Holothuria polii Extract as a Potential Anticoccidial Agent: Evidence of Its MUC2 Regulatory Impact in Murine Jejunum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Holothuria polii
2.2. Gas Chromatography-Mass Spectrometry (GC-MS) of HpE
2.3. Experimental Design
2.4. Number of Oocysts
2.5. Oocyst Dimentional Analysis
2.6. Number of Parasitic Stages
2.7. The Response of Goblet Cells during Eimeriosis
2.8. Oxidative Status in Jejunum
2.9. Gene Expression Analysis
2.10. Statistical Analysis
3. Results
3.1. Chemical Analysis of HpE
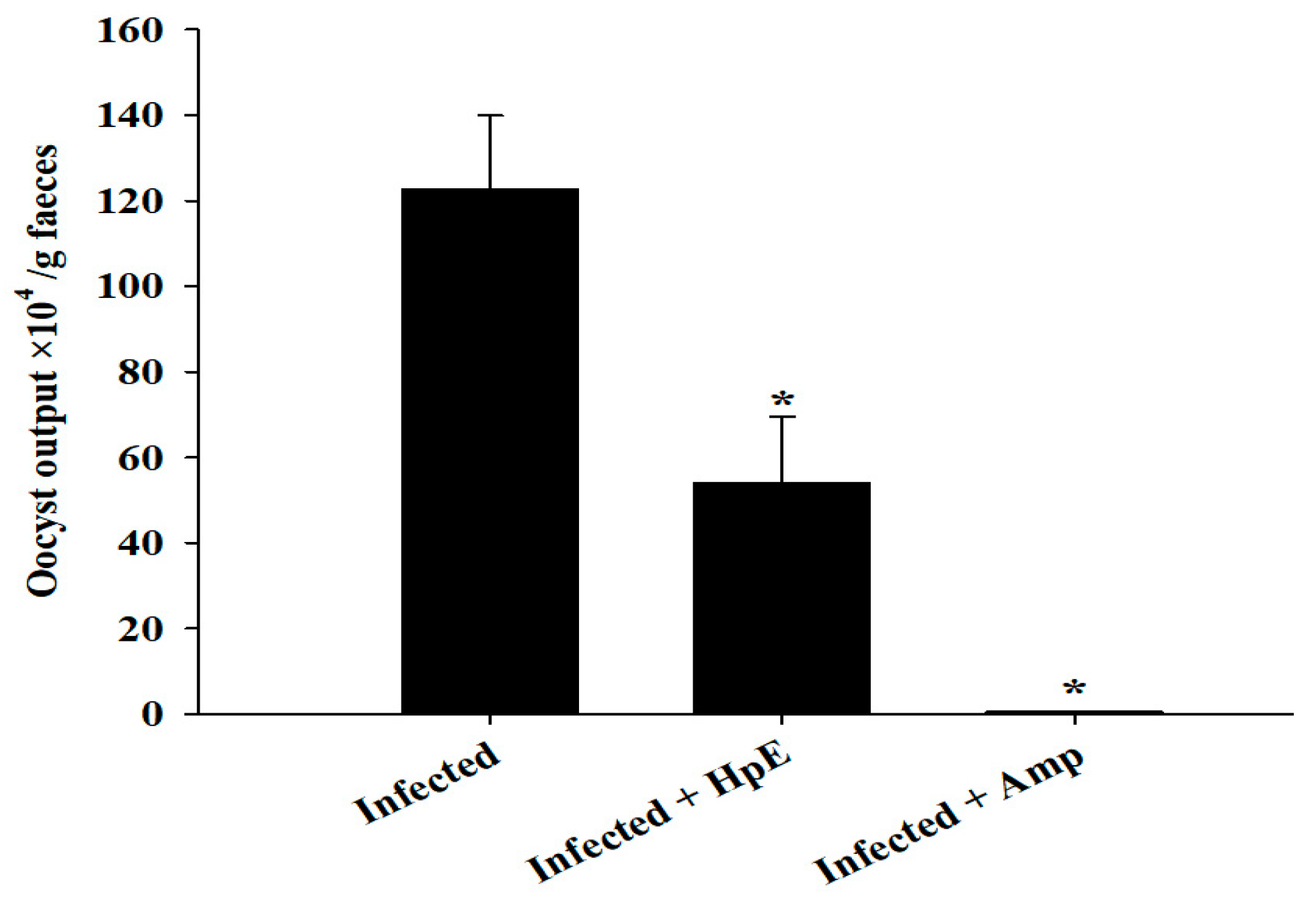
3.2. Oocyst Output
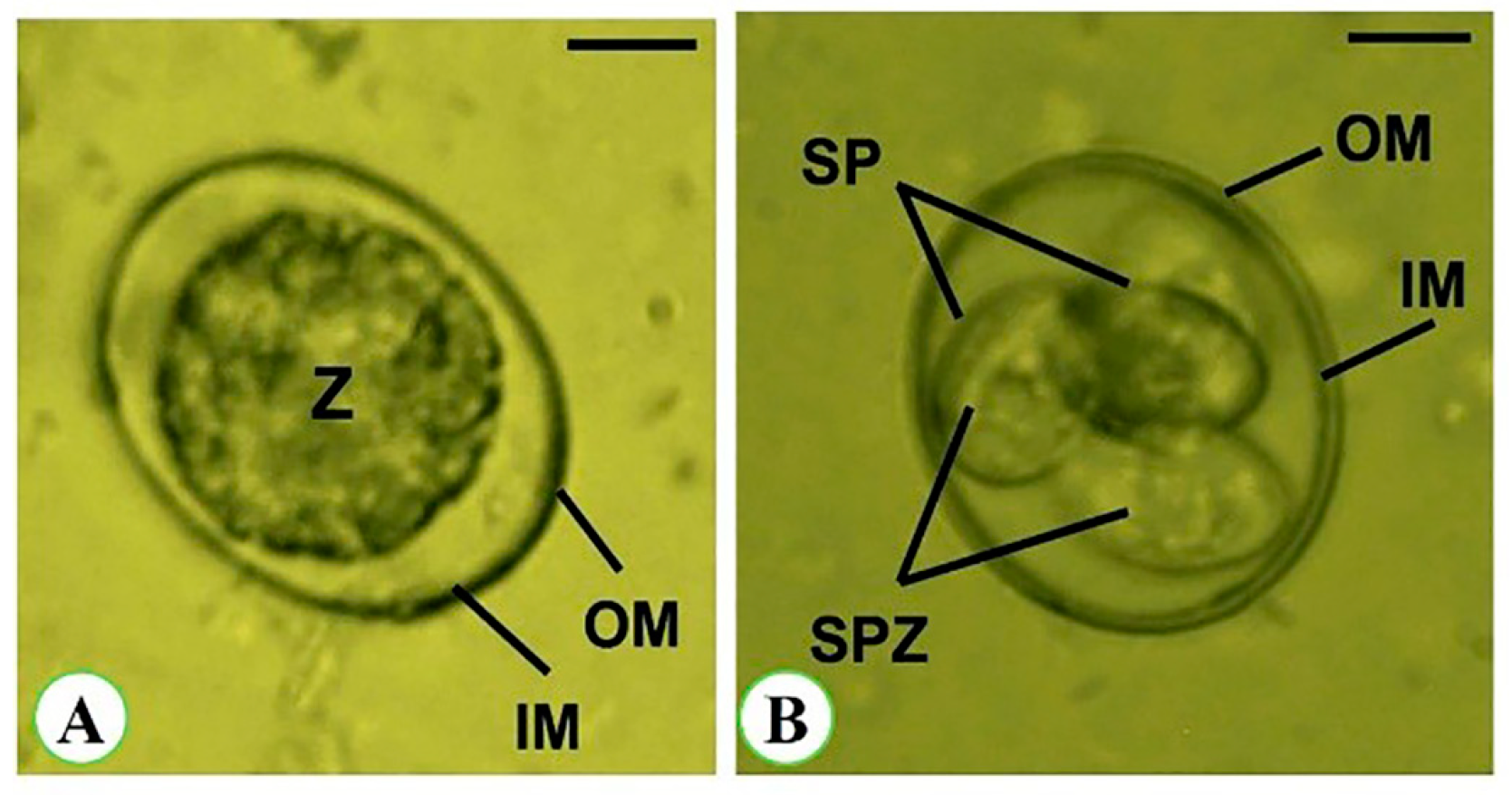
3.3. Oocyst Morphometry
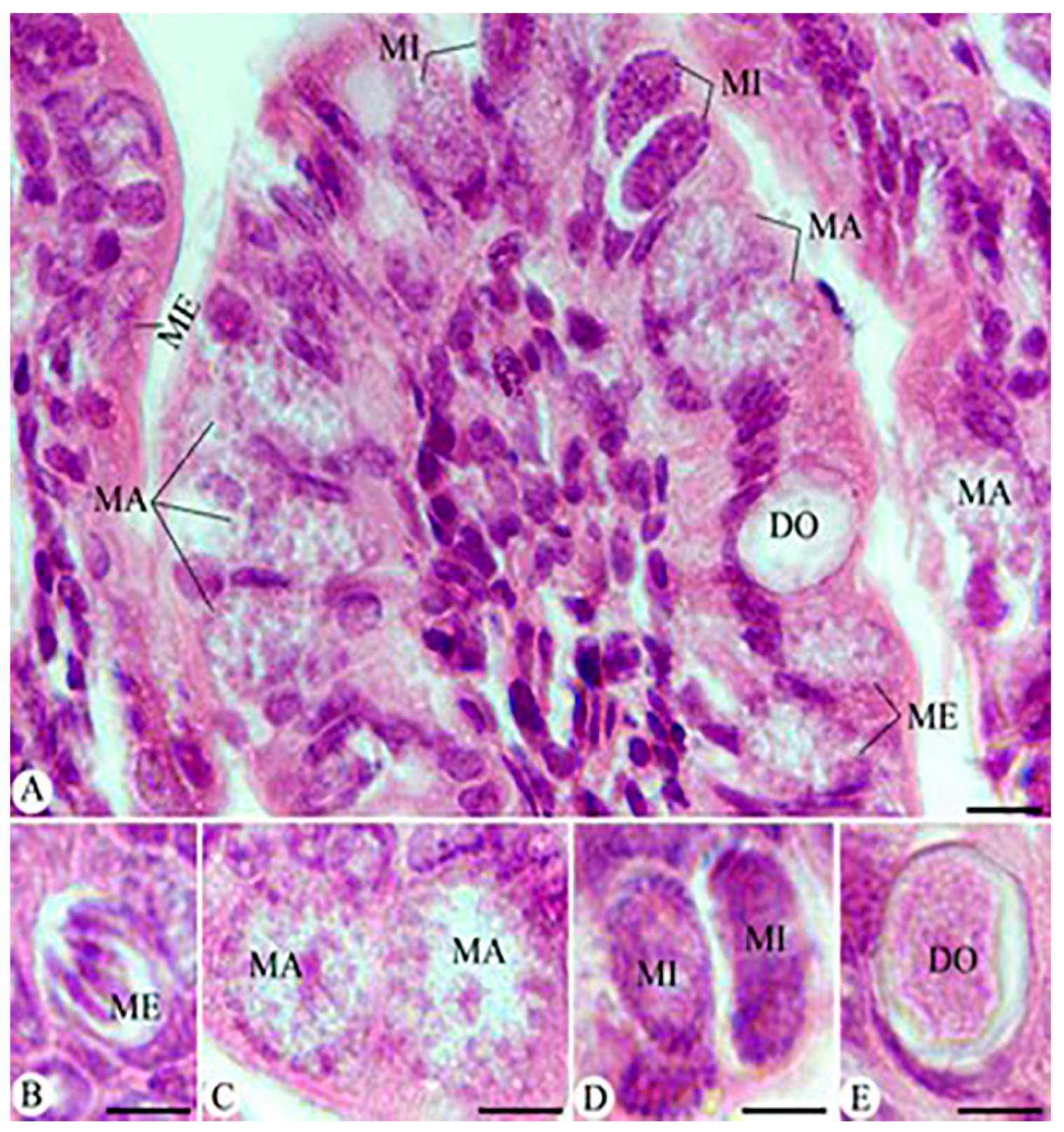
3.4. Parasitic Stages
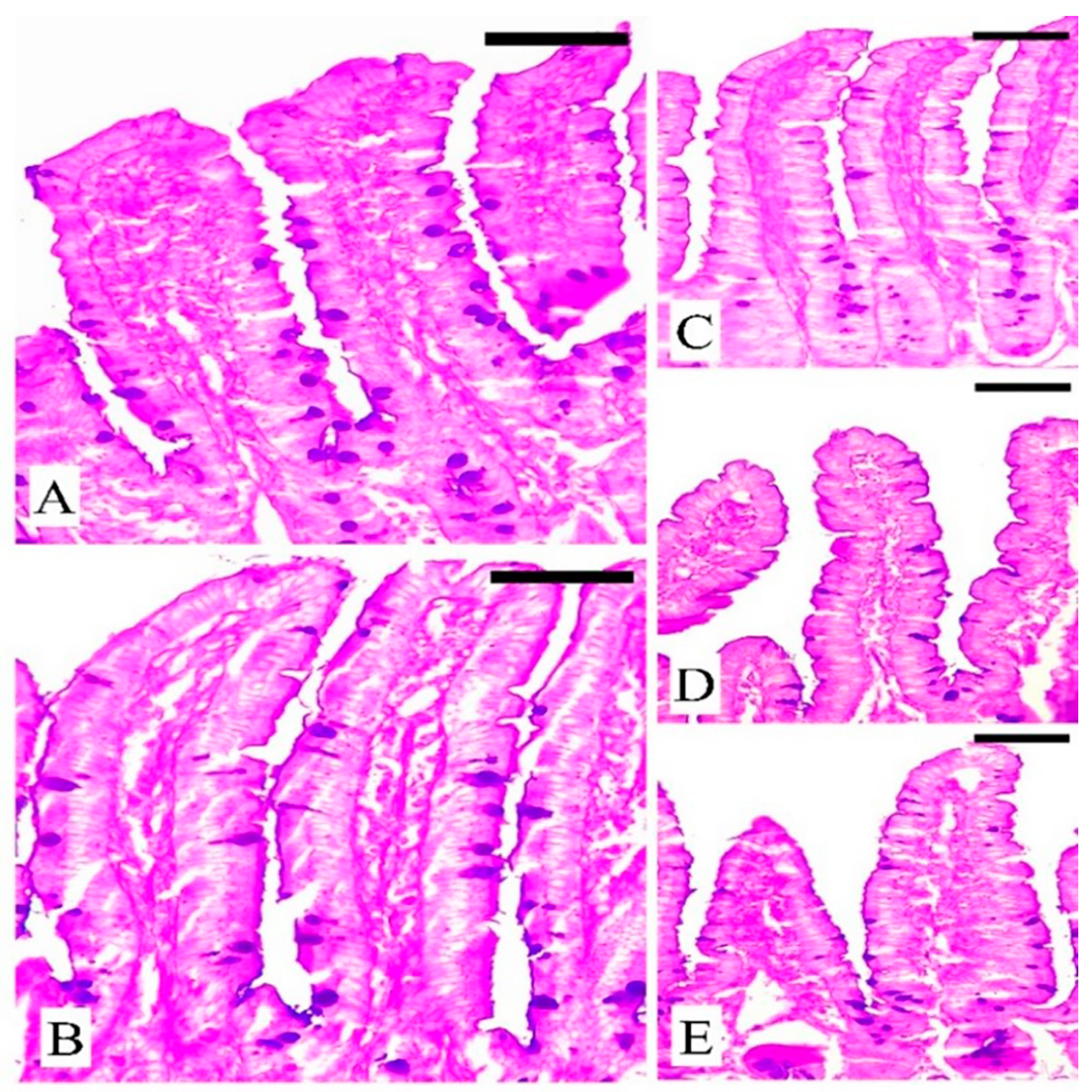
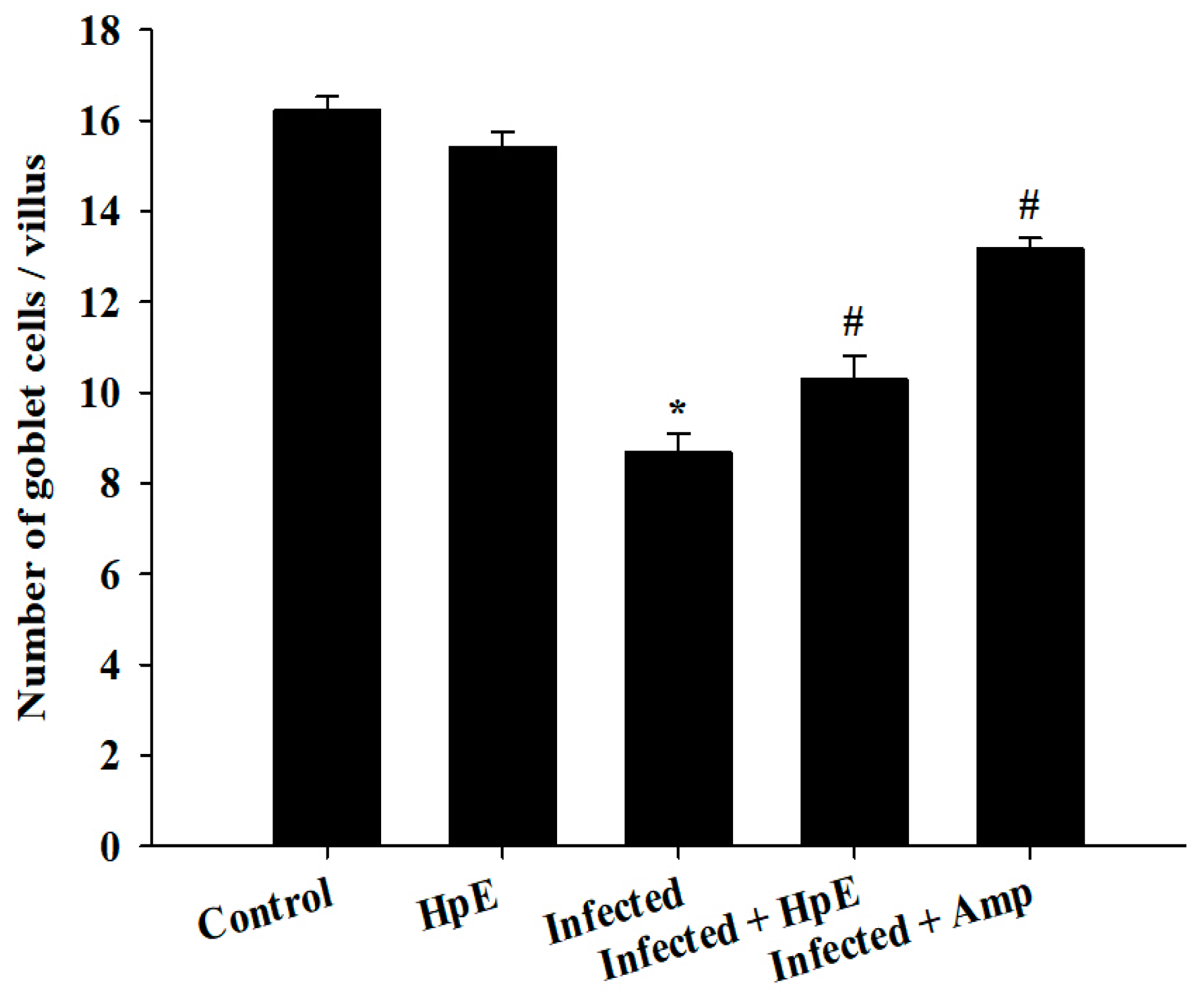
3.5. The Response of Goblet Cells (GCs) during Eimeriosis
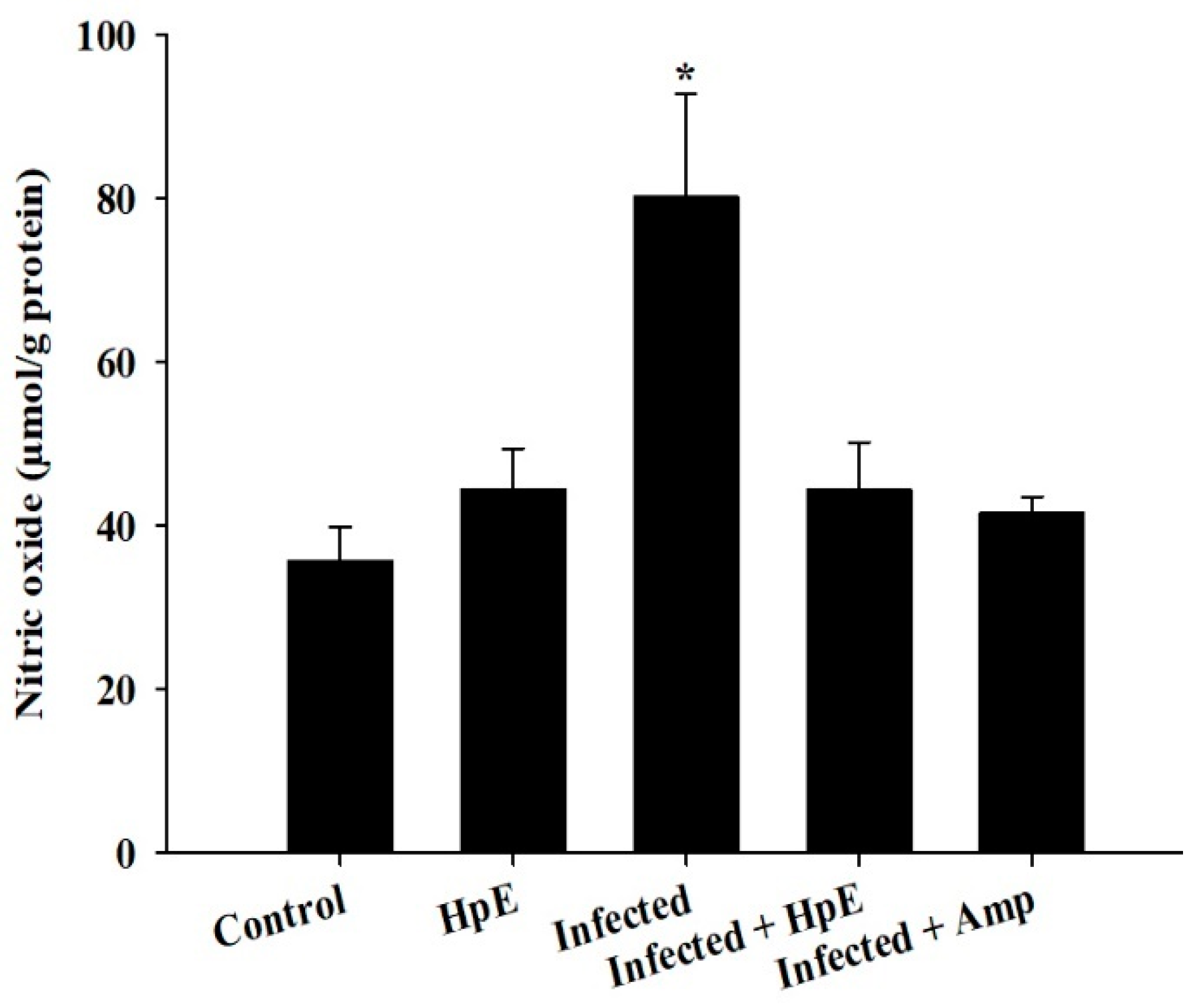
3.6. Assessment of NO Oxidative Stress Biomarkers
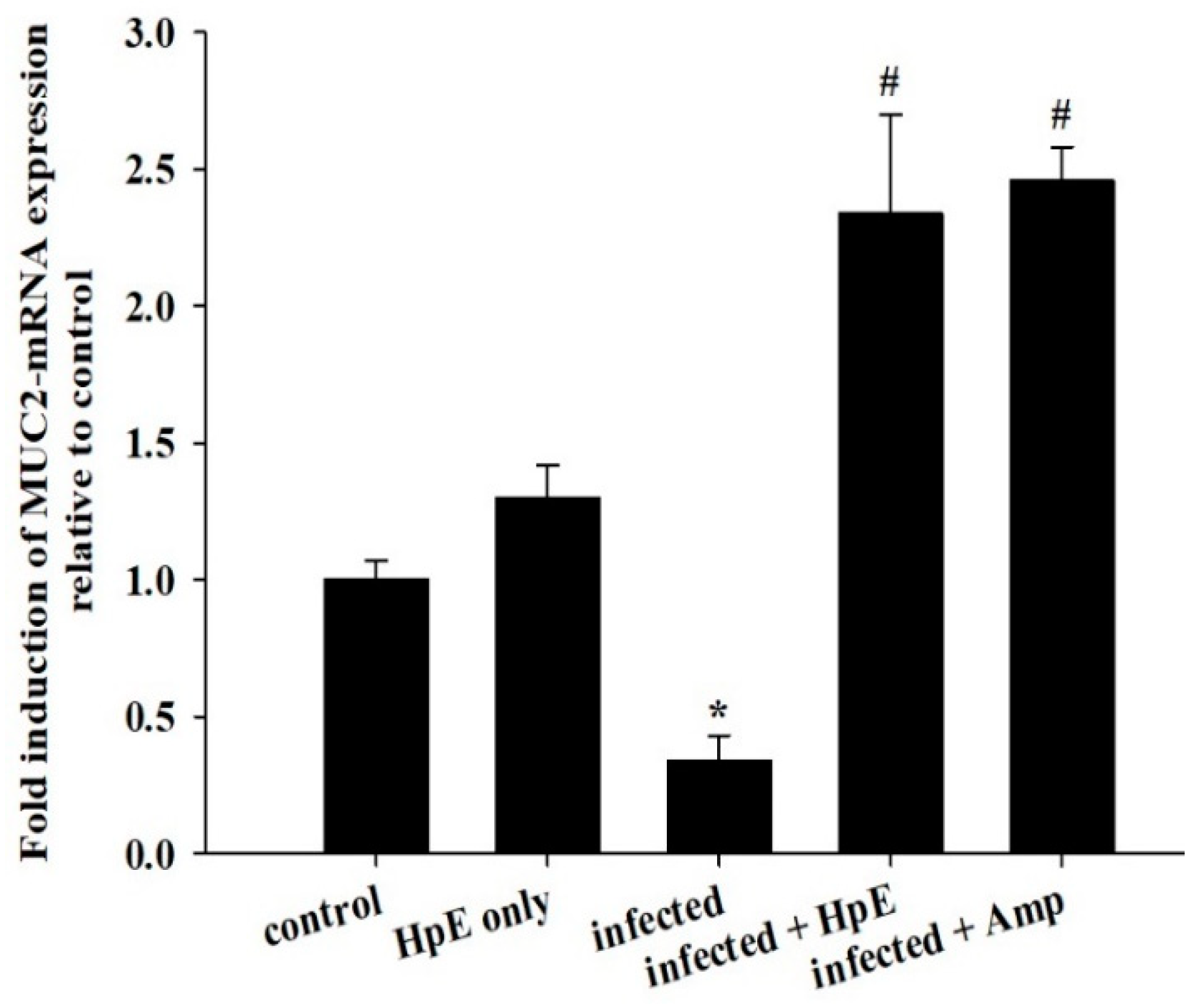
3.7. Mucin Genes Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, R.B. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken reproduction industry. Int. J. Parasitol. 1999, 29, 1209–1229. [Google Scholar] [CrossRef]
- Mehlhorn, H. (Ed.) Encyclopedic Reference of Parasitology, 4th ed.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Schito, M.L.; Barta, J.R.; Chobotar, B. Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J. Parasitol. 1996, 82, 255–262. [Google Scholar] [CrossRef]
