Simple Summary
Protozoan parasites of the genus Sarcocystis are characterized by a mandatory two-host, prey–predator life cycle. Several Sarcocystis species are known to form macroscopic or microscopic sarcocysts in the muscle tissues of domestic sheep and goats. It has been long considered that Sarcocystis species parasitizing farm animals are specific to intermediate hosts. However, some studies have recently reported the unexpected detection of Sarcocystis species in animals that are not considered to be their canonical hosts. In the current investigation, muscle samples of sheep and goats from Lithuania were molecularly tested for species previously described in such hosts and for other non-canonical Sarcocystis spp. Based on DNA sequence analysis, along with canonical Sarcocystis species present in their respective hosts, non-canonical (atypical) species, such as S. capracanis and S. morae, were detected in sheep, while S. arieticanis and S. tenella were found in goats. Possible explanations of the obtained results are discussed.
Abstract
Contradictory data is available on the intermediate host specificity of Sarcocystis spp. in farm animals. Therefore, the current work aimed at molecularly testing samples of sheep and goats reared in Lithuania to identify Sarcocystis species described in other intermediate hosts but suspected to be non-canonical parasites to these small ruminants. For this purpose, muscle samples from 47 domestic sheep and nine goats were examined. Sarcocystis species were identified using direct and nested PCR targeting cox1 and sequencing of positive amplified products. Along with the detection of the canonical Sarcocystis spp. in their respective intermediate hosts, the DNA of S. capracanis and S. morae was detected in sheep, although these species were previously thought to be specific to goats and deer, respectively. In addition, DNA from S. arieticanis and S. tenella was found in goats, even though these two species were believed to be sheep-specific. Notably, under light microscopy, only sarcocysts of S. capracanis specific to goats were observed. Thus, future research on the life cycle and host-specificity of Sarcocystis spp. examined is warranted.
1. Introduction
Parasites of the genus Sarcocystis have a mandatory two-host life cycle that is usually rooted in an ecological prey–predator relationship. Typically, herbivores such as sheep (Ovis aries) and goats (Capra hircus), act as intermediate hosts, while carnivores, such as Felidae and Canidae, are definitive hosts. Specific intermediate hosts can become infected by ingesting food or water contaminated with Sarcocystis oocysts or sporocysts. Depending on the Sarcocystis spp., either microscopic or macroscopic tissue cysts (sarcocysts) will develop and allocate (especially) in the striated muscle of the intermediate host. When meat containing mature sarcocysts is consumed by a predator or scavenger, the parasite undergoes sexual reproduction and forms isosporoid oocysts in the intestines of the definitive host [1].
A significant number of reports of Sarcocystis spp. infection in sheep is available worldwide [2,3,4,5]. Comprehensive studies indicate that at least four Sarcocystis species, S. arieticanis, S. tenella, S. gigantea, and S. medusiformis, use sheep as intermediate hosts [6]. Both S. arieticanis and S. tenella form microscopic sarcocysts in the muscles of sheep and use canids as definitive hosts [1]. Meanwhile, S. gigantea and S. medusiformis form macroscopic sarcocysts in the muscles of sheep and use felids as definitive hosts [7]. Two additional species, S. mihoensis and S. microps, proposed to have dogs as the definitive host, have only been identified in two reports from Japan and China, respectively, and available molecular and biological details are scarce [8,9]. Usually, Sarcocystis infection is non-lethal to the ruminant intermediate host; nonetheless, the economic impact on profits associated with reduced production and carcass condemnation should be taken into consideration [1,2]. By contrast, significantly fewer reports on Sarcocystis spp. infecting domestic goats are available. Three different Sarcocystis species, S. capracanis, S. hircicanis, and S. moulei, are proposed to use goats as intermediate hosts. Two species, S. capracanis and S. hircicanis, form microscopic sarcocysts and use canids as definitive hosts, while S. moulei constitutes macroscopic sarcocysts and uses felids as definitive hosts (reviewed by [10]). However, most of the research has been conducted on S. capracanis, considered to be the most frequent and of most pathogenicity in goats [11]. To date, no zoonotic Sarcocystis spp. have been detected in small ruminants.
Recent studies indicate that the 18S rRNA molecular marker has limited discriminatory power when investigating closely related Sarcocystis spp. infecting ungulates as intermediate hosts. In contrast, cox1 was successfully used to identify Sarcocystis species present in sheep and goats [12,13,14]. Accurate species identification based on appropriate molecular markers and further sequencing confirmation procedures are key elements for a comprehensive investigation of the complex epidemiological scenario of the Sarcocystis genus.
For a long time, it has been widely assumed that Sarcocystis spp. found in farm animals are strictly specific to the intermediate host [1]. However, Sarcocystis species have recently been increasingly reported in non-specific hosts. For instance, goat-specific S. capracanis has been reported in domestic sheep [15], while S. moulei that also utilizes goats as an intermediate host has been reported both in domestic sheep [16,17] and water buffalo (Bubalus bubalis) [18]. Moreover, sheep-specific S. tenella has been detected in wild goats (Capra aegagrus) [11,19]. Nevertheless, the outcomes of some of these findings are currently under discussion, and additional molecular and ultrastructural analyses are required [20].
