The Effect of Dietary Plant-Derived Omega 3 Fatty Acids on the Reproductive Performance and Gastrointestinal Health of Female Rabbits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Diets
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
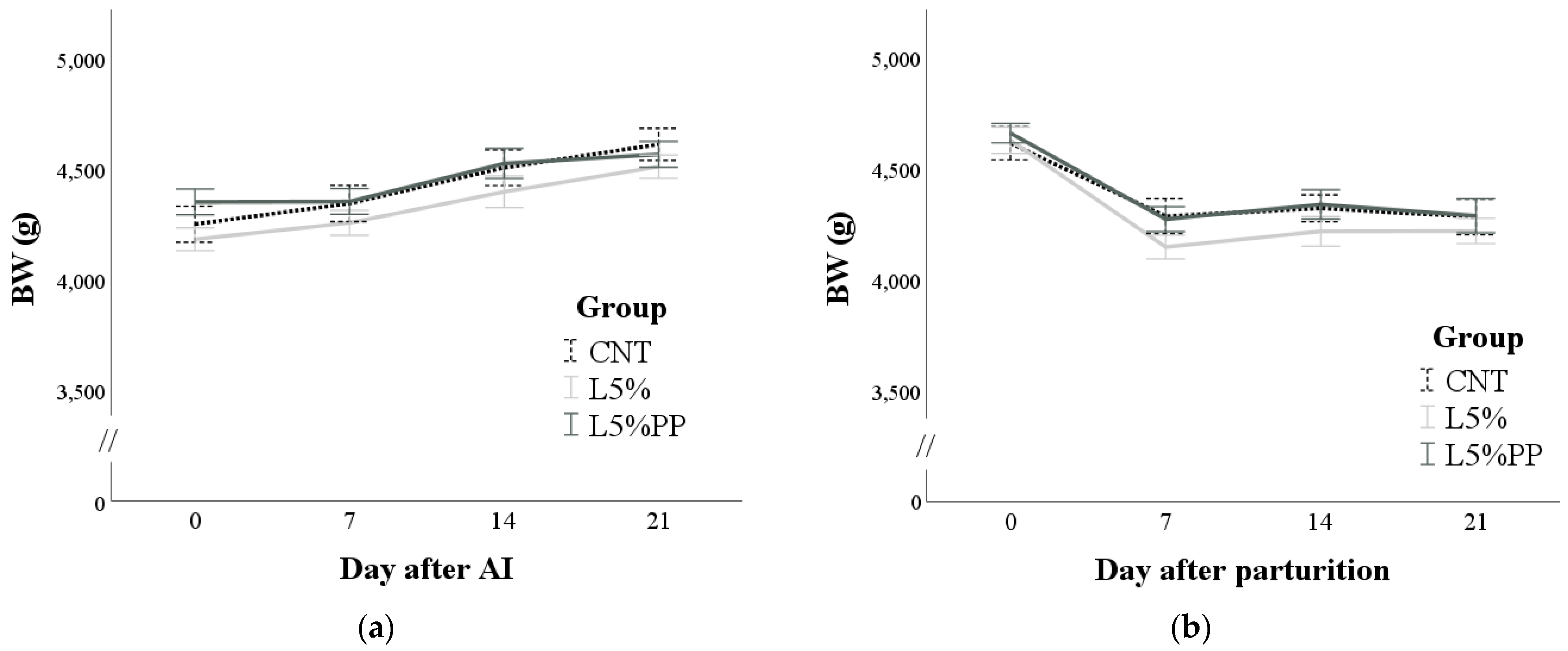
3.1. Feed Intake and Body Weight
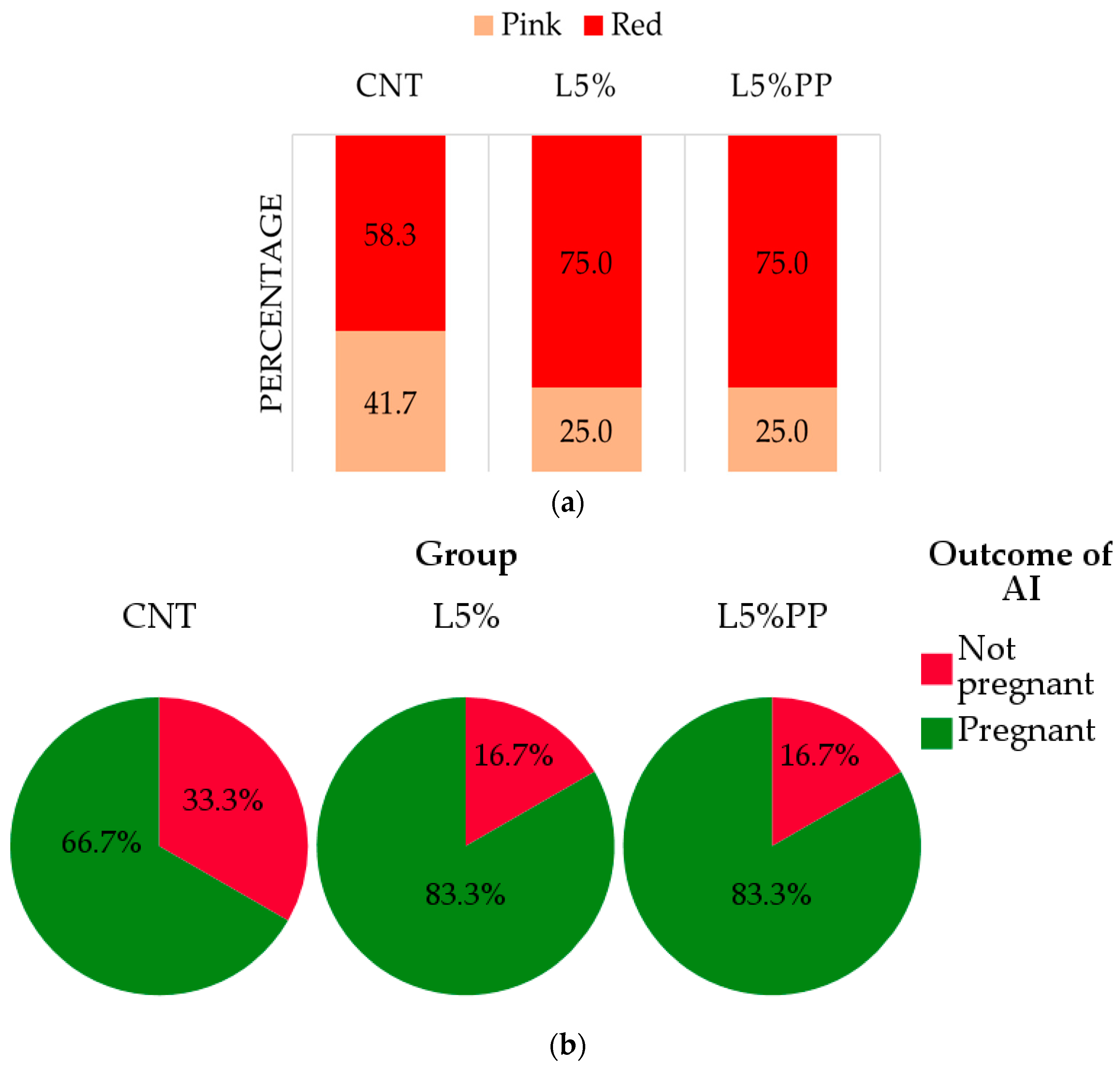
3.2. Reproductive Performance
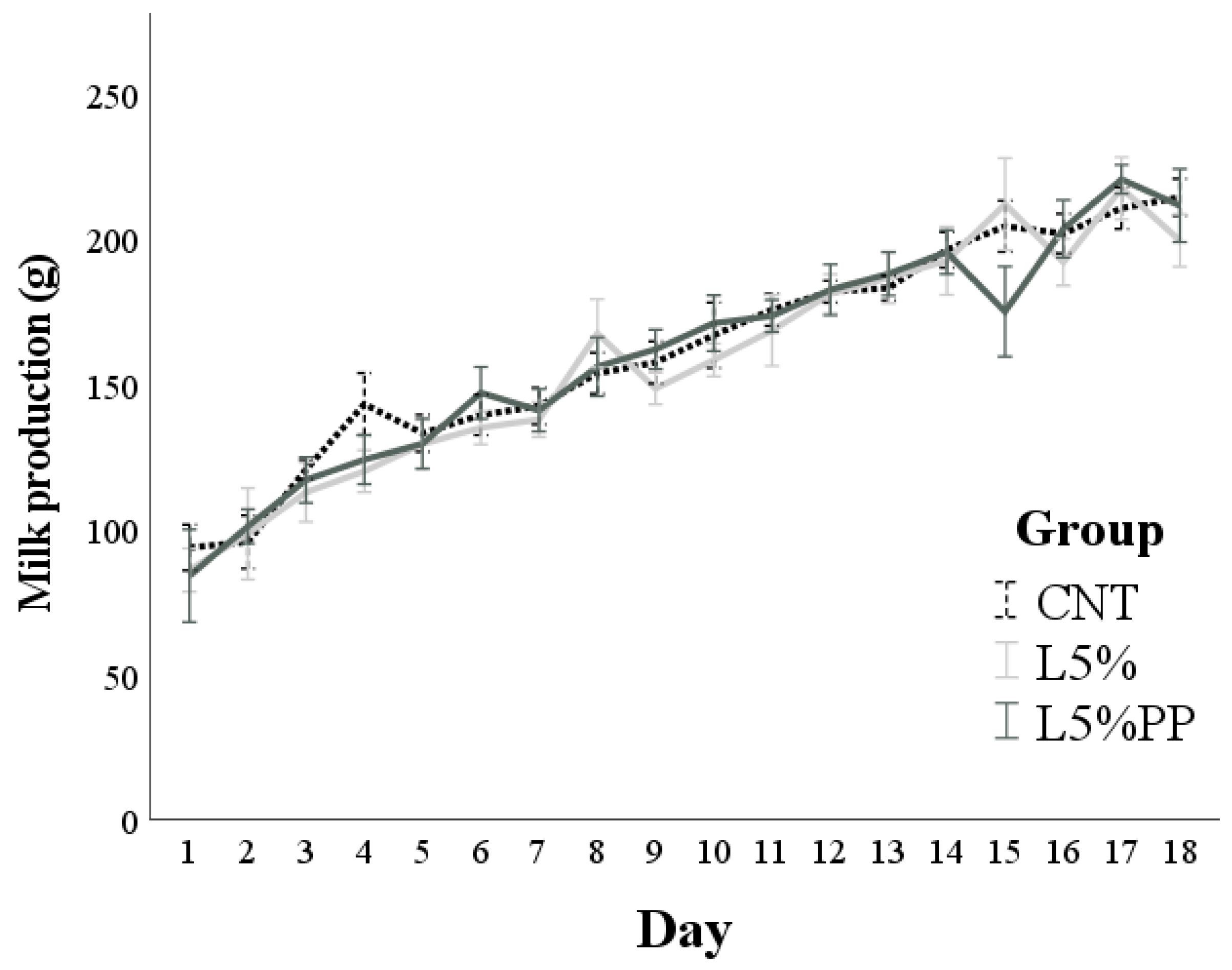
3.3. Milk Production
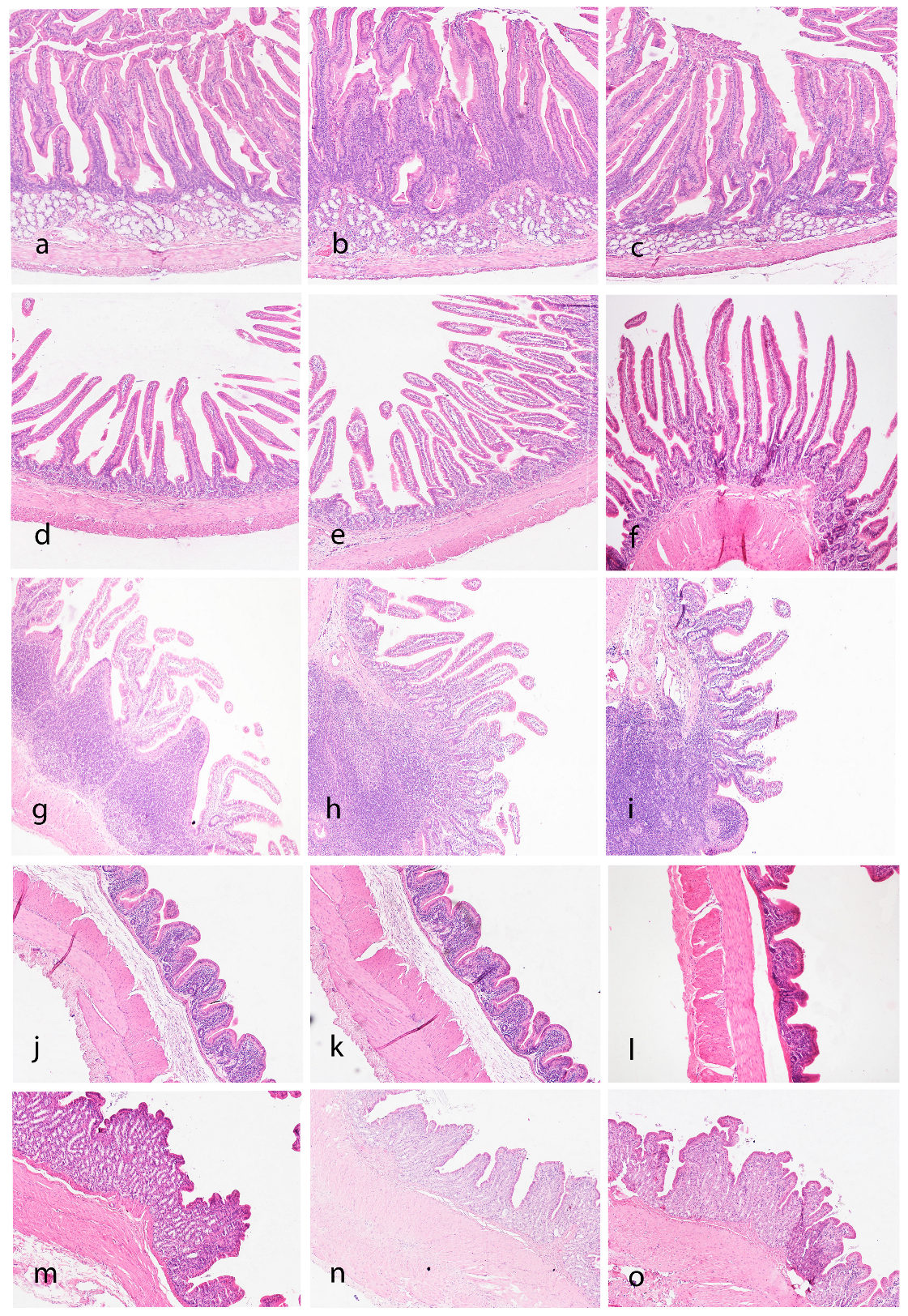
3.4. Histological Examination of the Gastrointestinal Tract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fortun-Lamothe, L. Energy Balance and Reproductive Performance in Rabbit Does. Anim. Reprod. Sci. 2006, 93, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C. Reproductive Activity and Welfare of Rabbit Does. Ital. J. Anim. Sci. 2007, 6, 743–747. [Google Scholar] [CrossRef]
- Menchetti, L.; Andoni, E.; Barbato, O.; Canali, C.; Quattrone, A.; Vigo, D.; Codini, M.; Curone, G.; Brecchia, G. Energy Homeostasis in Rabbit Does during Pregnancy and Pseudopregnancy. Anim. Reprod. Sci. 2020, 218, 106505. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Dal Bosco, A.; Arias-Álvarez, M.; Lorenzo, P.L.; Cardinali, R.; Rebollar, P.G. The Main Factors Affecting the Reproductive Performance of Rabbit Does: A Review. Anim. Reprod. Sci. 2010, 122, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Vecchione, L.; Filipescu, I.; Petrescu, V.F.; Fioretti, B.; Beccari, T.; Ceccarini, M.R.; Codini, M.; Quattrone, A.; Trabalza-Marinucci, M.; et al. Effects of Goji Berries Supplementation on the Productive Performance of Rabbit. Livest. Sci. 2019, 220, 123–128. [Google Scholar] [CrossRef]
- Andoni, E.; Curone, G.; Agradi, S.; Barbato, O.; Menchetti, L.; Vigo, D.; Zelli, R.; Cotozzolo, E.; Ceccarini, M.R.; Faustini, M.; et al. Effect of Goji Berry (Lycium Barbarum) Supplementation on Reproductive Performance of Rabbit Does. Animals 2021, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Castrica, M.; Agradi, S.; Curone, G.; Vigo, D.; Di Giancamillo, A.; Modina, S.C.; Riva, F.; Balzaretti, C.M.; De Bellis, R.; et al. Antioxidant Activity of Different Tissues from Rabbits Fed Dietary Bovine Colostrum Supplementation. Animals 2023, 13, 850. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘Nutraceuticals’: Neither Nutritious nor Pharmaceutical: Defining ‘Nutraceuticals’. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- El-Sabrout, K.; Khalifah, A.; Ciani, F. Current Applications and Trends in Rabbit Nutraceuticals. Agriculture 2023, 13, 1424. [Google Scholar] [CrossRef]