- Lang, C.E.; Macdonald, J.R.; Reisman, D.S.; Boyd, L.; Jacobson Kimberley, T.; Schindler-Ivens, S.M.; Hornby, T.G.; Ross, S.A.; Scheets, P.L. Observation of amounts of movement practice provided during stroke rehabilitation. Arch. PM&R 2009, 90, 1692–1698. [Google Scholar]
- Amer, O.S.O.; Dkhil, M.A.; Hikal, W.M.; Al-Quraishy, S. Antioxidant and anti-inflammatory activities of Pomegranate (Punica granatum) on Eimeria papillata induced infection in mice. J. Biomed Res. Int. 2015, 1–7. [Google Scholar]
- Burrell, T.; Wood, S.L.; Cherry, N.M.; Muir, J.P.; Smith, W.B. 388 Bayou Beef: A random effects model of crustacean waste as a feedstock. J. Anim. Sci. 2019, 97, 154–155. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Thagfan, F.A.; Hassan, A.S.; Al-Shaebi, E.M.; Abdel-Gaber, R.; Al-Quraishy, S. Anthelmintic, anticoccidial and antioxidant activity of Salvadora persica root extracts. Saudi J. Biol. Sci. 2019, 26, 1223–1226. [Google Scholar]
- Amit Koparde, A.; Chandrashekar Doijad, R.; Shripal Magdum, C. Natural Products in Drug Discovery; IntechOpen: London, UK, 2019. [Google Scholar]
- Al-Otaibi, T.; Abu Hawsah, M.; Alojayri, G.; Al-Shaebi, E.M.; Dkhil, M.A.; Thagfan, F.; Elkhadragy, M.F.; Al-Quraishy, S.; Abdel-Gaber, R. Biological activities of Persea americana: In vitro and in vivo studies. J. FS&T 2023, 43, E123722. [Google Scholar]