In Lithuania, among farm animals, cattle (Bos taurus), horses (Equus ferus caballus), pigs (Sus scrofa), and sheep were previously examined for the presence of Sarcocystis spp. by means of morphological [21] and molecular methods [22,23]. Specifically, investigations carried out on sarcocysts excised from various analyzed muscles (oesophagus, diaphragm, and heart) of sheep by means of cox1 sequence analysis revealed the occurrence of S. arieticanis and S. tenella [23]. By contrast, no previous studies have been conducted on the identification of Sarcocystis spp. in goats bred in the country.
Given the contradictory data on the host-specificity of Sarcocystis species forming sarcocysts in sheep and goats, the present study aims at characterizing morphologically and genetically Sarcocystis sarcocysts that are present in the muscles of domestic goats, and to detect through robust cox1 sequence analysis the potential presence of other non-canonical Sarcocystis spp. in Lithuanian sheep and goat muscle homogenates.
2. Materials and Methods
2.1. Sample Collection
Between 2019 and 2021, diaphragm, oesophagus, and heart samples were collected from 47 healthy domestic sheep following the slaughter process. Furthermore, three diaphragms, five hearts, and eight oesophagi were collected from healthy goats. The entire organs were obtained to examine the presence of Sarcocystis spp. infection. According to the Government system of animal registration, among the sheep, seven were under a year old, and 40 were adults. Among the goats, four were younger than one year, while the remaining four were older than two years. Sheep and goats were slaughtered for the purpose of meat consumption at the licensed slaughterhouse “Alantos agroservisas, UAB”, located in the village of Alanta, Molėtai district. The investigated sheep and goats were mainly raised in the eastern part of Lithuania. Muscle samples of slaughtered animals were taken by the company veterinarian and delivered to the Laboratory of Molecular Ecology, Nature Research Centre (Vilnius, Lithuania) for detailed morphological and molecular analysis of Sarcocystis spp. The transported muscle samples were stored frozen (at −20 °C) until further analysis. The general information about sheep and goats is known to the company’s veterinarian, and the farmers’ consents have been provided to the company. None of the animals were intentionally euthanized for the purpose of this research, and all sample collection procedures adhered to accepted animal welfare guidelines.
2.2. Microscopic Examination for the Presence of Sarcocysts in Goat Tissues
The collected tissue samples from goats were visually checked for macroscopic Sarcocystis-like structures. Subsequently, a morphological analysis of the microscopic sarcocysts was conducted using freshly squashed muscle samples. A microscopical examination was performed under a Nikon ECLIPSE 8oi light microscope (Nikon Corp., Tokyo, Japan). Sarcocysts excised from fresh muscle samples were preserved individually in separate tubes containing 96% of ethyl alcohol at −20 °C for the molecular examination.
2.3. Molecular Analysis of Sarcocysts Excised from Goat Tissues
DNA extraction of sarcocysts (n = 2) was carried out with the GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) in accordance with the manufacturer’s recommendations.
Partial cox1 sequences were amplified by conventional PCR using forward SF1 [24] and reverse SsunR3 [20] primers. Each PCR reaction was carried out in a 25 μL mixture containing 12.5 μL of DreamTaq PCR Master Mix (Thermo Fisher Scientific Baltics, Vilnius, Lithuania), 5 μL of DNA template, 0.5 μM of both forward and reverse primers and nuclease-free water. The cycling conditions were the same as described [23] previously. The amplified products were visualized by horizontal 1% agarose gel electrophoresis. Positive samples were purified with exonuclease ExoI and phosphatase FastAP (Thermo Fisher Scientific Baltics, Vilnius, Lithuania).
Sequencing of the selected PCR samples was performed using a Big-Dye®Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) and a 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA), following the manufacturer’s specifications. The resulting cox1 sequences of Sarcocystis spp. were compared with the most similar ones available in NCBI GenBank using the Nucleotide BLAST function (http://blast.ncbi.nlm.nih.gov/, accessed on 12 June 2023).
2.4. Acid-Pepsin Digestion of Muscle Samples from Sheep and Goats
Aiming for sensitive detection of Sarcocystis spp., muscle samples from 47 sheep (n = 141) and nine goats (n = 16) were subjected to identification of Sarcocystis spp. by combined acid-pepsin digestion and PCR techniques [23]. Artificial digestion was carried out by following a modified protocol that was initially designed for the isolation of Toxoplasma gondii from animal tissues [25]. Samples were prepared by cutting several pieces of tissue from different parts of the organ. Twenty-five g of each muscle sample were added to 150 mL of saline and blended. Further procedures of pepsin digestion were performed according to our previous study [23].
2.5. Molecular Detection of Specific Sarcocystis spp. in the Tissue Digests
All digested samples were subjected to the extraction of the genomic DNA using a PureLink Microbiome DNA Purification Kit (Invitrogen by Thermo Fisher Scientific, Waltham, MA, USA). For the specific detection of the canonical species that are present in domestic sheep (S. arieticanis, S. gigantea, S. medusiformis, S. mihoensis, and S. tenella) and goats (S. capracanis, S. hircicanis, and S. moulei) and to investigate their degree of unspecificity, conventional and nested PCR (nPCR) assays were employed (see below). In addition, given the previous reports of the wide host range of S. morae [26,27,28], specific tests were carried out. Primers used for direct and nPCR are compiled in Table 1. Due to the absence of available cox1 data in the GenBank regarding S. moulei when the primers were designed, the 28S rRNA gene was chosen as the molecular target for investigating this particular species of Sarcocystis.