- Menchetti, L.; Canali, C.; Castellini, C.; Boiti, C.; Brecchia, G. The Different Effects of Linseed and Fish Oil Supplemented Diets on Insulin Sensitivity of Rabbit Does during Pregnancy. Res. Vet. Sci. 2018, 118, 126–133. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Signorini, C.; Cotozzolo, E.; Noto, D.; Moretti, E.; Brecchia, G.; Dal Bosco, A.; Belmonte, G.; Durand, T.; et al. Effect of Dietary n-3 Source on Rabbit Male Reproduction. Oxidative Med. Cell. Longev. 2019, 2019, 3279670. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated Fatty Acids in Male and Female Reproduction1. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; O’Neal, D.N.; Best, J.D.; Beilin, L.J. Purified Eicosapentaenoic and Docosahexaenoic Acids Have Differential Effects on Serum Lipids and Lipoproteins, LDL Particle Size, Glucose, and Insulin in Mildly Hyperlipidemic Men. Am. J. Clin. Nutr. 2000, 71, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Storlien, L.H.; Jenkins, A.B.; Chisholm, D.J.; Pascoe, W.S.; Khouri, S.; Kraegen, E.W. Influence of Dietary Fat Composition on Development of Insulin Resistance in Rats: Relationship to Muscle Triglyceride and ω-3 Fatty Acids in Muscle Phospholipid. Diabetes 1991, 40, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Nordøy, A. Dietary Fatty Acids and Coronary Heart Disease. Lipids 1999, 34, S19–S22. [Google Scholar] [CrossRef]
- Leaf, A. The Electrophysiologic Basis for the Antiarrhythmic and Anticonvulsant Effects of n-3 Polyunsaturated Fatty Acids: Heart and Brain. Lipids 2001, 36, S107–S110. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D. Fatty Acids and Immune Responses—A New Perspective in Searching for Clues to Mechanism. Annu. Rev. Nutr. 2000, 20, 431–456. [Google Scholar] [CrossRef]
- Salem, N.; Litman, B.; Kim, H.; Gawrisch, K. Mechanisms of Action of Docosahexaenoic Acid in the Nervous System. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef]
- Menchetti, L.; Barbato, O.; Sforna, M.; Vigo, D.; Mattioli, S.; Curone, G.; Tecilla, M.; Riva, F.; Brecchia, G. Effects of Diets Enriched in Linseed and Fish Oil on the Expression Pattern of Toll-Like Receptors 4 and Proinflammatory Cytokines on Gonadal Axis and Reproductive Organs in Rabbit Buck. Oxidative Med. Cell. Longev. 2020, 2020, 4327470. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Dal Bosco, A.; Maranesi, M.; Petrucci, L.; Rebollar, P.G.; Castellini, C. Dietary Fish Oil and Flaxseed for Rabbit Does: Fatty Acids Distribution and Δ6-Desaturase Enzyme Expression of Different Tissues. Animal 2019, 13, 1934–1942. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Al-Soufi, S.; García, J.; Muíños, A.; López-Alonso, M. Marine Macroalgae in Rabbit Nutrition—A Valuable Feed in Sustainable Farming. Animals 2022, 12, 2346. [Google Scholar] [CrossRef]
