Post-Thaw Parameters of Buck Semen Quality after Soy Lecithin Extender Supplementation with Fumaric Acid
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Bucks, Semen Collection and Handling
2.2. Semen Extender, Fumaric Acid Addition
2.3. Semen Cryopreservation
2.4. Semen Evaluation
2.4.1. Spermatozoa Motility
2.4.2. Spermatozoa Viability
2.4.3. Plasma Membrane Functional Integrity
2.4.4. Acrosome Integrity
2.4.5. Mitochondrial Membrane Function Combined with Viability
2.5. Statistical Analysis
3. Results
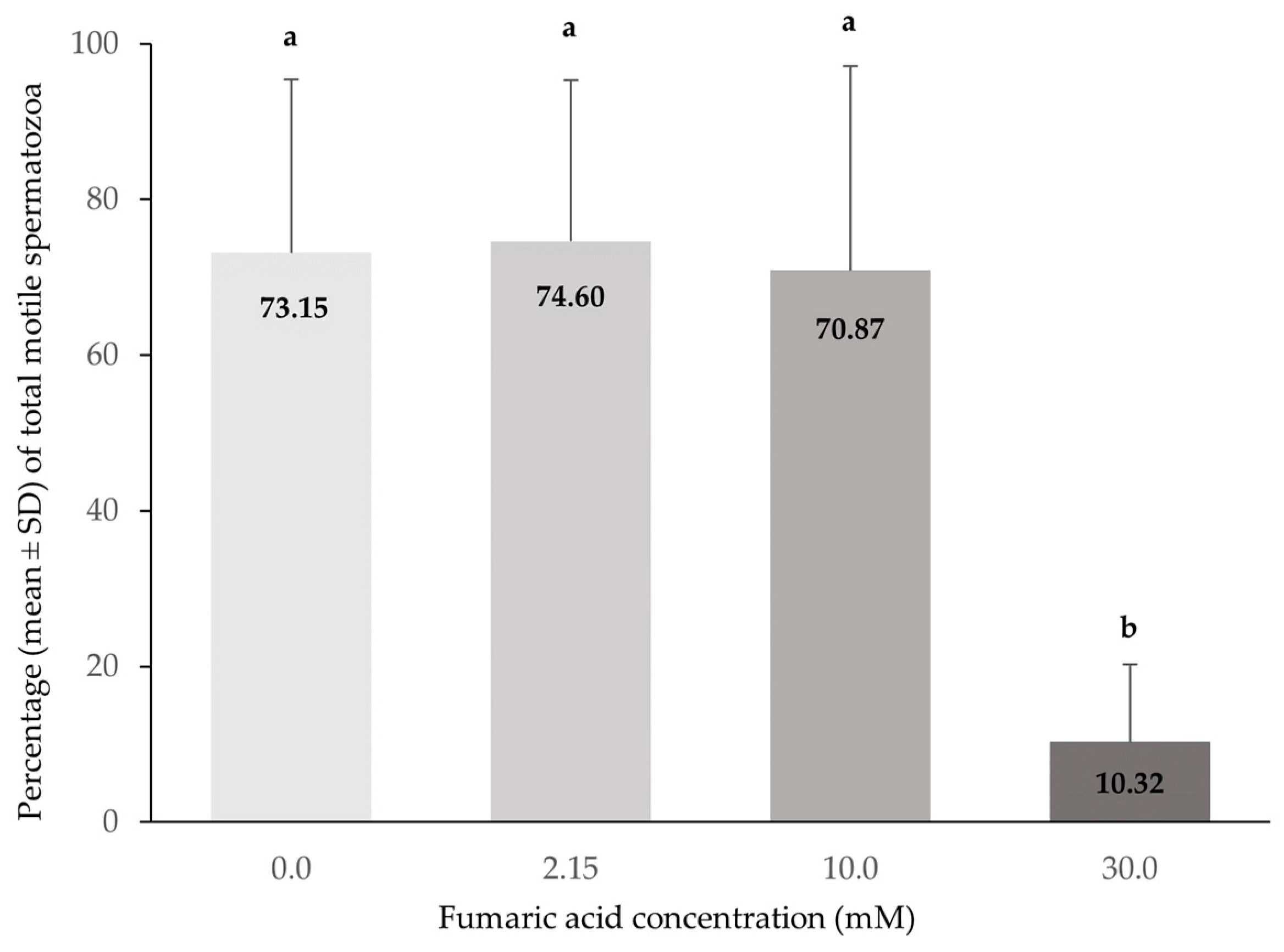
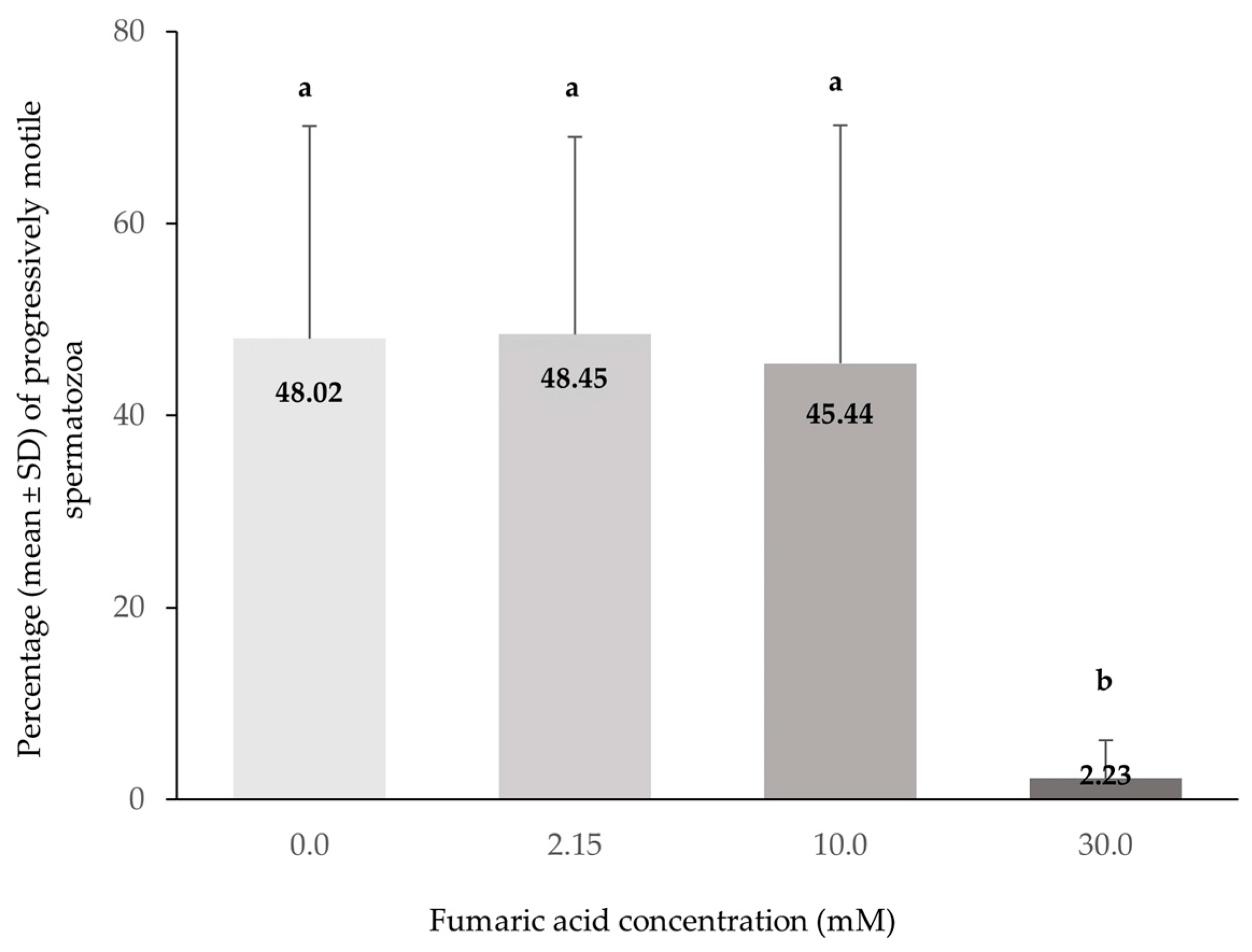
3.1. CASA Motility
3.2. Spermatozoa Viability (Eosin–Nigrosin)
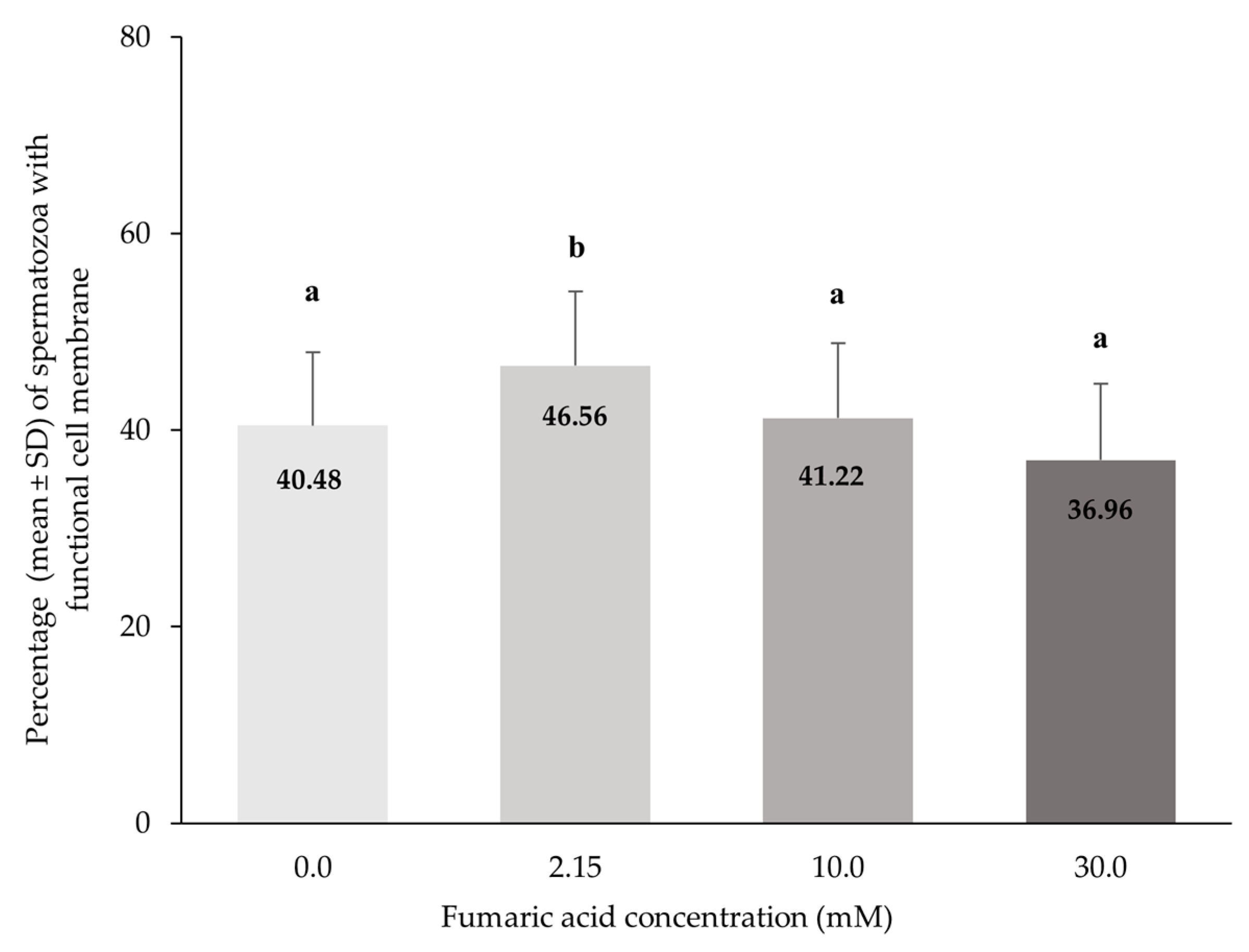
3.3. Plasma Membrane Functional Integrity
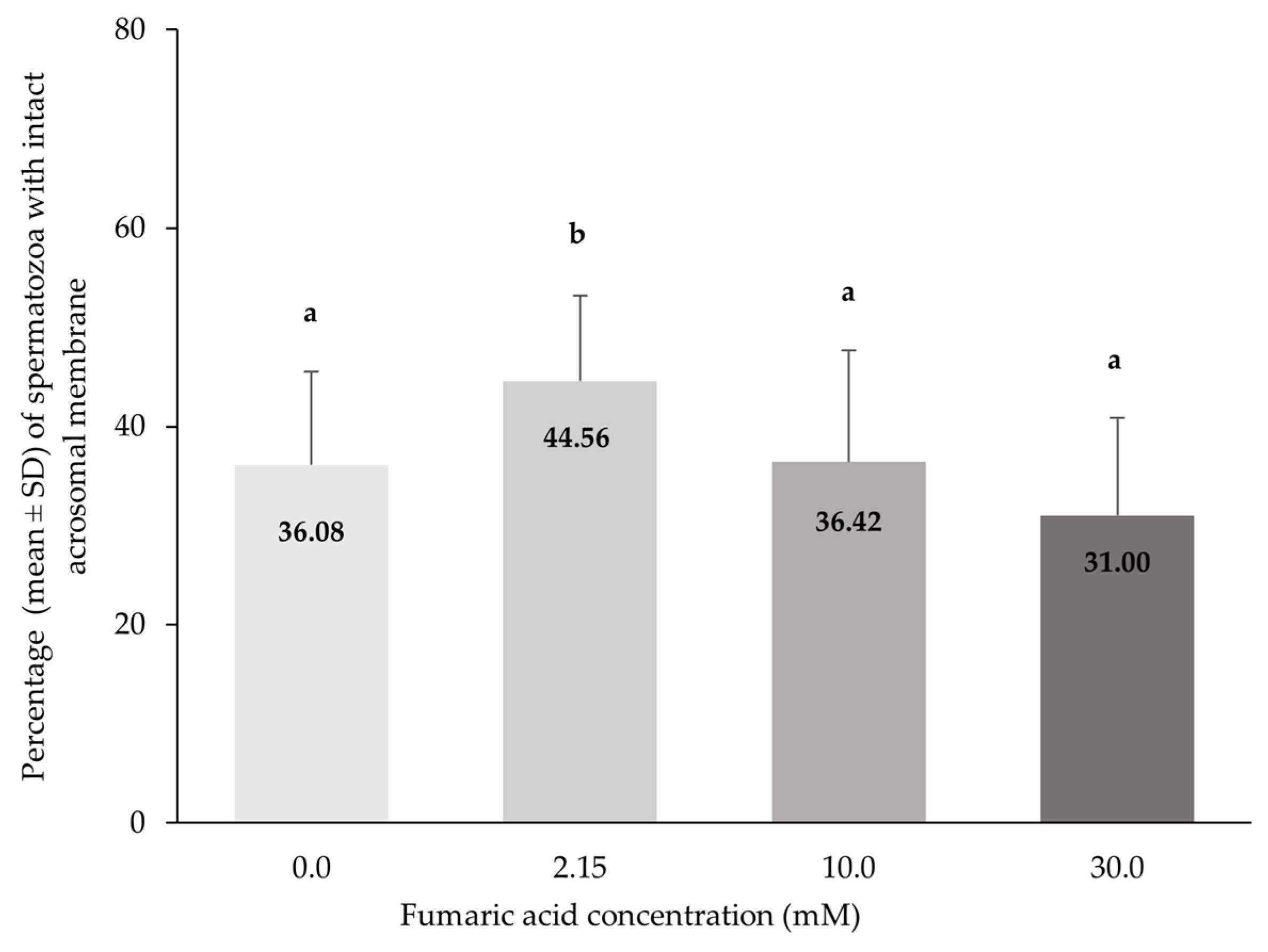
3.4. Acrosome Integrity
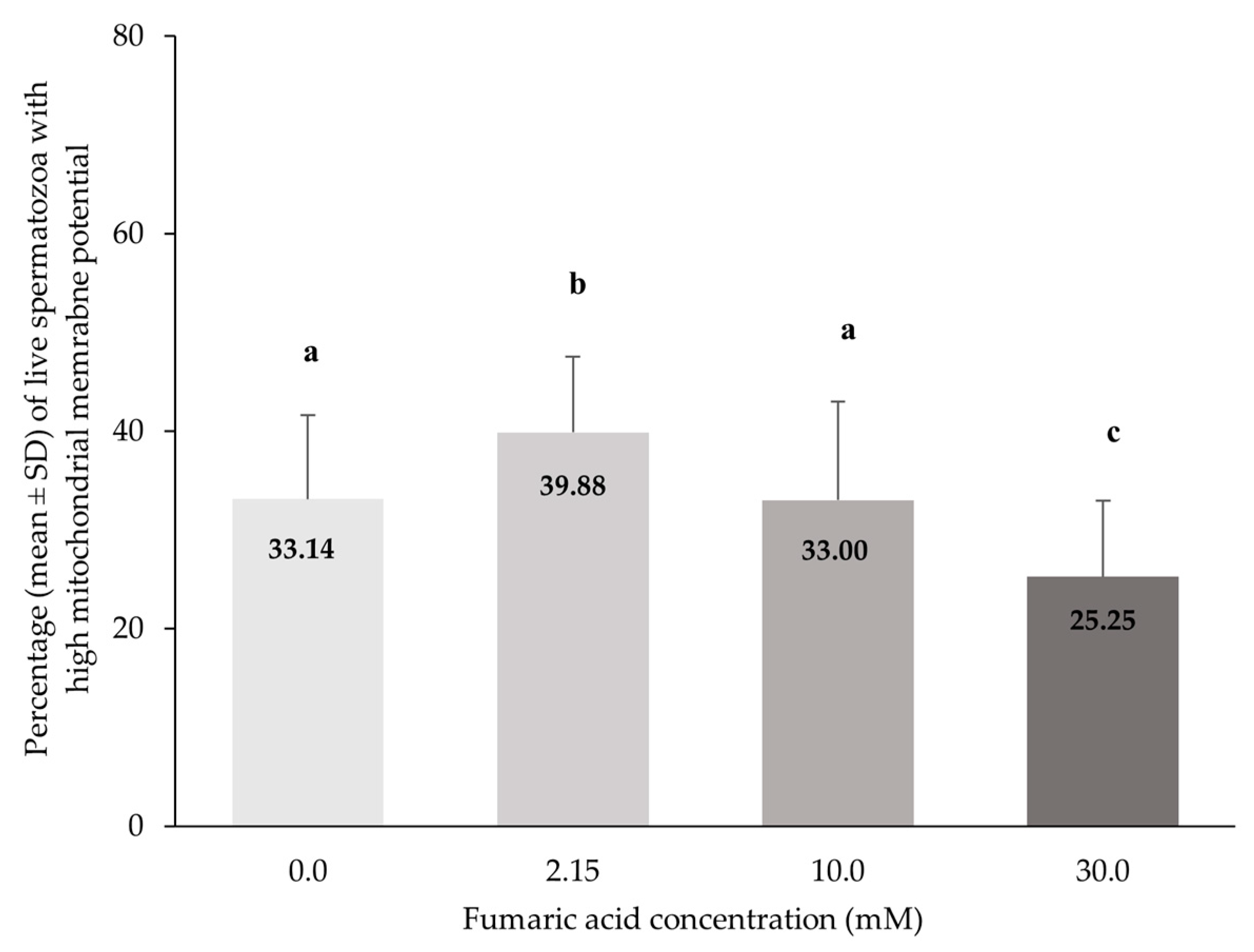
3.5. Mitochondrial Membrane Function Combined with Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gangwar, C.; Kharche, S.D.; Kumar, S.; Jindal, S.K. Cryopreservation of Goat Semen: Status and Prospects. Indian J. Small Ruminants. 2016, 22, 1–10. [Google Scholar] [CrossRef]
- Leboeuf, B.; Restall, B.; Salamon, S. Production and Storage of Goat Semen for Artificial Insemination. Anim. Reprod. Sci. 2000, 62, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The Causes of Reduced Fertility with Cryopreserved Semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Evans, G.; Maxwell, W.M.C. Flow Cytometric Evaluation of Sperm Parameters in Relation to Fertility Potential. Theriogenology 2005, 63, 445–457. [Google Scholar] [CrossRef]
- Dorado, J.; Hidalgo, M.; Muñoz, A.; Rodríguez, I. Assessment of Goat Semen Freezability According to the Spermatozoa Characteristics from Fresh and Frozen Samples. Anim. Reprod. Sci. 2009, 112, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, J.; Zhang, Y.; Liu, Y.; Sun, X.; Quan, G.; Alvarez Rodriguez, M.; Zhou, G. Editorial: Cryopreservation of Mammalian Gametes and Embryos: Implications of Oxidative and Nitrosative Stress and Potential Role of Antioxidants. Front. Vet. Sci. 2023, 10, 1174756. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Sakin, F.; Ateşşahin, A.; Kulaksız, R.; Çevik, M. The Effect of Antioxidants on Post-Thawed Angora Goat (Capra Hircus Ancryrensis) Sperm Parameters, Lipid Peroxidation and Antioxidant Activities. Small Rumin. Res. 2010, 89, 24–30. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Impact of Reactive Oxygen Species on Spermatozoa: A Balancing Act between Beneficial and Detrimental Effects. Hum. Reprod. 1995, 10, 15–21. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C. The Effect of Reactive Oxygen Species on Equine Sperm Motility, Viability, Acrosomal Integrity, Mitochondrial Membrane Potential, and Membrane Lipid Peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar]