Table 1.
List of PCR primers, their sequences, annealing temperatures, predicted size of amplicons, and target genes.
Positive DNA controls for S. arieticanis, S. capracanis, S. morae, and S. tenella were obtained from the individual sarcocysts acquired during our previous investigations [20,23,27]. The specificity of the primers was evaluated by testing them with DNA taken from sarcocysts of the particular Sarcocystis species being studied. Only the DNA from the targeted species produced amplicons of the expected sizes using the designed primers.
Each sample was subjected to conventional and nPCR assays. For both direct PCR and nPCR, the same conditions were employed as previously described [23]. Except for the annealing temperatures (53–61 °C), which were chosen according to the primer pair used for the reaction (Table 1), negative controls were employed as described in our earlier study [23].
2.6. Phylogenetic and Statistical Analysis
The cox1 sequences of Sarcocystis spp. from sheep and goat tissues and from the individual sarcocysts of goat samples were deposited in GenBank with the accession numbers OR258580-OR258681. The obtained sequences were compared with those of the most closely related Sarcocystis spp. using Nucleotide BLAST (http://blast.ncbi.nlm.nih.gov/, accessed on 12 June 2023). For the phylogenetic analysis, sequences from this work were aligned with those gathered from the GenBank database by using the MUSCLE algorithm available in the MEGA7 software [29]. The final alignment consisted of 241 nucleotide positions and 29 taxa. The selection of the nucleotide evolutionary model (the Kimura 2-parameter + G) [30] and the construction of a phylogenetic tree under Bayesian inference were carried out by TOPALi v2.5 software [31]. For the analysis, general settings were as follows: 2 runs, 1,000,000 generations, 10 sample frequencies, and 25% burn-in. The phylogenetic tree was rooted in S. truncata from wild ungulates.
3. Results
3.1. Morphological Observations and Molecular Identification of Sarcocysts Present in Goat Tissues
By fresh squeezing, Sarcocystis-like sarcocysts were found in a single oesophagus specimen. One type of microcyst similar to those of S. capracanis was observed under a light microscope. Sarcocysts were elongated and spindle-shaped, measuring 500–1310 × 65–150 µm (948 ± 237 × 109 ± 24 µm; n = 20) in size (Figure 1a). The sarcocyst wall was thick with radial striations and finger-like protrusions, which were 2.8–4.6 µm (3.9 ± 0.5 µm; n = 20) in length (Figure 1b). Cysts were septated, and their inner compartments were filled with elongated, banana-shaped bradyzoites measuring 11.2–15.9 × 2.5–4.7 µm (14.0 ± 1.2 × 3.7 ± 0.5 µm; n = 60) in size (Figure 1c).
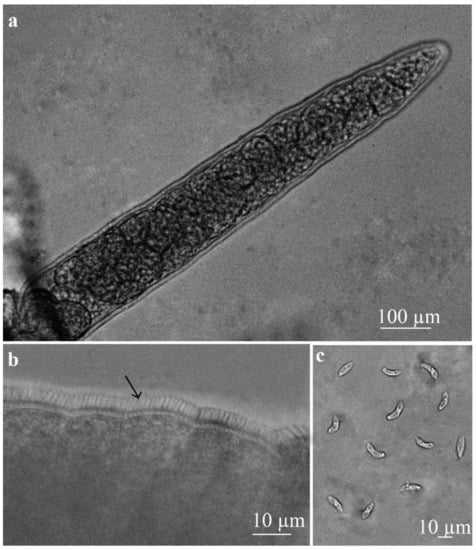
Figure 1.
Light microscopy morphology of S. capracanis excised from the oesophagus of a goat. Fresh preparations. (a) An elongated, spindle-shaped fragment of sarcocyst. (b) A portion of sarcocyst wall with finger-like protrusions to which arrow is pointed. (c) Banana-shaped bradyzoites.
Two sarcocysts were isolated from the oesophagus of a single goat (ChLTC33st.1 and ChLTC33st.2) and genetically characterized within cox1. The obtained 894 bp sequences showed 99.1% identity compared with each other. In comparison to the sequences available in the GenBank, they shared 98.0–99.6% identity with those of S. capracanis and demonstrated 93.5–94.4% similarity to the sister species S. tenella of sheep.
3.2. Molecular Identification of Sarcocystis spp. in the Digested Samples of Sheep and Goats Using Species-Specific PCR
Based on the species-specific PCRs using primers targeting cox1, two species, S. arieticanis and S. tenella, were identified in sheep tissues, and S. capracanis was confirmed in the muscles of goats by both conventional and nPCR (Table 2). By contrast, attempts to amplify S. gigantea, S. medusiformis, and S. mihoensis in samples of sheep and S. hircicanis and S. moulei in samples of goats using the designed primers did not yield any positive results. Apart from Sarcocystis species specific to their respective intermediate hosts, cox1 fragments of S. capracanis, S. morae, and an undescribed genetic variant, Sarcocystis sp. OaLT1, as well as those of S. arieticanis and S. tenella, were amplified in sheep and goats, respectively. In the cases of all molecularly confirmed Sarcocystis spp., the intraspecific variability values did not overlap with those of interspecific variability. In addition, similar values of intraspecific variability were obtained when comparing sequences of the same species from both sheep and goats. For instance, the cox1 sequences of S. tenella from sheep and goats demonstrated 96.0–100% and 95.7–100% similarity, respectively, compared to sequences of this species deposited in NCBI GenBank.