- de Blas, C.; Wiseman, J. (Eds.) Nutrition of the Rabbit, 2nd ed.; CABI: Wallingford UK; Cambridge, MA, USA, 2010; ISBN 978-1-84593-669-3. [Google Scholar]
- Okab, A.B.; Samara, E.M.; Abdoun, K.A.; Rafay, J.; Ondruska, L.; Parkanyi, V.; Pivko, J.; Ayoub, M.A.; Al-Haidary, A.A.; Aljumaah, R.S.; et al. Effects of Dietary Seaweed (Ulva Lactuca) Supplementation on the Reproductive Performance of Buck and Doe Rabbits. J. Appl. Anim. Res. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Vizzarri, F.; Chiapparini, S.; Corino, C.; Casamassima, D.; Palazzo, M.; Parkanyi, V.; Ondruska, L.; Rossi, R. Dietary Supplementation with Natural Extracts Mixture: Effects on Reproductive Performances, Blood Biochemical and Antioxidant Parameters in Rabbit Does. Ann. Anim. Sci. 2020, 20, 565–578. [Google Scholar] [CrossRef]
- El Maghraby, D.M.; Fakhry, E.M. Lipid Content and Fatty Acid Composition of Mediterranean Macro-Algae as Dynamic Factors for Biodiesel Production. Oceanologia 2015, 57, 86–92. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis, 20th ed.; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Soest, P.J.V.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. AOAC Int. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Maertens, L.; Moermans, R.; Groote, G. Prediction of the Apparent Digestible Energy Content of Commercial Pelleted Feeds for Rabbits. J. Appl. Rabbit Res. 1988, 11, 60–67. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. A Simple Procedure for Rapid Transmethylation of Glycerolipids and Cholesteryl Esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Brecchia, G.; Muça, G.; Munga, A.; Menchetti, L.; Galosi, L.; Rossi, G.; Barbato, O.; Pastorelli, G.; Agradi, S.; Serra, V.; et al. Goji Berry in the Diet of the Rabbit Buck: Effects on Semen Quality, Oxidative Status and Histological Features of the Reproductive Tract. Antioxidants 2023, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Garson, G.D. Testing Statistical Assumptions; Statistical Associates Publishing: Asheboro, NC, USA, 2012. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS: And Sex and Drugs and Rock’n’roll, 3rd ed.; Sage: Los Angeles, CA, USA, 2012; ISBN 978-1-84787-906-6. [Google Scholar]
