Interaction Network Characterization of Infectious Bronchitis Virus Nsp2 with Host Proteins
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Strains, and Antibodies
2.2. Plasmids
2.3. Construction and Identification of a cDNA Library from Chicken Kidney Tissue
2.4. Yeast Two-Hybrid Screening for Nsp2 Interacting Proteins
2.5. Molecular Docking
2.6. Confocal Laser Scanning Microscopy Assay
2.7. Co-Immunoprecipitation (Co-IP)
2.8. Bioinformatic Analysis of Potential Protein Functions
2.9. siRNAs and Transfection
2.10. Quantitative Real-Time PCR (qRT-PCR)
2.11. Statistical Analysis
3. Results
3.1. cDNA Library Construction and Quality Assessment
3.2. Yeast Two-Hybrid Screen
3.3. Docking Analysis and Prediction of the Interaction Between Nsp2 and Host Proteins
3.4. Nsp2 Interacts with ATP1B3/DNAJA1/ISCA1 in Cells
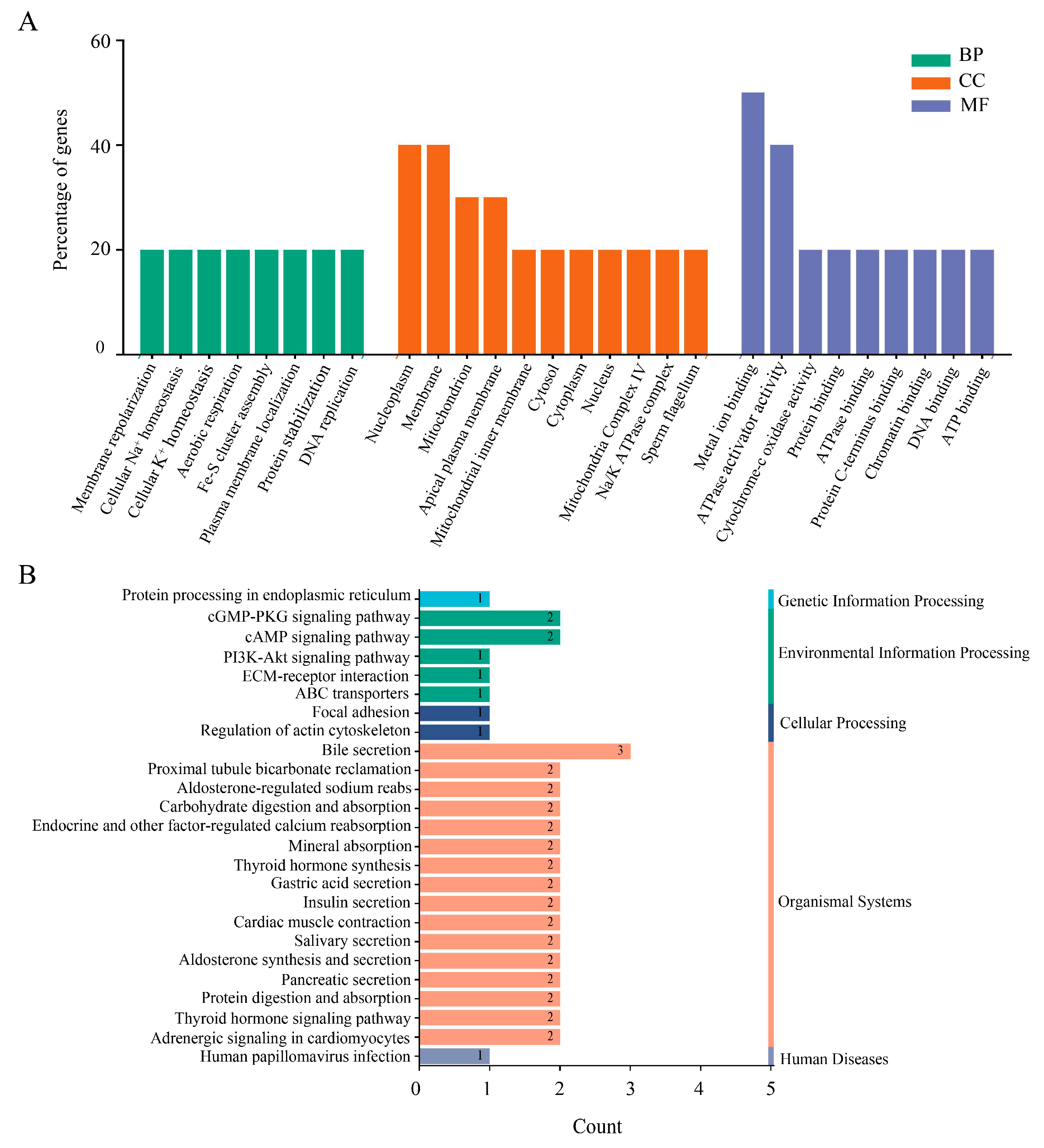
3.5. GO and KEGG Pathway Analysis of Nsp2-Interacting Proteins
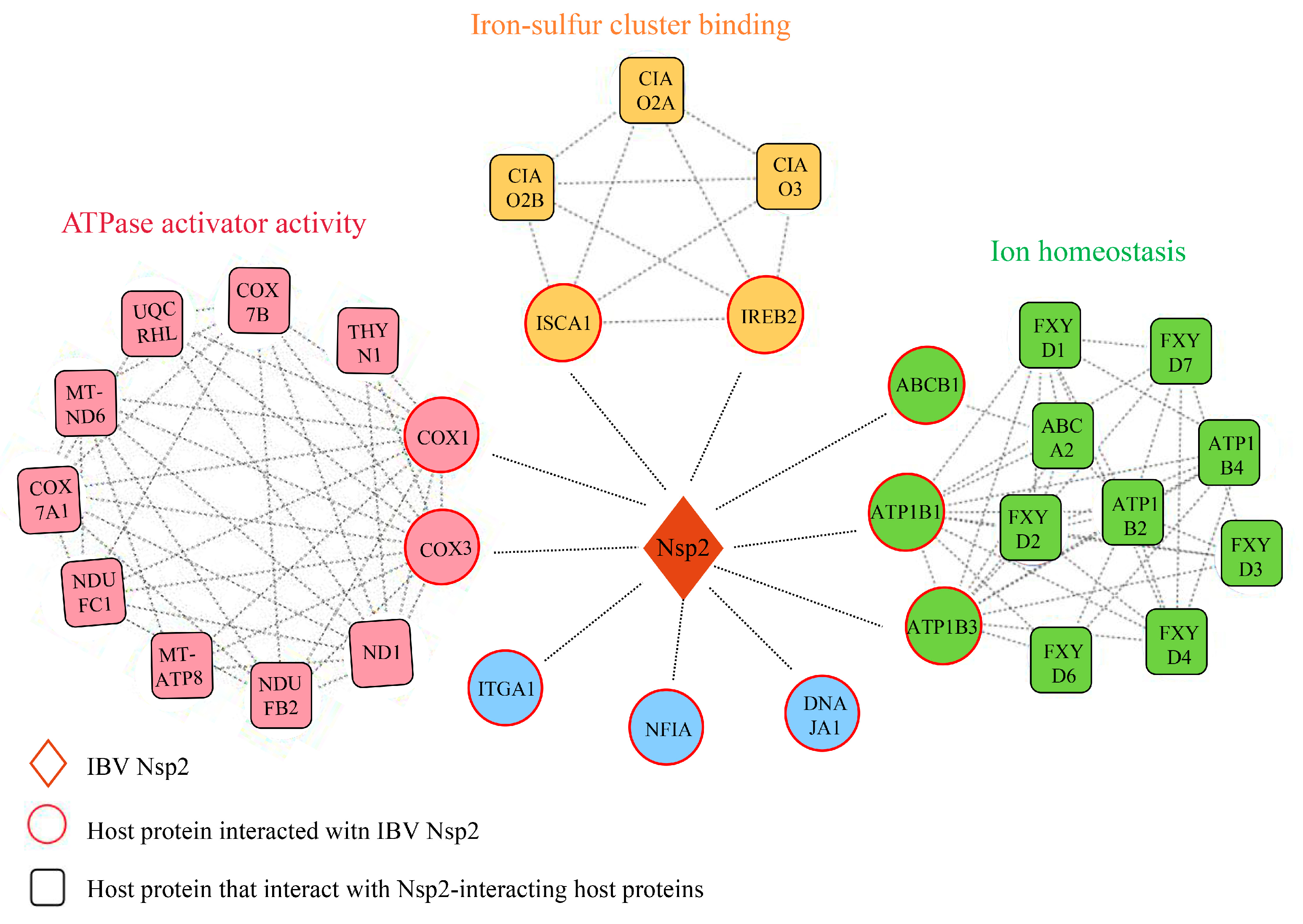
3.6. Construction of the Nsp2-Host Protein Interaction Network
3.7. IBV Upregulates DNAJA1 to Facilitate Viral Replication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Najimudeen, S.M.; Hassan, M.S.H.; Cork, S.C.; Abdul-Careem, M.F. Infectious Bronchitis Coronavirus Infection in Chickens: Multiple System Disease with Immune Suppression. Pathogens 2020, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wang, X.; Liu, Z.; Bo, Z.; Zhang, C.; Guo, M.; Zhang, X.; Wu, Y. QX-type infectious bronchitis virus infection in roosters can seriously injure the reproductive system and cause sex hormone secretion disorder. Virulence 2023, 14, 2185380. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, B.H.; Jukneliene, D.; Kanjanahaluethai, A.; Bechill, J.; Severson, K.M.; Smith, C.M.; Rota, P.A.; Baker, S.C. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J. Virol. 2004, 78, 13600–13612. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; van Geelen, A.; Buckley, A.C.; O’Brien, A.; Pillatzki, A.; Lager, K.M.; Faaberg, K.S.; Baker, S.C. Coronavirus Endoribonuclease Activity in Porcine Epidemic Diarrhea Virus Suppresses Type I and Type III Interferon Responses. J. Virol. 2019, 93, e02000-18. [Google Scholar] [CrossRef] [PubMed]