Table 2.
Identification of Sarcocystis spp. detected by conventional and nPCR in tissues from domestic sheep and goats and comparison of genetic variability.
In addition to the previously genetically characterized Sarcocystis species, an undescribed Sarcocystis sp. OaLT1 genetic variant was observed in the diaphragm muscles of two sheep. Two 100% identical 241 bp sequences of Sarcocystis sp. OaLT1 were obtained using direct PCR and primers designed for the identification of S. morae (Table 1). These sequences displayed 80.3–83.8% similarity to S. tenella, 78.6–81.9% similarity to S. capracanis, and 80.4% similarity to S. heydorni of cattle (Table 2).
Based on a short fragment of cox1, Sarcocystis sp. OaLT1 clustered together with Sarcocystis spp. using ungulates and canids as their intermediate hosts and definitive hosts, respectively (Figure 2). The exact phylogenetic position of Sarcocystis sp. OaLT1 was not resolved because a low posterior probability value (58) was obtained for the grouping of the newly identified Sarcocystis sp. OaLT1 variant with S. heydorni of cattle. However, the phylogenetic analysis showed close relationships of Sarcocystis sp. OaLT1 and Sarcocystis spp. parasitizing sheep (S. tenella), goat (S. capracanis), cattle (S. heydorni) [32], moose (Alces alces) (S. alces) [33] and roe deer (Capreolus capreolus) (S. gracilis) [34].
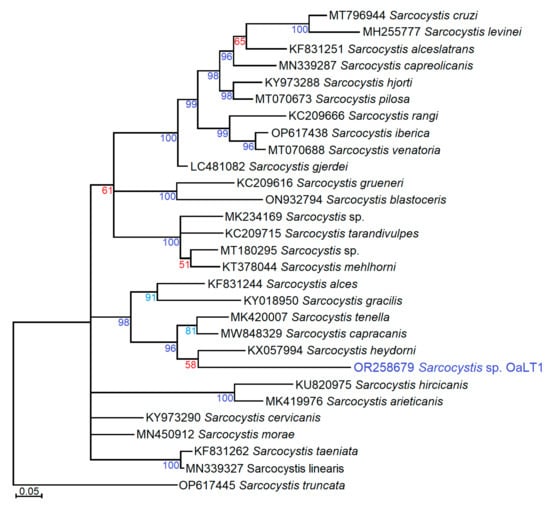
Figure 2.
The phylogenetic tree of some Sarcocystis spp. using cox1 sequences and Bayesian methods, displaying the placement of new variant Sarcocystis sp. OaLT1 from sheep detected in the present study. The tree was rooted on S. truncata. Percentage posterior probability values higher than 50, 70, and 95 were presented in red, turquoise, and indigo, respectively.
3.3. The Detection Rates of Sarcocystis spp. in Sheep Tissues by Direct and Nested PCR
The occurrence of S. arieticanis and S. tenella in the diaphragm, heart, and oesophagus of sheep ranged from 83.0% to 100% and from 10.6% to 59.6% by nPCR and direct PCR, respectively. At the same time, the occurrence of S. capracanis and S. morae in three types of sheep muscles was in the range of 10.6–42.6% and 10.6–31.9%, respectively (Table 3).

Table 3.
The distribution of Sarcocystis spp. in different organs of sheep determined by nPCR and direct PCR. Values in cells indicate the number of positive samples, and values in brackets show the percentage ratio.
Positive samples indicating the presence of S. capracanis and S. morae were validated through the sequencing of positive amplicons obtained from both conventional PCR and nPCR. In the cases of S. arieticanis and S. tenella, two samples from the oesophagus, diaphragm, and heart were obtained through conventional PCR and subjected to sequencing. The same sequencing procedure was applied to the positive amplicons derived from the nPCR of the respective Sarcocystis species under investigation.
4. Discussion
The present study revealed the first identification of Sarcocystis species in goats raised in Lithuania. Based on molecular examination, S. capracanis was observed in three out of nine (33.3%) goats tested. In addition, S. arieticanis was detected in a single goat (11.15%), and S. tenella was found in four (44.4%) individuals (Table 2). Both species are known to use domestic sheep as intermediate hosts [1]. In total, results of molecular analyses showed that 8/9 (88.9%) of goats were infected with Sarcocystis spp. Notably, goats are infrequently reared for meat in Lithuania, and the number of goats in the country is almost 10 times lower than the number of sheep (https://osp.stat.gov.lt/lietuvos-aplinka-zemes-ukis-ir-energetika-2022/zemes-ukis/gyvulininkyste, accessed on 7 July 2023).