- Simopoulos, A. Omega-3 Fatty Acids in Health and Disease and in Growth and Development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef] [PubMed]
- Agradi, S.; Sulce, M.; Menchetti, L.; Vigo, D.; Castrica, M.; Barbato, O.; Andoni, E.; Quattrone, A.; Munga, A.; Marongiu, M.L.; et al. Dietary Supplementation with n-3 Polyunsaturated Fatty Acids: Effects on Reproductive and Productive Performance and Meat Quality in Rabbit Breeding. Anim. Nutr. 2023, 14, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, P.G.; García-García, R.M.; Arias-Álvarez, M.; Millán, P.; Rey, A.I.; Rodríguez, M.; Formoso-Rafferty, N.; De La Riva, S.; Masdeu, M.; Lorenzo, P.L.; et al. Reproductive Long-Term Effects, Endocrine Response and Fatty Acid Profile of Rabbit Does Fed Diets Supplemented with n-3 Fatty Acids. Anim. Reprod. Sci. 2014, 146, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mordenti, A.L.; Sardi, L.; Bonaldo, A.; Pizzamiglio, V.; Brogna, N.; Cipollini, I.; Tassinari, M.; Zaghini, G. Influence of Marine Algae (Schizochytrium spp.) Dietary Supplementation on Doe Performance and Progeny Meat Quality. Livest. Sci. 2010, 128, 179–184. [Google Scholar] [CrossRef]
- Xiccato, G.; Trocino, A.; Sartori, A.; Queaque, P.I. Effect of Parity Order and Litter Weaning Age on the Performance and Body Energy Balance of Rabbit Does. Livest. Prod. Sci. 2004, 85, 239–251. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Sartori, A.; Bohatir, P.; Rémignon, H.; Ricci, R. Effect of Dietary Supplementation of Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) on Growth Performance, Apparent Digestibility and Health Status of Companion Dwarf Rabbits. Livest. Sci. 2013, 152, 182–191. [Google Scholar] [CrossRef]
- Rodríguez, M.; García-García, R.M.; Arias-Álvarez, M.; Millán, P.; Febrel, N.; Formoso-Rafferty, N.; López-Tello, J.; Lorenzo, P.L.; Rebollar, P.G. Improvements in the Conception Rate, Milk Composition and Embryo Quality of Rabbit Does after Dietary Enrichment with n-3 Polyunsaturated Fatty Acids. Animal 2018, 12, 2080–2088. [Google Scholar] [CrossRef]
- Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Dal Bosco, A.; Riva, F.; Cremonesi, P.; Agradi, S.; Mattioli, S.; Castiglioni, B.; et al. Could Dietary Supplementation with Different Sources of N-3 Polyunsaturated Fatty Acids Modify the Rabbit Gut Microbiota? Antibiotics 2022, 11, 227. [Google Scholar] [CrossRef]