- Alkhudhayri, A.; Al-Shaebi, E.M.; Qasem, M.A.A.; Murshed, M.; Mares, M.M.; Al-Quraishy, S.; Dkhil, M.A. Antioxidant and anti-apoptotic effects of selenium nanoparticles against murine eimeriosis. J. Anais Acad. Brasil. Ci. 2020, 92, e20191107. [Google Scholar] [CrossRef]
- Abdel-Gaber, R.; Hawsah, M.A.; Al-Otaibi, T.; Alojayri, G.; Al-Shaebi, E.M.; Mohammed, O.B.; Elkhadragy, M.F.; Al-Quraishy, S.; Dkhil, M.A. Biosynthesized selenium nanoparticles to rescue coccidiosis mediated oxidative stress, apoptosis and inflammation in the jejunum of mice. Front. Immunol. 2023, 14, 1139899. [Google Scholar] [CrossRef]
- Ndjonka, D.; Abladam, E.D.; Djafsia, B.; Ajonina-Ekoti, I.; Achukwi, M.D.; Liebau, E. Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J. Helminthol. 2014, 88, 481–488. [Google Scholar] [CrossRef]
- Toral-Granda, M.V. Requiem for the Galápagos sea cucumber fishery? SPC Beche-De-Mer Inf. Bull. 2005, 21, 5–8. [Google Scholar]
- Bahrami, Y.; Zhang, W.; Franco, C. Discovery of novel saponins from the viscera of the sea cucumber Holothuria lessoni. J. Mar. Drugs. 2014, 12, 2633–2667. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, W.H.; Hellal, A.M.; Attia, M.S.; Youssef, F.S. Sea Cucumber “Holothuria (Thymiosycia) arenicola” induced-autotomy for sustainable development in Egypt: Histological, Ultrastructure, and Chemical studies. Egypt. J. Aquatic Biol. Fish. 2022, 26, 1459–1491. [Google Scholar] [CrossRef]
- Hamed, M.A.; Abou El-Naga, E.H.; Youssef, N.M.; El-Sakka, S.S. Effect of sea cucumbers Holothuria atra extract on hematological parameters and cardio enzymes in rats. J. Egypt. Acad. Soc. Environ. 2017, 18, 11–19. [Google Scholar]