Studies on Sarcocystis spp. in goats have been mostly carried out in Asia and Africa. In Kunming (China), 174 of 225 (77.3%) goats were confirmed to be infected with Sarcocystis spp. using cox1 region. The prevailing S. capracanis was detected in 74.6% of goats, and S. hircicanis was found in 33.3% of individuals [35]. Meanwhile, it was reported that the frequency of microscopic Sarcocystis in Saudi Arabia was lower (42.9%; 36/84 goats), as only S. capracanis was distinguished by cox1 [14]. Only S. capracanis was identified in Malaysian goats by PCR targeting 18S rRNA, revealing a level of infection of around 90.5% [36]. Other surveys conducted exclusively by light microscopy examination reported occurrences in Egypt, Iraq, and Iran ranging from 79.4–100.0% [37,38,39,40,41,42]. In general, given the small sample size of goats collected in Lithuania, reliable conclusions on the infection rates of Sarcocystis spp. are difficult to be drawn.
As commented above, an important number of recent studies suggest that the intermediate host-specificity of several Sarcocystis species may not be as strict as previously believed [1]. The reliability of the findings of some studies [18,43] is in doubt due to the absence of DNA sequencing data in the GenBank database. A report originating from Egypt indicated that S. hjorti, a parasite known to infect cervids [44], was found in cattle [43]. Nevertheless, according to the presented data, the sequence similarity only reaches 96% when compared to other S. hjorti sequences, suggesting that it is likely an undescribed Sarcocystis sp. rather than S. hjorti [43]. There are a couple of studies about S. gigantea, a species that typically parasitizes sheep [7], infecting cattle [45] or horses [46]. While the obtained 18S rRNA sequences exhibited high similarities to S. medusiformis and S. moulei, they consistently displayed distinguishable differences from Sarcocystis species identified in cattle and horses (e.g., absence of secondary cyst wall). There is also an investigation showing that S. cruzi, which typically infects cattle, was detected based on 18S rRNA in another bovid species, the wood bison (Bison bison athabascae) [47]. Regardless, there is a need to enrich previously described data on 18S rRNA with additional investigation on other genetic regions, such as the cox1 gene, as it possesses significant variability and serves as the most accurate method to differentiate taxonomically close Sarcocystis species [48].
Some existing reports mentioned that S. tenella and S. capracanis can parasitize both sheep and goats [35,49]. Furthermore, S. tenella has been detected in chamois (Rupicapra rupicapra tatrica) [19], and S. tenella and S. capracanis have been identified in Barbary sheep (Ammotragus lervia) [50]. However, both host species do not belong to the Ovis or Capra genera, thus raising questions about Sarcocystis species-specificity for their intermediate host.
During this study, DNA of non-specific Sarcocystis spp. has been detected in heart, oesophagus, and diaphragm samples from sheep and goats (Table 2). To the best of our knowledge, this study represents the first detection of S. morae DNA in sheep as well as the first confirmation of S. arieticanis DNA in goats. Even though parasite DNA was detected in non-canonical hosts, no sarcocysts of the aforementioned Sarcocystis species have been found in any of the muscle samples.
Several hypotheses arise herein to explain the results of the current study. The first and simplest explanation could be the contamination of the laboratory equipment and environment. However, such an explanation is unlikely for the following reasons: (i) the agarose gel of all negative controls remained clean; (ii) not all samples were positive for atypical/unexpected species; (iii) the genetic identity of all positive samples for atypical species was confirmed by the Sanger sequencing method showing multiple single peaks in each chromatogram; (iv) the obtained sequences of non-host-specific Sarcocystis species demonstrated intraspecific variation. The second hypothesis raised suggests that S. capracanis and S. morae form microscopic sarcocysts in the muscle tissues of sheep, while S. arieticanis and S. tenella form sarcocysts in the muscle tissues of goats. However, the load of sarcocysts of non-specific Sarcocystis species is negligible compared to the load of sarcocysts of the predominant typical species. A similar case has already been described when DNA of S. hominis was detected in digested muscle tissues of 14/102 cattle, but sarcocysts of this species specific to cattle were not found in diaphragm samples [22]. Real-time PCR would help to answer the question of the relative quantities of the identified species in the test samples [48]. In sum, the findings of the present study indicate that there are still many unanswered questions about the life cycle and host-specificity of Sarcocystis species infecting farm animals.
5. Conclusions
Microscopical and molecular identification of S. capracanis in excised sarcocysts from goats constitutes the first report of Sarcocystis spp. infection in such a host in Lithuania. Species-specific PCR tests allowed the detection of two non-typical Sarcocystis species in sheep, S. capracanis and S. morae, in the muscles of sheep, and two non-typical goat Sarcocystis species, S. arieticanis and S. tenella, in the muscles of goats. Additional molecular data showed the presence of a new Sarcocystis genetic variant (Sarcocystis sp. OaLT1) with 78.6–83.8% similarity to the closest Sarcocystis species cycling in ungulates as intermediate hosts in the muscle samples of two sheep.
Evidence of a lower intermediate host-specificity identified here warrants the need for further investigations, including additional effort on direct examination for individual sarcocysts and, when possible, experimental infections to demonstrate such cross-infections.
