small intestine, 6 large intestine) | 7–14 | 9 f, 2 fn, 8 m, 2 mn | 15 tubular, 4 mucinous, 1 solid, 1 mixed solid-tubular |
| Liver (n = 21; 18 hepatocellular; 3 cholangiocarcinoma) | 7–16 | 5 f, 10 fn, 5 m, 1 mn | 9 solid, 7 trabecular, 2 clear-cell, 2 tubulopapillary, 1 mixed solid-tubular |
| Lungs (n = 19) | 6–13 | 5 f, 4 fn, 4 m, 6 mn | 11 tubulopapillary, 7 mixed solid-tubular, 1 lepidic |
| Mammary gland (n = 20) | 4–13 | 10 f, 10 fn | 11 tubulopapillary, 4 solid, 5 anaplastic, additional lymph node metastases for each case |
| Nasal cavity (n = 21) | 3–13 | 6 f, 3 fn, 8 m, 4 mn | 9 tubular, 7 solid, 5 mixed solid-tubular |
| Oral epithelium (n = 18) | 5–14 | 7 f, 7 fn, 1 m, 3 mn | 16 conventional, 2 papillary |
| Ovary (n = 20) | 3–14 | 13 f, 7 fn | 17 tubulopapillary, 2 solid, 1 mixed solid-tubular |
| Prostate (n = 21) | 7–13 | 5 m, 16 mn | 7 mixed solid-tubular, 4 tubulopapillary, 1 acinar, 9 prostatic urothelial |
| Thyroid gland (n = 21) | 5–15 | 7 f, 10 fn, 2 m, 2 mn | 16 mixed follicular and solid, 5 follicular |
| Urinary bladder (n = 22) | 7–14 | 6 f, 9 fn, 4 m, 3 mn | 15 papillary, 6 solid, 1 mixed solid-papillary |
| Primary Antibody | Anti-BRAF V600E | Anti-BRAF V600E |
|---|---|---|
| Clone | VE1 Mouse Monoclonal Antibody | VE1 Mouse Monoclonal Antibody |
| Manufacturer | Ventana® (Roche Diagnostics, material number: 08033706001, catalogue number: 760–5095) | Abcam® (ref. ab228461) |
| Isotype | IgG2a | IgG2b |
| Dilution | Diluted in buffer (pH 7.3) with carrier protein Brij 35 and the preservative 0.05% Pro Clin 300 | Diluted in buffer (pH 7.5) with carrier protein Tris and the preservative 0.1% Sodium azide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartel, A.; Aupperle-Lellbach, H.; Kehl, A.; Weidle, S.; Aeschlimann, L.; Klopfleisch, R.; Brot, S.d. Expression of Mutated BRAFV595E Kinase in Canine Carcinomas—An Immunohistochemical Study. Vet. Sci. 2024, 11, 584. https://doi.org/10.3390/vetsci11110584
Bartel A, Aupperle-Lellbach H, Kehl A, Weidle S, Aeschlimann L, Klopfleisch R, Brot Sd. Expression of Mutated BRAFV595E Kinase in Canine Carcinomas—An Immunohistochemical Study. Veterinary Sciences. 2024; 11(11):584. https://doi.org/10.3390/vetsci11110584
Chicago/Turabian StyleBartel, Annika, Heike Aupperle-Lellbach, Alexandra Kehl, Silvia Weidle, Leonore Aeschlimann, Robert Klopfleisch, and Simone de Brot. 2024. "Expression of Mutated BRAFV595E Kinase in Canine Carcinomas—An Immunohistochemical Study" Veterinary Sciences 11, no. 11: 584. https://doi.org/10.3390/vetsci11110584
APA StyleBartel, A., Aupperle-Lellbach, H., Kehl, A., Weidle, S., Aeschlimann, L., Klopfleisch, R., & Brot, S. d. (2024). Expression of Mutated BRAFV595E Kinase in Canine Carcinomas—An Immunohistochemical Study. Veterinary Sciences, 11(11), 584. https://doi.org/10.3390/vetsci11110584