- Rodríguez, M.; Carro, M.D.; Valiente, V.; Formoso-Rafferty, N.; Rebollar, P.G. Effects of Dietary Fish Oil Supplementation on Performance, Meat Quality, and Cecal Fermentation of Growing Rabbits1. J. Anim. Sci. 2017, 95, 3620–3630. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Carro, M.D.; Valiente, V.; Formoso-Rafferty, N.; Rebollar, P.G. Supplementation with Fish Oil Improves Meat Fatty Acid Profile Although Impairs Growth Performance of Early Weaned Rabbits. Animals 2019, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Tsiligianni, T.; Saratsi, A.; Besenfelder, U.; Anastasiadis, A.; Vainas, E.; Saratsis, P.; Brem, G. The Use of Cytological Examination of Vaginal Smears (CEVS) in the Selection of Rabbits for Superovulation. Theriogenology 2004, 61, 989–995. [Google Scholar] [CrossRef]
- Elkomy, A.E.; El-Speiy, M.E. Polyunsaturated Fatty Acids Combined with Equine Chorionic Gonadotropin to Enhance Reproductive Performance in Aged Rabbit Does. Ital. J. Anim. Sci. 2015, 14, 3535. [Google Scholar] [CrossRef]
- Rodríguez, M.; García-García, R.M.; Arias-Álvarez, M.; Formoso-Rafferty, N.; Millán, P.; López-Tello, J.; Lorenzo, P.L.; González-Bulnes, A.; Rebollar, P.G. A Diet Supplemented with -3 Polyunsaturated Fatty Acids Influences the Metabomscic and Endocrine Response of Rabbit Does and Their Offspring. J. Anim. Sci. 2017, 95, 2690. [Google Scholar] [CrossRef]
- Rodríguez, M.; Rebollar, P.G.; Mattioli, S.; Castellini, C. n-3 PUFA Sources (Precursor/Products): A Review of Current Knowledge on Rabbit. Animals 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Mattos, R.; Staples, C.R.; Arteche, A.; Wiltbank, M.C.; Diaz, F.J.; Jenkins, T.C.; Thatcher, W.W. The Effects of Feeding Fish Oil on Uterine Secretion of PGF2α, Milk Composition, and Metabolic Status of Periparturient Holstein Cows. J. Dairy Sci. 2004, 87, 921–932. [Google Scholar] [CrossRef]
- Nazir, G.; Ghuman, S.P.S.; Singh, J.; Honparkhe, M.; Ahuja, C.S.; Dhaliwal, G.S.; Sangha, M.K.; Saijpaul, S.; Agarwal, S.K. Improvement of Conception Rate in Postpartum Flaxseed Supplemented Buffalo with Ovsynch + CIDR Protocol. Anim. Reprod. Sci. 2013, 137, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C. Polyunsaturated Fatty Acid Metabolism, 1st ed.; Academic Press and AOCS Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-811230-4. [Google Scholar]
- Santos, J.E.P.; Thatcher, W.W.; Chebel, R.C.; Cerri, R.L.A.; Galvão, K.N. The Effect of Embryonic Death Rates in Cattle on the Efficacy of Estrus Synchronization Programs. Anim. Reprod. Sci. 2004, 82–83, 513–535. [Google Scholar] [CrossRef]