- Hagemeijer, M.C.; Verheije, M.H.; Ulasli, M.; Shaltiel, I.A.; de Vries, L.A.; Reggiori, F.; Rottier, P.J.; de Haan, C.A. Dynamics of coronavirus replication-transcription complexes. J. Virol. 2010, 84, 2134–2149. [Google Scholar] [CrossRef]
- Ogando, N.S.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Bredenbeek, P.J.; Posthuma, C.C.; Snijder, E.J. The Enzymatic Activity of the nsp14 Exoribonuclease Is Critical for Replication of MERS-CoV and SARS-CoV-2. J. Virol. 2020, 94, e01246-20. [Google Scholar] [CrossRef]
- Wang, M.; Bo, Z.; Zhang, C.; Guo, M.; Wu, Y.; Zhang, X. Deciphering the Genetic Variation: A Comparative Analysis of Parental and Attenuated Strains of the QXL87 Vaccine for Infectious Bronchitis. Animals 2024, 14, 1784. [Google Scholar] [CrossRef]
- Graham, R.L.; Sims, A.C.; Baric, R.S.; Denison, M.R. The nsp2 proteins of mouse hepatitis virus and SARS coronavirus are dispensable for viral replication. Adv. Exp. Med. Biol. 2006, 581, 67–72. [Google Scholar] [CrossRef]
- von Brunn, A.; Teepe, C.; Simpson, J.C.; Pepperkok, R.; Friedel, C.C.; Zimmer, R.; Roberts, R.; Baric, R.; Haas, J. Analysis of intraviral protein-protein interactions of the SARS coronavirus ORFeome. PLoS ONE 2007, 2, e459. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Wu, W.; Chen, Z. Structure and Function of N-Terminal Zinc Finger Domain of SARS-CoV-2 NSP2. Virol. Sin. 2021, 36, 1104–1112. [Google Scholar] [CrossRef]
- Xu, Z.; Choi, J.H.; Dai, D.L.; Luo, J.; Ladak, R.J.; Li, Q.; Wang, Y.; Zhang, C.; Wiebe, S.; Liu, A.C.H.; et al. SARS-CoV-2 impairs interferon production via NSP2-induced repression of mRNA translation. Proc. Natl. Acad. Sci. USA 2022, 119, e2204539119. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhao, P.; Xu, L.D.; Yu, J.Q.; Cai, H.L.; Zhang, C.; Tong, C.; Yang, Y.L.; Xu, P.; Sun, Q.; et al. Enteric coronavirus nsp2 is a virulence determinant that recruits NBR1 for autophagic targeting of TBK1 to diminish the innate immune response. Autophagy 2024, 20, 1762–1779. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Wei, L.; Zhao, W.; Xu, Y.; Rao, Z. Expression, crystallization and preliminary X-ray diffraction analysis of the N-terminal domain of nsp2 from avian infectious bronchitis virus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Ming, Z.; Li, Y.; Chen, C.; Bao, Z.; Ren, Z.; Liu, B.; Tao, W.; Rao, Z.; Lou, Z. Purification, crystallization and preliminary X-ray analysis of nonstructural protein 2 (nsp2) from avian infectious bronchitis virus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liao, Y.; Yap, P.L.; Png, K.J.; Tam, J.P.; Liu, D.X. Inhibition of protein kinase R activation and upregulation of GADD34 expression play a synergistic role in facilitating coronavirus replication by maintaining de novo protein synthesis in virus-infected cells. J. Virol. 2009, 83, 12462–12472. [Google Scholar] [CrossRef]
- V’kovski, P.; Gerber, M.; Kelly, J.; Pfaender, S.; Ebert, N.; Braga Lagache, S.; Simillion, C.; Portmann, J.; Stalder, H.; Gaschen, V.; et al. Determination of host proteins composing the microenvironment of coronavirus replicase complexes by proximity-labeling. eLife 2019, 11, e42037. [Google Scholar] [CrossRef]
- Cornillez-Ty, C.; Liao, L.; Yates, J.; Kuhn, P.; Buchmeier, M.J. Severe acute respiratory syndrome coronavirus nonstructural protein 2 interacts with a host protein complex involved in mitochondrial biogenesis and intracellular signaling. J. Virol. 2009, 83, 10314–10318. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Wang, L.; Kong, W.S.; Chen, H.; Wang, X.N.; Meng, Q.; Zhang, H.N.; Guo, S.J.; Jiang, H.W.; Tao, S.C. Nsp2 has the potential to be a drug target revealed by global identification of SARS-CoV-2 Nsp2-interacting proteins. Acta Biochim. Biophys. Sin. 2021, 53, 1134–1141. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Krogan, N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Daniloski, Z.; Jordan, T.X.; Wessels, H.H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2021, 184, 92–105.e16. [Google Scholar] [CrossRef]
- Hoenen, T.; Groseth, A. Virus-Host Cell Interactions. Cells 2022, 11, 804. [Google Scholar] [CrossRef]
- Walhout, A.J.; Vidal, M. High-throughput yeast two-hybrid assays for large-scale protein interaction mapping. Methods 2001, 24, 297–306. [Google Scholar] [CrossRef]