Author Contributions
Conceptualization, D.B. and P.P.; methodology, A.M.-P. and P.P.; software, N.G. and P.P.; validation, A.M.-P., D.B., R.C.-B. and P.P.; formal analysis, A.M.-P., N.G. and P.P.; investigation, A.M.-P., N.G., E.J.-N. and D.L.B.; resources, D.B., E.J.-N., M.K. and P.P.; writing—original draft preparation, A.M.-P., N.G., E.J.-N., D.L.B. and P.P.; writing—review and editing, A.M.-P., D.B., N.G., E.J.-N., D.L.B., M.K., R.C.-B. and P.P.; visualization, E.J.-N. and P.P.; supervision, D.B.; funding acquisition, A.M.-P. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the European Social Fund under the no. 09.3.3-LMT-K-712 “Development of Competences of Scientists, other Researchers, and Students through Practical Research Activities” measure under a grant agreement with the Research Council of Lithuania (LMTLT). Postdoc no.: 09.3.3-LMT-K-712-23-0064.
Institutional Review Board Statement
Samples of slaughtered small ruminants were taken by the veterinarian of the slaughterhouse “Alantos agroservisas, UAB”. The animals were slaughtered for meat production, not for research purposes. None of the authors of the manuscript were included in the slaughter process of the animals examined. Based on the laws of Lithuania, the pre- and post-slaughter examination of sheep and goats were performed in accordance with Regulation (EC) No. 854/2004 for the slaughterhouses of meat production companies. There is no need for other ethical approvals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The cox1 sequences of S. arieticanis, S. capracanis, S. morae, S. tenella and Sarcocystis sp. OaLT1 were submitted to the GenBank database under the accession numbers OR258580-OR258681.
Acknowledgments
The authors would like to thank G. Januškevičienė for her help in organizing the collection of muscle samples from sheep and goats.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Martínez-Navalón, B.; Anastasio-Giner, B.; Cano-Fructuoso, M.; Sanchez-Martínez, P.; Llopis-Morant, A.; Perez-Castarlenas, B.; Goyena, E.; de Larrea, E.B. Sarcocystis Infection: A Major Cause of Carcass Condemnation in Adult Sheep in Spain. Span. J. Agric. Res. 2012, 10, 388. [Google Scholar] [CrossRef]
- Dong, H.; Su, R.; Wang, Y.; Tong, Z.; Zhang, L.; Yang, Y.; Hu, J. Sarcocystis species in wild and domestic sheep (Ovis ammon and Ovis aries) from China. BMC Vet. Res. 2018, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Amairia, S.; Amdouni, Y.; Rouatbi, M.; Rjeibi, M.R.; Awadi, S.; Gharbi, M. First detection and molecular identification of Sarcocystis spp. in small ruminants in North-West Tunisia. Transbound. Emerg. Dis. 2018, 65, 441–446. [Google Scholar] [CrossRef]
- Bittencourt, M.V.; Meneses, I.D.S.; Ribeiro-Andrade, M.; de Jesus, R.F.; de Araújo, F.R.; Gondim, L.F.P. Sarcocystis spp. in sheep and goats: Frequency of infection and species identification by morphological, ultrastructural, and molecular tests in Bahia, Brazil. Parasitol. Res. 2016, 115, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B.; de la Fuente, C.; Alunda, J.M.; Luzón, M. Molecular characterization of five Sarcocystis species in domestic sheep (Ovis aries) from Spain. Parasitol. Res. 2020, 119, 215–231. [Google Scholar] [CrossRef]
- Hu, J.J.; Huang, S.; Wen, T.; Esch, G.W.; Liang, Y.; Li, H.L. Sarcocystis spp. in Domestic Sheep in Kunming City, China: Prevalence, Morphology, and Molecular Characteristics. Parasite 2017, 24, 30. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Shibata, Y.; Kubo, M.; Itagaki, H. Sarcocystis mihoensis n. sp. from Sheep in Japan. J. Vet. Med. Sci. 1997, 59, 103–106. [Google Scholar] [CrossRef]
- Wang, G.; Wei, T.; Wang, X.; Li, W.; Zhang, P.; Dong, M.; Xiao, H. The Morphology and Life Cycle of Sarcocystis microps n. sp. in Sheep of Qinghai in China. China Vet. Technol. 1988, 6, 9–11. [Google Scholar]
- Lindsay, D.S.; Dubey, J.P. Neosporosis, Toxoplasmosis, and Sarcocystosis in Ruminants: An Update. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 36, 205–222. [Google Scholar] [CrossRef]
- Hong, E.J.; Sim, C.; Chae, J.S.; Kim, H.C.; Park, J.; Choi, K.S.; Yu, D.H.; Park, C.H.; Yoo, J.G.; Park, B.K. Ultrastructural and molecular identification of Sarcocystis tenella (Protozoa, Apicomplexa) in naturally infected Korean native goats. Vet. Med. 2016, 61, 374–381. [Google Scholar] [CrossRef]
- Gjerde, B. Sarcocystis species in red deer revisited: With a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitology 2014, 141, 441–452. [Google Scholar] [CrossRef]