- Weems, Y.S.; Kim, L.; Humphreys, V.; Tsuda, V.; Blankfein, R.; Wong, A.; Weems, C.W. Effect of Luteinizing Hormone (LH), Pregnancy-Specific Protein B (PSPB), or Arachidonic Acid (AA) on Secretion of Progesterone and Prostaglandins (PG) E (PGE; PGE1 and PGE2) and F2α (PGF2α) by Ovine Corpora Lutea of the Estrous Cycle or Pregnancy in Vitro. Prostaglandins Other Lipid Mediat. 2007, 84, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Mattos, R. Effects of Dietary Fatty Acids on Reproduction in Ruminants. Rev. Reprod. 2000, 5, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, J.R.; Escobar, R.D.S.; Ferreira, C.F.; Silveira, P.P. Fetal and Neonatal Levels of Omega-3: Effects on Neurodevelopment, Nutrition, and Growth. Sci. World J. 2012, 2012, 202473. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Navarrete Santos, A.; Duranthon, V. Rabbit as a Reproductive Model for Human Health. Reproduction 2012, 144, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.; Anderson, G.J.; Connor, W.E. High Levels of the (n-6) Fatty Acid 4,7,10,13,16-Docosapentaenoate in the Retinas of Rabbits Are Reduced by Feeding Dietary Fish Oil from Birth to Adult Life. J. Nutr. 1991, 121, 1924–1931. [Google Scholar] [CrossRef]
- Mateo, R.D.; Carroll, J.A.; Hyun, Y.; Smith, S.; Kim, S.W. Effect of Dietary Supplementation of N-3 Fatty Acids and Elevated Concentrations of Dietary Protein on the Performance of Sows. J. Anim. Sci. 2009, 87, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Maertens, L.; Aerts, J.M.; Brabander, D.L.D. Effet d’un aliment riche en acides gras omega-3 sur les performances et la composition du lait des lapines et la viabilité de leur descendance. In Proceedings of the 11 èmes Journées de la Recherche Cunicole, Paris, France, 29–30 November 2005; pp. 205–208. [Google Scholar]
| Ingredients (g/kg) | Diets | ||
|---|---|---|---|
| CNT | L5% | L5%PP | |
| Wheat bran | 250.7 | 248.7 | 249.1 |
| Barley | 133.3 | 130 | 130 |
| Sunflower seed meal | 120 | 116.7 | 115 |
| Alfalfa | 108.3 | 130 | 130 |
| Sunflower husks | 100 | 100 | 100 |
| Beet pulp | 75 | 56.6 | 55 |
| Extruded linseed | - | 50 | 50 |
| Full-fat soybean | 50 | 29.5 | 31 |
| Wheat straw | 41.7 | 20 | 20 |
| Sugarcane molasses | 30 | 30 | 30 |
| Wheat | 25 | 25 | 25 |
| Grape seeds meal | 23.3 | 16.7 | 16.7 |
| Soya hulls | 0 | 1.67 | 1.67 |
| Calcium carbonate | 16 | 14.8 | 14.2 |
| Soybean oil | 7.8 | - | - |
| Sodium chloride | 4 | 4 | 4 |
| Palm oil | 3.3 | - | - |