- Pfefferle, S.; Schöpf, J.; Kögl, M.; Friedel, C.C.; Müller, M.A.; Carbajo-Lozoya, J.; Stellberger, T.; von Dall’Armi, E.; Herzog, P.; Kallies, S.; et al. The SARS-coronavirus-host interactome: Identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011, 7, e1002331. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chen, W.N.; Poon, K.M.; Zheng, B.J.; Lin, X.; Wang, Y.X.; Wen, Y.M. Interaction between SARS-CoV helicase and a multifunctional cellular protein (Ddx5) revealed by yeast and mammalian cell two-hybrid systems. Arch. Virol. 2009, 154, 507–512. [Google Scholar] [CrossRef]
- Yu, X.; Chen, S.; Hou, P.; Wang, M.; Chen, Y.; Guo, D. VHL negatively regulates SARS coronavirus replication by modulating nsp16 ubiquitination and stability. Biochem. Biophys. Res. Commun. 2015, 459, 270–276. [Google Scholar] [CrossRef]
- Li, S.W.; Wang, C.Y.; Jou, Y.J.; Yang, T.C.; Huang, S.H.; Wan, L.; Lin, Y.J.; Lin, C.W. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-β1 via ROS/p38 MAPK/STAT3 pathway. Sci. Rep. 2016, 6, 25754. [Google Scholar] [CrossRef]
- Ott, M.; Amunts, A.; Brown, A. Organization and Regulation of Mitochondrial Protein Synthesis. Annu. Rev. Biochem. 2016, 85, 77–101. [Google Scholar] [CrossRef]
- Hoque, M.N.; Sarkar, M.M.H.; Khan, M.A.; Hossain, M.A.; Hasan, M.I.; Rahman, M.H.; Habib, M.A.; Akter, S.; Banu, T.A.; Goswami, B.; et al. Differential gene expression profiling reveals potential biomarkers and pharmacological compounds against SARS-CoV-2: Insights from machine learning and bioinformatics approaches. Front. Immunol. 2022, 13, 918692. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Jing, L.; Ma, R.; Liu, X.; Gao, L.; Cao, L.; Duan, J.; Zhou, X.; Li, Y.; et al. Mitochondrial dysfunction, perturbations of mitochondrial dynamics and biogenesis involved in endothelial injury induced by silica nanoparticles. Environ. Pollut. 2018, 236, 926–936. [Google Scholar] [CrossRef]
- Gotoh, T.; Terada, K.; Oyadomari, S.; Mori, M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004, 11, 390–402. [Google Scholar] [CrossRef]