- Aitken, R.J. Reactive Oxygen Species as Mediators of Sperm Capacitation and Pathological Damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial Generation of Superoxide and Hydrogen Peroxide as the Source of Mitochondrial Redox Signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Horváth, A.; Szenci, O.; Nagy, K.; Végh, L.; Pribenszky, C. Stress Preconditioning of Semen before Cryopreservation Improves Fertility and Increases the Number of Offspring Born: A Prospective Randomised Study Using a Porcine Model. Reprod. Fertil. Dev. 2016, 28, 475. [Google Scholar] [CrossRef]
- Upadhyay, V.R.; Ramesh, V.; Dewry, R.K.; Kumar, G.; Raval, K.; Patoliya, P. Implications of Cryopreservation on Structural and Functional Attributes of Bovine Spermatozoa: An Overview. Andrologia 2021, 53, e14154. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, J.; Qazi, I.H.; Fang, Y.; Zhou, G. Use of Melatonin in Sperm Cryopreservation of Farm Animals: A Brief Review. Anim. Reprod. Sci. 2021, 233, 106850. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, H.; Fuchs, I.; Koess, C. Interrelationship Between Plasma Membrane Integrity, Mitochondrial Membrane Potential and DNA Fragmentation in Cryopreserved Bovine Spermatozoa. Reprod. Domest. Anim. 2008, 43, 189–195. [Google Scholar] [CrossRef]
- Griffin, R.A.; Baker, M.; Aitken, R.J.; Swegen, A.; Gibb, Z. What Makes a Fertile Sperm? Unique Molecular Attributes of Stallion Fertility. Reproduction 2019, 158, R125–R137. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of Oxidative Stress and Antioxidants on Semen Functions. Vet. Med. Int. 2011, 2011, 686137. [Google Scholar] [CrossRef]
- Emamverdi, M.; Zhandi, M.; Shahneh, A.Z.; Sharafi, M.; Akhlaghi, A.; Motlagh, M.K.; Dadkhah, F.; Davachi, N.D. Flow Cytometric and Microscopic Evaluation of Post-Thawed Ram Semen Cryopreserved in Chemically Defined Home-Made or Commercial Extenders. Anim. Prod. Sci. 2015, 55, 551. [Google Scholar] [CrossRef]
- Chaudhari, D.V.; Dhami, A.J.; Hadiya, K.K.; Patel, J.A. Relative Efficacy of Egg Yolk and Soya Milk-Based Extenders for Cryopreservation (−196 °C) of Buffalo Semen. Vet. World 2015, 8, 239–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tuncer, P.B.; Bucak, M.N.; Sarıözkan, S.; Sakin, F.; Yeni, D.; Çiğerci, İ.H.; Ateşşahin, A.; Avdatek, F.; Gündoğan, M.; Büyükleblebici, O. The Effect of Raffinose and Methionine