- Georgieva, N.V.; Koinarski, V.; Gadjeva, V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. J. Vet. 2006, 172, 488–492. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Delic, D.; Al-Quraishy, S. Goblet cells and mucin related gene expression in mice infected with Eimeria papillata. J. Sci. World. 2013, 3, 439–865. [Google Scholar] [CrossRef]
- Abdel-Tawab, H.; Abdel-Baki, A.S.; El-Mallah, A.M.; Al-Quraishy, S.; Abdel-Haleem, H.M. In vivo and in vitro anticoccidial efficacy of Astragalus membranaceus against Eimeria papillata infection. J. King Saud Univ. Sci. 2020, 32, 2269–2275. [Google Scholar] [CrossRef]
- Purcell, S.W.; Hair, C.A.; Mills, D.J. Sea cucumber culture, farming and sea ranching in the tropics: Progress, problems and opportunities. J. Aquac. Res. 2012, 368, 68–81. [Google Scholar] [CrossRef]
- Dakrory, A.I.; Fahmy, S.R.; Soliman, A.M.; Mohamed, A.S.; Amer, S.A.M. Protective and Curative Effects of the Sea Cucumber Holothuria atra Extract against DMBA-Induced Hepatorenal Diseases in Rats. Int. J. BioMed Res. 2015, 563652, 1–11. [Google Scholar]
- Kanthal, L.; Dey, A.; Satyavathi, K.; Bhojaraju, P. GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. J. Pharmacogn Res. 2014, 6, 58. [Google Scholar] [CrossRef]
- Murshed, M.; Aljawdah, H.M.; Mares, M.; Al-Quraishy, S. In Vitro: The Effects of the Anticoccidial Activities of Calotropis procera Leaf Extracts on Eimeria stiedae Oocysts Isolated from Rabbits. Molecules 2023, 28, 3352. [Google Scholar] [CrossRef]