| Carboxymethylcellulose | 3 | 3 | 3 |
| Oligo vitamin supplement 1 | 2.5 | 2.5 | 2.5 |
| Algae “Padina pavonica” extract | - | - | 2 |
| Lysine HCl | 1.6 | 1.7 | 1.7 |
| Liquid acidifier 2 | 1.5 | 1.5 | 1.5 |
| Magnesium oxide | 1 | 1 | 1 |
| Methionine hydroxyanalog | 0.6 | 0.7 | 0.7 |
| Liquid choline | 0.5 | 0.5 | 0.5 |
| Vitamin E 50% | 0.3 | 0.3 | 0.3 |
| L Threonine | 0.3 | 0.1 | 0.1 |
| DL Methionine | 0.3 | - | - |
| Chemical Composition (g/kg) of Dry Matter | Diets | ||
|---|---|---|---|
| CNT | L5% | L5%PP | |
| Dry matter | 893.4 | 894.9 | 899.4 |
| Crude protein | 177.2 | 183.3 | 185.9 |
| Ether extract | 39.5 | 62.1 | 52.2 |
| Ash | 79.9 | 76.9 | 82.1 |
| NDF 1 | 432.4 | 396.5 | 424 |
| ADF 2 | 279 | 257.2 | 255.3 |
| ADL 3 | 77.6 | 73.8 | 72.2 |
| DE 4 | 9.8324 | 9.8324 | 9.8324 |
| Chemical Composition (g/kg) | Extruded Linseed | Algae Padina pavonica Extract |
|---|---|---|
| Dry matter | 954.6 | 996.6 |
| Crude Protein | 227.6 | 2.5 |
| Ether extract | 449.4 | 1.6 |
| Ash | 31.1 | 778.6 |
| NDF 1 | 132.4 | 28.3 |
| ADF 2 | 108.1 | 19.9 |
| ADL 3 | 40.6 | 5.6 |
| Fatty Acid (g/100 g of Fatty Acids) | Raw Material | Diet | |||
|---|---|---|---|---|---|
| Extruded Linseed | Algae Padina pavonica Extract | CNT | L5% | L5%PP | |
| C12:0 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 |
| C14:0 | 0.05 | 1.62 | 0.18 | 0.20 | 0.20 |
| C15:0 | 0.02 | 0.00 | 0.09 | 0.08 | 0.08 |
| C16:0 | 5.97 | 27.95 | 13.92 | 11.14 | 11.65 |
| C16:1cis9 | 0.08 | 1.51 | 0.18 | 0.20 | 0.18 |
| C17:0 | 0.06 | 0.00 | 0.12 | 0.11 | 0.10 |
| C17:1 | 0.03 | 0.00 | 0.05 | 0.05 | 0.05 |
| C18:0 | 4.54 | 12.52 | 3.07 | 3.82 | 3.48 |
| C18:1 | 20.93 | 25.84 | 25.39 | 23.89 | 23.98 |
| C18:2cis n-6, LA 1 | 15.11 | 9.72 | 47.63 | 32.41 | 33.53 |
| C20:0 | 0.15 | 0.00 | 0.28 | 0.22 | 0.22 |
| C18:3 n-6, γ-ALA 2 | 0.01 | 0.00 | 0.21 | 0.12 | 0.19 |
| C18:3 n-3, α-ALA 2 | 52.19 | 8.98 | 6.53 | 23.25 | 22.63 |
| C20:2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 |
| C20:3n-3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 |
| C22:0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.23 |
| C22:1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 |
| C20:4n-6, AA 3 | 0.00 | 0.34 | 0.00 | 0.00 | 0.08 |
| C22:2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| C20:5n-3, EPA 4 | 0.00 | 0.03 | 0.00 | 0.00 | 0.13 |
| C24:0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C24:1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 |
| C22:5n-3, DPA 5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 |