- Beilschmidt, L.K.; Ollagnier de Choudens, S.; Fournier, M.; Sanakis, I.; Hograindleur, M.A.; Clémancey, M.; Blondin, G.; Schmucker, S.; Eisenmann, A.; Weiss, A.; et al. ISCA1 is essential for mitochondrial Fe4S4 biogenesis in vivo. Nat. Commun. 2017, 8, 15124. [Google Scholar] [CrossRef]
- Beilschmidt, L.K.; Puccio, H.M. Mammalian Fe-S cluster biogenesis and its implication in disease. Biochimie 2014, 100, 48–60. [Google Scholar] [CrossRef]
- Pantopoulos, K.; Gray, N.K.; Hentze, M.W. Differential regulation of two related RNA-binding proteins, iron regulatory protein (IRP) and IRPB. RNA 1995, 1, 155–163. [Google Scholar]
- Bednash, J.S.; Kagan, V.E.; Englert, J.A.; Farkas, D.; Tyurina, Y.Y.; Tyurin, V.A.; Samovich, S.N.; Farkas, L.; Elhance, A.; Johns, F.; et al. Syrian hamsters as a model of lung injury with SARS-CoV-2 infection: Pathologic, physiologic, and detailed molecular profiling. Transl. Res. 2022, 240, 1–16. [Google Scholar] [CrossRef]
- Liu, L.; Du, J.; Yang, S.; Zheng, B.; Shen, J.; Huang, J.; Cao, L.; Huang, S.; Liu, X.; Guo, L.; et al. SARS-CoV-2 ORF3a sensitizes cells to ferroptosis via Keap1-NRF2 axis. Redox Biol. 2023, 63, 102752. [Google Scholar] [CrossRef]
- Xia, H.; Wu, Y.; Zhao, J.; Cheng, C.; Lin, J.; Yang, Y.; Lu, L.; Xiang, Q.; Bian, T.; Liu, Q. N6-Methyladenosine-modified circSAV1 triggers ferroptosis in COPD through recruiting YTHDF1 to facilitate the translation of IREB2. Cell Death Differ. 2023, 30, 1293–1304. [Google Scholar] [CrossRef]
- Kaplan, J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef]
- Cui, X.; Sun, Z.R.; Ren, G.W.; Wang, G.L.; Qi, Y.; Ma, Y.P.; Ruan, Q. Interaction between human cytomegalovirus UL136 protein and ATP1B1 protein. Braz. J. Med. Biol. Res. 2011, 44, 1251–1255. [Google Scholar] [CrossRef]
- Margier, M.; Le May, C.; Antoine, T.; Halimi, C.; Nowicki, M.; Lespine, A.; Reboul, E. P-glycoprotein (ABCB1) is involved in vitamin K efflux. Food Chem. 2021, 343, 128510. [Google Scholar] [CrossRef]
- Assad, M.; Parveen, Z.; Farman, S.; Khurshid, B.; Hashmi, M.A.; Khan, K.M.; Khurshid, A. In Vitro Screening and MD Simulations of Thiourea Derivatives against SARS-CoV-2 in Association with Multidrug Resistance ABCB1 Transporter. ACS Omega 2022, 7, 47671–47679. [Google Scholar] [CrossRef]