- Duszynski, D.W.; Wilber, P.G. A Guideline for the Preparation of Species Descriptions in the Eimeriidae. J. Parasitol. 1997, 83, 333. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, G.A. Theory and Practice of Histological Techniques, 2nd ed.; Churchill Livingstone: London, UK, 1990. [Google Scholar]
- Adamu, M.; Boonkaewwan, C.; Gongruttananun, N.; Vongpakorn, M. Hematological, biochemical and histopathological changes caused by coccidiosis in chickens. J. Kasetsart Nat. Sci. 2013, 47, 238–246. [Google Scholar]
- Allen, A.; Hutton, D.A.; Leonard, A.J.; Pearson, J.P.; Sellers, L.A. The role of mucus in the protection of the gastroduodenal mucosa. Scand. J. Gastroenterol. 1986, 125, 71–78. [Google Scholar] [CrossRef]
- Tsakiris, S.; Schulipis, K.H.; Marinou, K.; Behrakis, P. Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+,K+-ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. J. Pharmacol. Res. 2004, 49, 475–479. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Tomley, F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef]
- Quiroz-Castañeda, R.E.; Dantán-González, E. Control of Avian Coccidiosis: Future and Present Natural Alternatives. Int. J. BioMed Res. 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2, 4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef]
- Dimian, A.C.; Omota, F.; Bliek, A. Entrainer-Assisted Reactive Distillation: Application to Process Synthesis for Fatty Esters. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2002; Volume 10, pp. 175–180. [Google Scholar]
- Chowdhury, R.; Steur, M.; Patel, P.S.; Franco, O.H. Individual fatty acids in cardiometabolic disease. In Handbook of Lipids in Human Function; AOCS Press: Champaign, IL, USA, 2016; pp. 207–318. [Google Scholar]
- Hu, W.; Fitzgerald, M.; Topp, B.; Alam, M.; O’Hare, T.J. A review of biological functions, health benefits, and possible de novo biosynthetic pathway of palmitoleic acid in macadamia nuts. JFF 2019, 62, 103520. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Du, X.; Ma, X.; Gao, Y. The physiological function of squalene and its application prospects in animal husbandry. Front. Vet. Sci. 2024, 10, 1284500. [Google Scholar] [CrossRef] [PubMed]
- Linh, B.K.; Hayashi, T.; Horii, Y. Eimeria vermiformis infection reduces goblet cells by multiplication in the crypt cells of the small intestine of C57BL/6 mice. J. Parasitol. Res. 2008, 104, 789–794. [Google Scholar] [CrossRef]
- Abdel-Latif, M.; Abdel-Haleem, H.M.; Abdel-Baki, A.A.S. Anticoccidial activities of Chitosan on Eimeria papillata-infected mice. J. Parasitol. Res. 2016, 115, 2845–2852. [Google Scholar] [CrossRef] [PubMed]
- Esch, K.J.; Petersen, C.A. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 2013, 26, 58–85. [Google Scholar] [CrossRef]