- El-Morsey, A.; Abdo, W.; Sultan, K.; Elhawary, N.M.; AbouZaid, A.A. Ultrastructural and Molecular Identification of the sarcocysts of Sarcocystis tenella and Sarcocystis arieticanis Infecting Domestic Sheep (Ovis aries) from Egypt. Acta Parasitol. 2019, 64, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Metwally, D.M.; Al-Damigh, M.A.; Al-Turaiki, I.M.; El-Khadragy, M.F. Molecular Characterization of Sarcocystis Species Isolated from Sheep and Goats in Riyadh, Saudi Arabia. Animals 2019, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Formisano, P.; Aldridge, B.; Alony, Y.; Beekhuis, L.; Davies, E.; Del Pozo, J.; Dunn, K.; English, K.; Morrison, L.; Sargison, N.; et al. Identification of Sarcocystis capracanis in cerebrospinal fluid from sheep with neurological disease. Vet. Parasitol. 2013, 193, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, N.; Khaksar, M.; Ghaffari, S.; Hamidekish, S.M. Molecular Analysis of Sarcocystis Spp. Isolated from Sheep (Ovis aries) in Babol Area, Mazandaran Province, Northern Iran. Iran. J. Parasitol. 2016, 11, 73–80. [Google Scholar] [PubMed]
- Swar, S.O.; Shnawa, B.H. Ultrastructural and Molecular Characterization of Sarcocystis Species Derived from Macroscopic Sarcocysts of Domestic Sheep and Goats in Soran City, Erbil, Iraq. Worlds Vet. J. 2020, 10, 540–550. [Google Scholar] [CrossRef]
- Dakhil, H.G.; Abdallah, B.H.; Abdallah, F.A. Molecular identification of Sarcocystis fusiformis and S. moulei infecting water buffaloes (Bubalus bubalis) in southern Iraq. World J. Pharm. Res. 2017, 6, 215–229. [Google Scholar]
- Kolenda, R.; Schierack, P.; Zieba, F.; Zwijacz-Kozica, T.; Bednarski, M. First Molecular Characterization of Sarcocystis tenella in Tatra Chamois (Rupicapra rupicapra tatrica) in Poland. Parasitol. Res. 2015, 114, 3885–3892. [Google Scholar] [CrossRef]
- Prakas, P.; Rehbein, S.; Rudaitytė-Lukošienė, E.; Butkauskas, D. Molecular Identification of Sarcocystis species in Diaphragm Muscle Tissue of European mouflon (Ovis gmelini musimon) from Austria. Parasitol. Res. 2021, 120, 2695–2702. [Google Scholar] [CrossRef]
- Januškevičius, V.; Januškevičienė, G.; Prakas, P.; Butkauskas, D.; Petkevičius, S. Prevalence and Intensity of Sarcocystis spp. Infection in Animals Slaughtered for Food in Lithuania. Vet. Med. 2019, 64, 149–157. [Google Scholar] [CrossRef]
- Prakas, P.; Strazdaitė-Žielienė, Ž.; Januškevičius, V.; Chiesa, F.; Baranauskaitė, A.; Rudaitytė-Lukošienė, E.; Servienė, E.; Petkevičius, S.; Butkauskas, D. Molecular Identification of Four Sarcocystis species in Cattle from Lithuania, Including S. hominis, and Development of a Rapid Molecular Detection Method. Parasites Vectors 2020, 13, 610. [Google Scholar] [CrossRef] [PubMed]
- Marandykina-Prakienė, A.; Butkauskas, D.; Gudiškis, N.; Juozaitytė-Ngugu, E.; Januškevičius, V.; Rudaitytė-Lukošienė, E.; Prakas, P. Molecular Identification of Sarcocystis Species in Sheep from Lithuania. Animals 2022, 12, 2048. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Phylogenetic Relationships among Sarcocystis Species in Cervids, Cattle and Sheep Inferred from the Mitochondrial Cytochrome C Oxidase Subunit I Gene. Int. J. Parasitol. 2013, 43, 579–591. [Google Scholar] [CrossRef]
- Dubey, J.P. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet. Parasitol. 1998, 74, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B.; Luzón, M.; Alunda, J.M.; de la Fuente, C. Morphological and molecular characteristics of six Sarcocystis spp. from red deer (Cervus elaphus) in Spain, including Sarcocystis cervicanis and three new species. Parasitol. Res. 2017, 116, 2795–2811. [Google Scholar] [CrossRef]
- Rudaitytė-Lukošienė, E.; Prakas, P.; Strazdaitė-Žielienė, Ž.; Servienė, E.; Januškevičius, V.; Butkauskas, D. Molecular identification of two Sarcocystis species in fallow deer (Dama dama) from Lithuania. Parasitol. Int. 2020, 75, 102044. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Rehbein, S.; Rudaitytė-Lukošienė, E.; Butkauskas, D. Molecular identification of Sarcocystis species in sika deer (Cervus nippon) of free-ranging populations in Germany and Austria. Vet. Res. Commun. 2023. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Milne, I.; Wright, F.; Rowe, G.; Marshall, D.F.; Husmeier, D.; McGuire, G. TOPALi: Software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 2004, 20, 1806–1807. [Google Scholar] [CrossRef] [PubMed]
- Rubiola, S.; Chiesa, F.; Zanet, S.; Civera, T. Molecular identification of Sarcocystis spp. in cattle: Partial sequencing of Cytochrome C Oxidase subunit 1 (COI). Ital. J. Food Saf. 2019, 7, 7725. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, S.S.; Gjerde, B. Sarcocystis in moose (Alces alces): Molecular identification and phylogeny of six Sarcocystis species in moose, and a morphological description of three new species. Parasitol. Res. 2008, 103, 93–110. [Google Scholar] [CrossRef]