- Lacasse, É.; Gudimard, L.; Dubuc, I.; Gravel, A.; Allaeys, I.; Boilard, É.; Flamand, L. SARS-CoV-2 Nsp2 Contributes to Inflammation by Activating NF-κB. Viruses 2023, 15, 334. [Google Scholar] [CrossRef]
- Zhang, Y.; Gargan, S.; Roche, F.M.; Frieman, M.; Stevenson, N.J. Inhibition of the IFN-α JAK/STAT Pathway by MERS-CoV and SARS-CoV-1 Proteins in Human Epithelial Cells. Viruses 2022, 14, 667. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Zhang, X.; Wang, A.; Wang, L.; Yang, Y.; Deng, R.; Zhang, G.P. MicroRNA 373 Facilitates the Replication of Porcine Reproductive and Respiratory Syndrome Virus by Its Negative Regulation of Type I Interferon Induction. J. Virol. 2017, 91, e01311-16. [Google Scholar] [CrossRef]
- Lu, Y.; Hou, H.; Wang, F.; Qiao, L.; Wang, X.; Yu, J.; Liu, W.; Sun, Z. ATP1B3: A virus-induced host factor against EV71 replication by up-regulating the production of type-I interferons. Virology 2016, 496, 28–34. [Google Scholar] [CrossRef]
- Cao, W.; Guo, Y.; Cheng, Z.; Xu, G.; Zuo, Q.; Nie, L.; Huang, Y.; Liu, S.; Zhu, Y. Inducible ATP1B1 Upregulates Antiviral Innate Immune Responses by the Ubiquitination of TRAF3 and TRAF6. J. Immunol. 2021, 206, 2668–2681. [Google Scholar] [CrossRef]
- Billings, L.K.; Hsu, Y.H.; Ackerman, R.J.; Dupuis, J.; Voight, B.F.; Rasmussen-Torvik, L.J.; Hercberg, S.; Lathrop, M.; Barnes, D.; Langenberg, C.; et al. Impact of common variation in bone-related genes on type 2 diabetes and related traits. Diabetes 2012, 61, 2176–2186. [Google Scholar] [CrossRef]
- Gething, M.J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef]
- Cao, M.; Wei, C.; Zhao, L.; Wang, J.; Jia, Q.; Wang, X.; Jin, Q.; Deng, T. DnaJA1/Hsp40 is co-opted by influenza A virus to enhance its viral RNA polymerase activity. J. Virol. 2014, 88, 14078–14089. [Google Scholar] [CrossRef]
- Wang, R.Y.; Huang, Y.R.; Chong, K.M.; Hung, C.Y.; Ke, Z.L.; Chang, R.Y. DnaJ homolog Hdj2 facilitates Japanese encephalitis virus replication. Virol. J. 2011, 8, 471. [Google Scholar] [CrossRef]