on Frozen/Thawed Angora Buck (Capra Hircus Ancryrensis) Semen Quality, Lipid Peroxidation and Antioxidant Enzyme Activities. Cryobiology 2010, 61, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Alcay, S.; Gokce, E.; Toker, M.B.; Onder, N.T.; Ustuner, B.; Uzabacı, E.; Gul, Z.; Cavus, S. Freeze-Dried Egg Yolk Based Extenders Containing Various Antioxidants Improve Post-Thawing Quality and Incubation Resilience of Goat Spermatozoa. Cryobiology 2016, 72, 269–273. [Google Scholar] [CrossRef]
- Purdy, P.H. A Review on Goat Sperm Cryopreservation. Small Rumin. Res. 2006, 63, 215–225. [Google Scholar] [CrossRef]
- Bergeron, A.; Manjunath, P. New Insights towards Understanding the Mechanisms of Sperm Protection by Egg Yolk and Milk. Mol. Reprod. Dev. 2006, 73, 1338–1344. [Google Scholar] [CrossRef]
- Sun, L.; Fan, W.; Wu, C.; Zhang, S.; Dai, J.; Zhang, D. Effect of Substituting Different Concentrations of Soybean Lecithin and Egg Yolk in Tris-Based Extender on Goat Semen Cryopreservation. Cryobiology 2020, 92, 146–150. [Google Scholar] [CrossRef]
- Wollina, U. Fumaric Acid Esters in Dermatology. Indian Dermatol. Online J. 2011, 2, 111. [Google Scholar] [CrossRef]
- Dzarbusynov, B.U.; Merkusheva, N.V.; Shalekenov, B.U. The determination of Krebs cycle carboxylic acids in the ejaculate by chromatography. Klin. Lab. Diagn. 1993, 6, 11–13. [Google Scholar]
- Velho, A.L.C.; Menezes, E.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Metabolomic Markers of Fertility in Bull Seminal Plasma. PLoS ONE 2018, 13, e0195279. [Google Scholar] [CrossRef]
- Ahuja, M.; Ammal Kaidery, N.; Yang, L.; Calingasan, N.; Smirnova, N.; Gaisin, A.; Gaisina, I.N.; Gazaryan, I.; Hushpulian, D.M.; Kaddour-Djebbar, I.; et al. Distinct Nrf2 Signaling Mechanisms of Fumaric Acid Esters and Their Role in Neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Experimental Parkinson’s-Like Disease. J. Neurosci. 2016, 36, 6332–6351. [Google Scholar] [CrossRef]
- Nour, O.A.; Shehatou, G.S.G.; Rahim, M.A.; El-Awady, M.S.; Suddek, G.M. Antioxidant and Anti-Inflammatory Effects of Dimethyl Fumarate in Hypercholesterolemic Rabbits. Egypt. J. Basic. Appl. Sci. 2017, 4, 153–159. [Google Scholar] [CrossRef]
- Lee, K.; Alcaraz, A.; Soung, J. Fumaric Acid Esters in Dermatology. In Biologic and Systemic Agents in Dermatology; Yamauchi, P.S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 199–208. ISBN 978-3-319-66883-3. [Google Scholar]