- Fatoba, A.J.; Adeleke, M.A. Diagnosis and control of chicken coccidiosis: A recent update. J. Parasit. Dis. 2018, 42, 483–493. [Google Scholar] [CrossRef]
- Lake, C.R.; Sternberg, D.E.; Van Kammen, D.P.; Ballenger, J.C.; Ziegler, M.G.; Post, R.M.; Kopin, I.J.; Bunney, W.E. Schizophrenia: Elevated cerebrospinal fluid norepinephrine. Science 1980, 207, 331–333. [Google Scholar] [CrossRef]
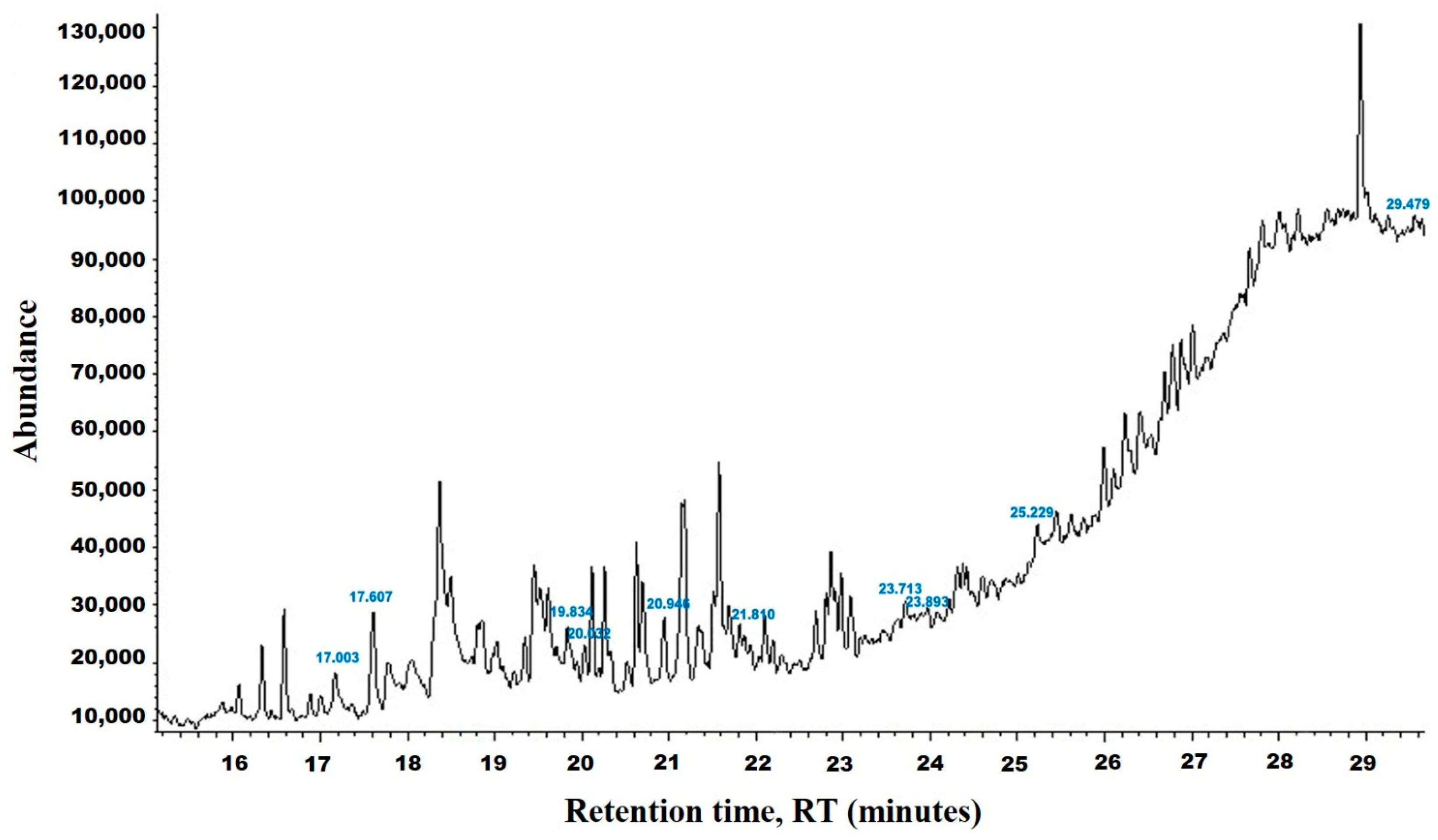
| tR (min) | Compound | MW | Peak Area | Peak Area (%) | Structure | Formula |
|---|---|---|---|---|---|---|
| 17.049 | 2,4-Di-tert-butylphenol | 206 | 126,864 | 5.90 | 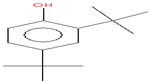 | C14H22O |
| 17.638 | Dodecanoic acid | 200 | 736,245 | 34.23 | 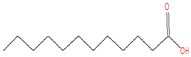 | C12H24O2 |
| 19.932 | Tetradecanoic acid | 228 | 224,314 | 10.43 |  | C14H28O2 |
| 20.005 | n-Hexadecanoic acid | 256 | 182,407 | 8.48 |  | C16H32O2 |
| 20.994 | Pentadecanoic acid | 242 | 380,350 | 17.68 |  | C15H30O2 |
| 21.815 | Palmitoleic acid | 254 | 91,151 | 4.24 | 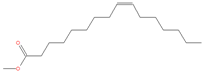 | C16H30O2 |
| 23.667 | 9,12-Octadecadienoic acid (Z,Z)- | 280 | 116,629 | 5.42 |  | C18H32O2 |
| 23.898 | Octadecanoic acid | 284 | 69,120 | 3.21 | 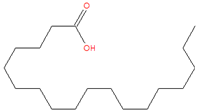 | C18H36O2 |
| 25.193 | 5,8,11,14-Eicosatetraenoic acid, methyl ester, (all-Z)- | 318 | 187,140 | 8.70 |  | C21H34O2 |
| 29.443 | Squalene | 410 | 36,859 | 1.71 | 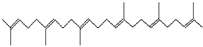 | C30H50 |
| Oocysts | Sporulated Oocyst | Non-Sporulated Oocyst |
|---|---|---|