- Dziuba, N.; Ferguson, M.R.; O’Brien, W.A.; Sanchez, A.; Prussia, A.J.; McDonald, N.J.; Friedrich, B.M.; Li, G.; Shaw, M.W.; Sheng, J.; et al. Identification of cellular proteins required for replication of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 2012, 28, 1329–1339. [Google Scholar] [CrossRef]
- Tomita, Y.; Mizuno, T.; Díez, J.; Naito, S.; Ahlquist, P.; Ishikawa, M. Mutation of host DnaJ homolog inhibits brome mosaic virus negative-strand RNA synthesis. J. Virol. 2003, 77, 2990–2997. [Google Scholar] [CrossRef]
- Sharma, K.; Tripathi, S.; Ranjan, P.; Kumar, P.; Garten, R.; Deyde, V.; Katz, J.M.; Cox, N.J.; Lal, R.B.; Sambhara, S.; et al. Influenza A virus nucleoprotein exploits Hsp40 to inhibit PKR activation. PLoS ONE 2011, 6, e20215. [Google Scholar] [CrossRef]





| Primer | Sequence | Size (bp) |
|---|---|---|
| Nsp2-F | 5′-ATGGCTTCAAGCCTAAAACAGG-3′ | 2025 |
| Nsp2-R | 5′-TTAACCCGCTTTGCAAATAACAT-3′ | |
| T7-F | 5′-TAATACGACTCACTATAGGGC-3′ | 200–2000 |
| AD-R | 5′-GTGAACTTGCGGGGTTTTTC-3′ | |
| ATP1B3-F | 5′-ATGAGCAAGGAGACGAAGAAG-3′ | 846 |
| ATP1B3-R | 5′-TCACTATTCAGTCATCTCAACTTTGAAGGC-3′ | |
| DNAJA1-F | 5′-ATGGTGAAGGAGACCACGTACTAC-3′ | 1194 |
| DNAJA1-R | 5′-TCATGATGTCTGACATTGAACACCT-3′ | |
| ISCA1-F | 5′-ATGGCATCGTCGGTGGTG-3′ | 388 |
| ISCA1-R | 5′-TAATGTTAAAGCTTTCTCCACA-3′ |
| Gene | Sequence | Size (bp) |
|---|---|---|
| COX3-F | 5′-TGACCAATCTTCGGCGCA-3′ | 211 |
| COX3-R | 5′-AAAGGATTATTCCGTATCGTAGGC-3′ | |
| NFIA-F | 5′-ATGTATTCTCCGCTCTGTCTC-3′ | 180 |
| NFIA-R | 5′-CAGTTCGTCCTTCACGGCTCT-3′ | |
| ITGA1-F | 5′-GAAAATGAGGAAGGAAAATGGGT-3′ | 148 |
| ITGA1-R | 5′-GCACTGAAGTAGCGTCTGGTAAAT-3′ | |
| COX1-F | 5′-CGTAGAAGCTGGGGCCGG-3′ | 230 |
| COX1-R | 5′-GGATGGCAGTAATGAGGACGGA-3′ | |
| ATP1B1-F | 5′-CTGCAAGTTCAAACGTGAGTGG-3′ | 195 |
| ATP1B1-R | 5′-CAGTGGACAGGGATGAGATAGGG-3′ | |
| ABCB1-F | 5′-GAAATACATATGAGATCGCTA-3′ | 101 |
| ABCB1-R | 5′-CGGGCTGACCATTTGAGGCT-3′ | |
| ISCA1-F | 5′-GGCATCGTCGGTGGTGCGGGC-3′ | 137 |
| ISCA1-R | 5′-CTACATGCTCAGGCTGGTCTT-3′ | |
| DNAJA1-F | 5′-TGGCACTGAAGTACCACCCC-3′ | 85 |
| DNAJA1-R | 5′-TCGGACAGCACCTCATACGC-3′ | |
| ATP1B3-F | 5′-ATGAGCAAGGAGACGAAGAAGC-3′ | 154 |
| ATP1B3-R | 5′-CCGCGAGGAAGCCATAAAAT-3′ | |