- Hayes, J.D.; McMahon, M. NRF2 and KEAP1 Mutations: Permanent Activation of an Adaptive Response in Cancer. Trends Biochem. Sci. 2009, 34, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses Via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Litjens, N.H.; Van Strijen, E.; Van Gulpen, C.; Mattie, H.; Van Dissel, J.T.; Thio, H.B.; Nibbering, P.H. In Vitro Pharmacokinetics of Anti-Psoriatic Fumaric Acid Esters. BMC Pharmacol. 2004, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.-H.; Ryan, S.; Van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric Acid Esters Exert Neuroprotective Effects in Neuroinflammation via Activation of the Nrf2 Antioxidant Pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Shivanandappa, T.B.; Kumar, M.; Kushwah, A.S. Fumaric Acid Protect the Cadmium-Induced Hepatotoxicity in Rats: Owing to Its Antioxidant, Anti-Inflammatory Action and Aid in Recast the Liver Function. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1911–1920. [Google Scholar] [CrossRef]
- Thiessen, A.; Schmidt, M.M.; Dringen, R. Fumaric Acid Dialkyl Esters Deprive Cultured Rat Oligodendroglial Cells of Glutathione and Upregulate the Expression of Heme Oxygenase 1. Neurosci. Lett. 2010, 475, 56–60. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dringen, R. Fumaric Acid Diesters Deprive Cultured Primary Astrocytes Rapidly of Glutathione. Neurochem. Int. 2010, 57, 460–467. [Google Scholar] [CrossRef]
- Fahim, A.T.; Abd El-Fattah, A.A.; Sadik, N.A.H.; Ali, B.M. Resveratrol and Dimethyl Fumarate Ameliorate Testicular Dysfunction Caused by Chronic Unpredictable Mild Stress-Induced Depression in Rats. Arch. Biochem. Biophys. 2019, 665, 152–165. [Google Scholar] [CrossRef]
- Szcześniak-Fabiańczyk, B.; Bochenek, M.; Smorag, Z.; Ryszka, F. Effect of Antioxidants Added to Boar Semen Extender on the Semen Survival Time and Sperm Chromatin Structure. Reprod. Biol. 2003, 3, 81–87. [Google Scholar]
- Rekkas, C.; Saratsi, A.; Panagiotidis, I.; Samartzi, F.; Basioura, A.; Tsiokos, D.; Ligda, C. Effect of Fumaric Acid Addition to Soy Lecithin Extender on Post-Thaw Parameters of Buck Semen Quality; Reproduction in Domestic Animals: Thessaloniki, Greece, 2022; Volume 57, (Suppl. 4), p. 86. [Google Scholar]
- Fordyce, G.; Entwistle, K.; Norman, S.; Perry, V.; Gardiner, B.; Fordyce, P. Standardising Bull Breeding Soundness Evaluations and Reporting in Australia. Theriogenology 2006, 66, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Taraboletti, A.; Shriver, L.P. Dimethyl Fumarate Modulates Antioxidant and Lipid Metabolism in Oligodendrocytes. Redox Biol. 2015, 5, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sebök, B.; Bonnekoh, B.; Geisel, J.; Mahrle, G. Antiproliferative and Cytotoxic Profiles of Antipsoriatic Fumaric Acid Derivatives in Keratinocyte Cultures. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1994, 270, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.F.; Torres, C.A.A.; Maffili, V.V.; Borges, A.M.; Santos, A.D.F.; Rodrigues, M.T.; Oliveira, R.F.M. The Hypoosmotic Swelling Test in Fresh Goat Spermatozoa. Anim. Reprod. 2005, 2, 139–144. [Google Scholar]