| Length (µm) | 17.62 ± 2.18 | 19.63 ± 2.27 |
| Width (µm) | 15.52 ± 1.84 | 17.22 ± 2.49 |
| L/W index | 1.08 | 1.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, Y.A.; Abdel-Moneim, A.E.; Taha, R.G.; Khalil, M.F.; Abdel-Gaber, R.; Thagfan, F.A.; Al-Malki, E.S.; Dkhil, M.A. Holothuria polii Extract as a Potential Anticoccidial Agent: Evidence of Its MUC2 Regulatory Impact in Murine Jejunum. Vet. Sci. 2024, 11, 490. https://doi.org/10.3390/vetsci11100490
El-Sayed YA, Abdel-Moneim AE, Taha RG, Khalil MF, Abdel-Gaber R, Thagfan FA, Al-Malki ES, Dkhil MA. Holothuria polii Extract as a Potential Anticoccidial Agent: Evidence of Its MUC2 Regulatory Impact in Murine Jejunum. Veterinary Sciences. 2024; 11(10):490. https://doi.org/10.3390/vetsci11100490
Chicago/Turabian StyleEl-Sayed, Youssef A., Ahmed E. Abdel-Moneim, Rania G. Taha, Mona F. Khalil, Rewaida Abdel-Gaber, Felwa A. Thagfan, Esam S. Al-Malki, and Mohamed A. Dkhil. 2024. "Holothuria polii Extract as a Potential Anticoccidial Agent: Evidence of Its MUC2 Regulatory Impact in Murine Jejunum" Veterinary Sciences 11, no. 10: 490. https://doi.org/10.3390/vetsci11100490
APA StyleEl-Sayed, Y. A., Abdel-Moneim, A. E., Taha, R. G., Khalil, M. F., Abdel-Gaber, R., Thagfan, F. A., Al-Malki, E. S., & Dkhil, M. A. (2024). Holothuria polii Extract as a Potential Anticoccidial Agent: Evidence of Its MUC2 Regulatory Impact in Murine Jejunum. Veterinary Sciences, 11(10), 490. https://doi.org/10.3390/vetsci11100490