| IREB2-F | 5′-ATACAGAACGCCCCGAACCCT-3′ | 204 |
| IREB2-R | 5′-AAGGTGGAAAGGGCAGAGGA-3′ | |
| β-actin-F | 5′-CTGTGCCCATCTATGAAGGCTA-3′ | 139 |
| β-actin-R | 5′-ATTTCTCTCTCGGCTGTGGTG-3′ |
| Gene | NCBI Accession | Function |
|---|---|---|
| COX1 | QFK69789.1 | Component regarding the cytochrome c oxidase. The last enzyme in the mitochondrial electron transport chain drives oxidative phosphorylation. |
| COX3 | QFK69793.1 | |
| NFIA | XM_038183087.1 | DNA-binding transcription factor activity. |
| ITGA1 | NM_205069.1 | Involved in the anchorage-dependent, negative regulation of EGF-stimulated cell growth. |
| ATP1B1 | NM_205520.4 | Catalyzes the hydrolysis of ATP coupled with the exchange of Na+ and K+ ions across the plasma membrane. |
| ATP1B3 | NM_205535.1 | |
| ABCB1 | XM_025147038.1 | Translocate drugs and phospholipids across the membrane. |
| ISCA1 | NM_001271936.1 | Involved in the maturation of mitochondrial 4Fe-4S proteins and functioning late in the iron-sulfur cluster assembly. |
| DNAJA1 | NM_001012945 | Plays a role in protein transport into mitochondria. |
| IREB2 | NM_001031454.1 | Binding to the IRE element in ferritin results in its repression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Bo, Z.; Zhang, C.; Guo, M.; Wu, Y.; Zhang, X. Interaction Network Characterization of Infectious Bronchitis Virus Nsp2 with Host Proteins. Vet. Sci. 2024, 11, 531. https://doi.org/10.3390/vetsci11110531
Wang M, Bo Z, Zhang C, Guo M, Wu Y, Zhang X. Interaction Network Characterization of Infectious Bronchitis Virus Nsp2 with Host Proteins. Veterinary Sciences. 2024; 11(11):531. https://doi.org/10.3390/vetsci11110531
Chicago/Turabian StyleWang, Mengmeng, Zongyi Bo, Chengcheng Zhang, Mengjiao Guo, Yantao Wu, and Xiaorong Zhang. 2024. "Interaction Network Characterization of Infectious Bronchitis Virus Nsp2 with Host Proteins" Veterinary Sciences 11, no. 11: 531. https://doi.org/10.3390/vetsci11110531
APA StyleWang, M., Bo, Z., Zhang, C., Guo, M., Wu, Y., & Zhang, X. (2024). Interaction Network Characterization of Infectious Bronchitis Virus Nsp2 with Host Proteins. Veterinary Sciences, 11(11), 531. https://doi.org/10.3390/vetsci11110531