- Van Der Horst, G.; Maree, L. SpermBlue®: A New Universal Stain for Human and Animal Sperm Which Is Also Amenable to Automated Sperm Morphology Analysis. Biotech. Histochem. 2010, 84, 299–308. [Google Scholar] [CrossRef]
- Garner, D.L.; Thomas, C.A.; Joerg, H.W.; DeJarnette, J.M.; Marshall, C.E. Fluorometric Assessments of Mitochondrial Function and Viability in Cryopreserved Bovine Spermatozoa1. Biol. Reprod. 1997, 57, 1401–1406. [Google Scholar] [CrossRef]
- Garner, D.L.; Johnson, L.A. Viability Assessment of Mammalian Sperm Using SYBR-14 and Propidium Iodide1. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- El-Kon, I.I.; Darwish, S.A. Effect of Glutathione (GSH) on Microscopic Parameters and DNA Integrity in Egyptian Buffalo Semen During Liquid and Frozen Storage. J. Reprod. Infertil. 2011, 2, 32–40. [Google Scholar]
- Sharafi, M.; Zhandi, M.; Akbari Sharif, A. Supplementation of Soybean Lecithin-Based Semen Extender by Antioxidants: Complementary Flowcytometric Study on Post-Thawed Ram Spermatozoa. Cell Tissue Bank. 2015, 16, 261–269. [Google Scholar] [CrossRef]
- Zhandi, M.; Sharafi, M. Negative Effect of Combined Cysteine and Glutathione in Soy Lecithin-Based Extender on Post-Thawed Ram Spermatozoa. Cell Tissue Bank. 2015, 16, 443–448. [Google Scholar] [CrossRef]
- Sanchez-Partida, L.; Maxwell, W.; Paleg, L.; Setchell, B. Proline and Glycine Betaine in Cryoprotective Diluents for Ram Spermatozoa. Reprod. Fertil. Dev. 1992, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Khalili, B.; Jafaroghli, M.; Farshad, A.; Paresh-Khiavi, M. The Effects of Different Concentrations of Glycine and Cysteine on the Freezability of Moghani Ram Spermatozoa. Asian Australas. J. Anim. Sci. 2010, 23, 318–325. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, M.-K.; Song, H.-J.; Kang, E.-J.; Ock, S.-A.; Mohana Kumar, B.; Balasubramanian, S.; Rho, G.-J. Effect of α-Tocopherol Supplementation during Boar Semen Cryopreservation on Sperm Characteristics and Expression of Apoptosis Related Genes. Cryobiology 2009, 58, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Minaei, M.B.; Barbarestani, M.; Nekoonam, S.; Abdolvahabi, M.A.; Takzare, N.; Asadi, M.H.; Hedayatpour, A.; Amidi, F. Effect of Trolox Addition to Cryopreservation Media on Human Sperm Motility. Iran. J. Reprod. Med. 2012, 10, 99–104. [Google Scholar] [PubMed]