- Dahlgren, S.S.; Gjerde, B. Sarcocystis in Norwegian roe deer (Capreolus capreolus): Molecular and morphological identification of Sarcocystis oviformis n. sp. and Sarcocystis gracilis and their phylogenetic relationship with other Sarcocystis species. Parasitol. Res. 2009, 104, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Liu, T.T.; Liu, Q.; Esch, G.W.; Chen, J.Q.; Huang, S.; Wen, T. Prevalence, morphology, and molecular characteristics of Sarcocystis spp. in domestic goats (Capra hircus) from Kunming, China. Parasitol. Res. 2016, 115, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Kutty, M.K.; Latif, B.; Muslim, A.; Hussaini, J.; Daher, A.M.; Heo, C.C.; Abdullah, S. Detection of sarcocystosis in goats in Malaysia by light microscopy, histology, and PCR. Trop. Anim. Health Prod. 2015, 47, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Latif, B.M.; Al-Delemi, J.K.; Mohammed, B.S.; Al-Bayati, S.M.; Al-Amiry, A.M. Prevalence of Sarcocystis spp. in meat-producing animals in Iraq. Vet. Parasitol. 1999, 84, 85–90. [Google Scholar] [CrossRef]
- Mahran, O. Sarcocystis infection in sheep and goats slaughtered in Shalatin abattoir, Red Sea Governorate, Egypt. Assiut Vet. Med. J. 2009, 55, 1–15. [Google Scholar] [CrossRef]
- Dehaghi, M.M.; Fathi, S.; Asl, E.N. Survey of Sarcocystis infection in slaughtered goats in Kerman Abattoir, Southeast of Iran. J. Anim. Vet. Adv. 2011, 10, 1205–1208. [Google Scholar]
- Morsy, K.; Saleh, A.; Al-Ghamdi, A.; Abdel-Ghaffar, F.; Al-Rasheid, K.; Bashtar, A.R.; Al Quraishy, S.; Mehlhorn, H. Prevalence pattern and biology of Sarcocystis capracanis infection in the Egyptian goats: A light and ultrastructural study. Vet. Parasitol. 2011, 181, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafeez, E.H.; Kamal, A.M.; Abdelgelil, N.H.; Abdel-Fatah, M. Parasites transmitted to human by ingestion of different types of meat, El-Minia city, El-Minia governorate, Egypt. J. Egypt. Soc. Parasitol. 2015, 45, 671–680. [Google Scholar] [CrossRef]
- Salehi, M.; Spotin, A.; Rostamian, M.; Adami, M. Prevalence and Molecular Assessment of Sarcocystis Infection in Livestock in Northeast Iran. Comp. Immunol. Microbiol. Infect. Dis. 2022, 80, 101738. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.M.; Hussein, N.M.; Hassan, A.A. First molecular characterization of Sarcocystis spp. in cattle in Qena Governorate, Upper Egypt. J. Parasit. Dis. 2018, 42, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, S.S.; Gjerde, B. Molecular characterization of five Sarcocystis species in red deer (Cervus elaphus), including Sarcocystis hjorti n. sp., reveals that these species are not intermediate host specific. Parasitology 2010, 137, 815–840. [Google Scholar] [CrossRef]
- Sarafraz, N.; Spotin, A.; Haniloo, A.; Fazaeli, A. Prevalence and molecular analysis of Sarcocystis infections in cattle in Northwest Iran and the first global report of S. gigantea in cattle. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101566. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Di Palma, S.; Gabrielli, S.; Morganti, G.; Milardi, G.L.; Middleton, B.; Lepri, E. Sarcocystis gigantea infection associated with granulomatous eosinophilic myositis in a horse. J. Vet. Diagn. Investig. 2020, 32, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Calero-Bernal, R.; Verma, S.K.; Seaton, C.T.; Sinnett, D.; Ball, E.; Dunams, D.; Rosenthal, B.M.; Dubey, J.P. Sarcocystis cruzi infection in wood bison (Bison bison athabascae). Vet. Parasitol. 2015, 210, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Hoeve-Bakker, B.J.A.; van der Giessen, J.W.B.; Franssen, F.F.J. Molecular identification targeting cox1 and 18S genes confirms the high prevalence of Sarcocystis spp. in cattle in the Netherlands. Int. J. Parasitol 2019, 49, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, M.; Kardooni, T. Molecular identification of Sarcocystis spp. in sheep and cattle by PCR-RFLP from Southwest of Iran. Jundishapur J. Microbiol. 2017, 10, 12798. [Google Scholar] [CrossRef]
- Delgado-de Las Cuevas, G.E.; Prakas, P.; Rudaitytė-Lukošienė, E.; García-Gil, M.L.; Martínez-González, M.; Butkauskas, D.; Mowery, J.D.; Dubey, J.P.; Habela, M.A.; Calero-Bernal, R. First description of Sarcocystis species infecting Barbary sheep (Ammotragus lervia). Parasitol. Res. 2021, 120, 2881–2886. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).