| C22:6n-3, DHA 6 | 0.00 | 7.63 | 0.00 | 0.00 | 0.06 |
| Tot | 99.16 | 96.25 | 97.64 | 95.50 | 97.00 |
| Others | 0.84 | 3.75 | 2.36 | 4.50 | 3.00 |
| Parameter | Group | p Value | ||
|---|---|---|---|---|
| CTN | L5% | L5%PP | ||
| Litter size at birth (total n) | 6.78 ± 0.70 | 7.67 ± 0.40 | 7.29 ± 0.62 | 0.576 |
| Litter size at weaning (n) | 5.89 ± 0.75 | 6.67 ± 0.41 | 6.79 ± 0.57 | 0.507 |
| Litter weight at birth (g) *† | 427 ± 25 | 440 ± 20 | 468 ± 19 | 0.394 |
| Litter weight at weaning (g) *† | 4987 ± 199 | 4814 ± 188 | 4965 ± 177 | 0.784 |
| Perinatal mortality (%) * | 6.67 c ± 0.86 | 2.78 b ± 0.56 | 0.00 a ± 0.00 | <0.001 |
| Pre-weaning mortality (%) * | 5.56 c ± 0.79 | 0.00 a ± 0.00 | 3.33 b ± 0.53 | 0.016 |
| Milk production (g/d) * | 161.9 ± 2.8 | 158.6 ± 2.6 | 159.90 ± 2.5 | 0.679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quattrone, A.; Belabbas, R.; Fehri, N.E.; Agradi, S.; Mazzola, S.M.; Barbato, O.; Dal Bosco, A.; Mattioli, S.; Failla, S.; Abdel-Kafy, E.-S.M.; et al. The Effect of Dietary Plant-Derived Omega 3 Fatty Acids on the Reproductive Performance and Gastrointestinal Health of Female Rabbits. Vet. Sci. 2024, 11, 457. https://doi.org/10.3390/vetsci11100457
Quattrone A, Belabbas R, Fehri NE, Agradi S, Mazzola SM, Barbato O, Dal Bosco A, Mattioli S, Failla S, Abdel-Kafy E-SM, et al. The Effect of Dietary Plant-Derived Omega 3 Fatty Acids on the Reproductive Performance and Gastrointestinal Health of Female Rabbits. Veterinary Sciences. 2024; 11(10):457. https://doi.org/10.3390/vetsci11100457
Chicago/Turabian StyleQuattrone, Alda, Rafik Belabbas, Nour Elhouda Fehri, Stella Agradi, Silvia Michela Mazzola, Olimpia Barbato, Alessandro Dal Bosco, Simona Mattioli, Sebastiana Failla, El-Sayed M. Abdel-Kafy, and et al. 2024. "The Effect of Dietary Plant-Derived Omega 3 Fatty Acids on the Reproductive Performance and Gastrointestinal Health of Female Rabbits" Veterinary Sciences 11, no. 10: 457. https://doi.org/10.3390/vetsci11100457
APA StyleQuattrone, A., Belabbas, R., Fehri, N. E., Agradi, S., Mazzola, S. M., Barbato, O., Dal Bosco, A., Mattioli, S., Failla, S., Abdel-Kafy, E.-S. M., Jemmali, B., Salem, I. B., Mandara, M. T., Giglia, G., Colin, M., Guillevic, M., Muça, G., Sulçe, M., Castrica, M., ... Menchetti, L. (2024). The Effect of Dietary Plant-Derived Omega 3 Fatty Acids on the Reproductive Performance and Gastrointestinal Health of Female Rabbits. Veterinary Sciences, 11(10), 457. https://doi.org/10.3390/vetsci11100457

