- Domínguez-Rebolledo, Á.E.; Fernández-Santos, M.R.; Bisbal, A.; Ros-Santaella, J.L.; Ramón, M.; Carmona, M.; Martínez-Pastor, F.; Garde, J.J. Improving the Effect of Incubation and Oxidative Stress on Thawed Spermatozoa from Red Deer by Using Different Antioxidant Treatments. Reprod. Fertil. Dev. 2010, 22, 856. [Google Scholar] [CrossRef]
- Wu, C.; Dai, J.; Zhang, S.; Sun, L.; Liu, Y.; Zhang, D. Effect of Thawing Rates and Antioxidants on Semen Cryopreservation in Hu Sheep. Biopreserv. Biobank. 2021, 19, 204–209. [Google Scholar] [CrossRef]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Álvarez, M.; Tamayo-Canul, J.; Anel, L.; De Paz, P.; Martínez-Pastor, F. Post-Thawing Quality and Incubation Resilience of Cryopreserved Ram Spermatozoa Are Affected by Antioxidant Supplementation and Choice of Extender. Theriogenology 2015, 83, 520–528. [Google Scholar] [CrossRef]
- Alvarez-Rodríguez, M.; Alvarez, M.; Anel-López, L.; Martínez-Rodríguez, C.; Martínez-Pastor, F.; Borragan, S.; Anel, L.; De Paz, P. The Antioxidant Effects of Soybean Lecithin- or Low-Density Lipoprotein-Based Extenders for the Cryopreservation of Brown-Bear (Ursus Arctos) Spermatozoa. Reprod. Fertil. Dev. 2013, 25, 1185. [Google Scholar] [CrossRef]
- Sarıözkan, S.; Bucak, M.N.; Tuncer, P.B.; Taşdemir, U.; Kinet, H.; Ulutaş, P.A. Effects of Different Extenders and Centrifugation/Washing on Postthaw Microscopic-Oxidative Stress Parameters and Fertilizing Ability of Angora Buck Sperm. Theriogenology 2010, 73, 316–323. [Google Scholar] [CrossRef]
- Del Valle, I.; Gomez-Duran, A.; Holt, W.V.; Muino-Blanco, T.; Cebrian-Perez, J.A. Soy Lecithin Interferes With Mitochondrial Function in Frozen-Thawed Ram Spermatozoa. J. Androl. 2012, 33, 717–725. [Google Scholar] [CrossRef]
- Layek, S.S.; Mohanty, T.K.; Kumaresan, A.; Parks, J.E. Cryopreservation of Bull Semen: Evolution from Egg Yolk Based to Soybean Based Extenders. Anim. Reprod. Sci. 2016, 172, 1–9. [Google Scholar] [CrossRef]
- Kwak, M.K.; Itoh, K.; Yamamoto, M.; Sutter, T.R.; Kensler, T.W. Role of Transcription Factor Nrf2 in the Induction of Hepatic Phase 2 and Antioxidative Enzymes in Vivo by the Cancer Chemoprotective Agent, 3H-1, 2-Dimethiole-3-Thione. Mol. Med. 2001, 7, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 Signaling in Regulation of Antioxidants and Phase 2 Enzymes in Cardiac Fibroblasts: Protection against Reactive Oxygen and Nitrogen Species-Induced Cell Injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, B.N.; Lawson, G.; Chan, J.Y.; Banuelos, J.; Cortés, M.M.; Hoang, Y.D.; Ortiz, L.; Rau, B.A.; Luderer, U. Knockout of the Transcription Factor NRF2 Disrupts Spermatogenesis in an Age-Dependent Manner. Free Radic. Biol. Med. 2010, 49, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Mai, Z.; Zhou, Y.; Gao, X.; Yu, B. Low NRF2 MRNA Expression in Spermatozoa from Men with Low Sperm Motility. Tohoku J. Exp. Med. 2012, 228, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Li, X.; Zhang, P.; Wang, J.; Zhu, D.; Chen, X.; Ye, L. Low Long Non-Coding RNA HOTAIR Expression Is Associated with down-Regulation of Nrf2 in the Spermatozoa of Patients with Asthenozoospermia or Oligoasthenozoospermia. Int. J. Clin. Exp. Pathol. 2015, 8, 14198–14205. [Google Scholar] [PubMed]
- Deng, S.-L.; Sun, T.-C.; Yu, K.; Wang, Z.-P.; Zhang, B.-L.; Zhang, Y.; Wang, X.-X.; Lian, Z.-X.; Liu, Y.-X. Melatonin Reduces Oxidative Damage and Upregulates Heat Shock Protein 90 Expression in Cryopreserved Human Semen. Free Radic. Biol. Med. 2017, 113, 347–354. [Google Scholar] [CrossRef]
- Pariz, J.R.; Ranéa, C.; Monteiro, R.A.C.; Evenson, D.P.; Drevet, J.R.; Hallak, J. Melatonin and Caffeine Supplementation Used, Respectively, as Protective and Stimulating Agents in the Cryopreservation of Human Sperm Improves Survival, Viability, and Motility after Thawing Compared to Traditional TEST-Yolk Buffer. Oxidative Med. Cell. Longev. 2019, 2019, 6472945. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.; Qin, L.; Reiter, R. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial Membrane Potential Profile and Its Correlation with Increasing Sperm Motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef]
- Ferramosca, A.; Provenzano, S.P.; Coppola, L.; Zara, V. Mitochondrial Respiratory Efficiency Is Positively Correlated With Human Sperm Motility. Urology 2012, 79, 809–814. [Google Scholar] [CrossRef]
- Moscatelli, N.; Spagnolo, B.; Pisanello, M.; Lemma, E.D.; De Vittorio, M.; Zara, V.; Pisanello, F.; Ferramosca, A. Single-Cell-Based Evaluation of Sperm Progressive Motility via Fluorescent Assessment of Mitochondria Membrane Potential. Sci. Rep. 2017, 7, 17931. [Google Scholar] [CrossRef]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive Oxygen Species and Sperm Function--In Sickness and In Health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Ogawa, K.; Mizuno, K.; Nagai, S.; Uchida, Y.; Ohta, S.; Fujie, M.; Suzuki, K.; Hirata, S.; Hoshi, K. Relationship between Sperm Mitochondrial Membrane Potential, Sperm Motility, and Fertility Potential. Asian J. Androl. 2002, 4, 97–103. [Google Scholar] [PubMed]
- Marchetti, P.; Ballot, C.; Jouy, N.; Thomas, P.; Marchetti, C. Influence of Mitochondrial Membrane Potential of Spermatozoa on in Vitro Fertilisation Outcome: Mitochondrial Membrane Potential and IVF Outcome. Andrologia 2012, 44, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, W.; Zou, P.; Jiang, F.; Zeng, Y.; Chen, Q.; Sun, L.; Yang, H.; Zhou, N.; Wang, X.; et al. Mitochondrial Functionality Modifies Human Sperm Acrosin Activity, Acrosome Reaction Capability and Chromatin Integrity. Hum. Reprod. 2019, 34, 3–11. [Google Scholar] [CrossRef]
- Darr, C.R.; Cortopassi, G.A.; Datta, S.; Varner, D.D.; Meyers, S.A. Mitochondrial Oxygen Consumption Is a Unique Indicator of Stallion Spermatozoal Health and Varies with Cryopreservation Media. Theriogenology 2016, 86, 1382–1392. [Google Scholar] [CrossRef]
- Losano, J.D.A.; Padín, J.F.; Méndez-López, I.; Angrimani, D.S.R.; García, A.G.; Barnabe, V.H.; Nichi, M. The Stimulated Glycolytic Pathway Is Able to Maintain ATP Levels and Kinetic Patterns of Bovine Epididymal Sperm Subjected to Mitochondrial Uncoupling. Oxidative Med. Cell. Longev. 2017, 2017, 1682393. [Google Scholar] [CrossRef]
- Hu, C.H.; Zhuang, X.J.; Wei, Y.M.; Zhang, M.; Lu, S.S.; Lu, Y.Q.; Yang, X.G.; Lu, K.H. Comparison of Mitochondrial Function in Boar and Bull Spermatozoa Throughout Cryopreservation Based on JC-1 Staining. Cryo Lett. 2017, 38, 75–79. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saratsi, A.; Samartzi, F.; Panagiotidis, I.; Basioura, A.; Tsiokos, D.; Ligda, C.; Rekkas, C.A. Post-Thaw Parameters of Buck Semen Quality after Soy Lecithin Extender Supplementation with Fumaric Acid. Vet. Sci. 2023, 10, 569. https://doi.org/10.3390/vetsci10090569
Saratsi A, Samartzi F, Panagiotidis I, Basioura A, Tsiokos D, Ligda C, Rekkas CA. Post-Thaw Parameters of Buck Semen Quality after Soy Lecithin Extender Supplementation with Fumaric Acid. Veterinary Sciences. 2023; 10(9):569. https://doi.org/10.3390/vetsci10090569
Chicago/Turabian StyleSaratsi, Aikaterini, Foteini Samartzi, Ioannis Panagiotidis, Athina Basioura, Dimitrios Tsiokos, Christina Ligda, and Constantinos A. Rekkas. 2023. "Post-Thaw Parameters of Buck Semen Quality after Soy Lecithin Extender Supplementation with Fumaric Acid" Veterinary Sciences 10, no. 9: 569. https://doi.org/10.3390/vetsci10090569
APA StyleSaratsi, A., Samartzi, F., Panagiotidis, I., Basioura, A., Tsiokos, D., Ligda, C., & Rekkas, C. A. (2023). Post-Thaw Parameters of Buck Semen Quality after Soy Lecithin Extender Supplementation with Fumaric Acid. Veterinary Sciences, 10(9), 569. https://doi.org/10.3390/vetsci10090569





