Beraprost and Overall Survival in Cats with Chronic Kidney Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Study Design
2.3. Data Analysis
3. Results
3.1. Baseline Characteristics of All Cats in Cohort A
3.2. Multivariable Analyses of All Cats in Cohort A
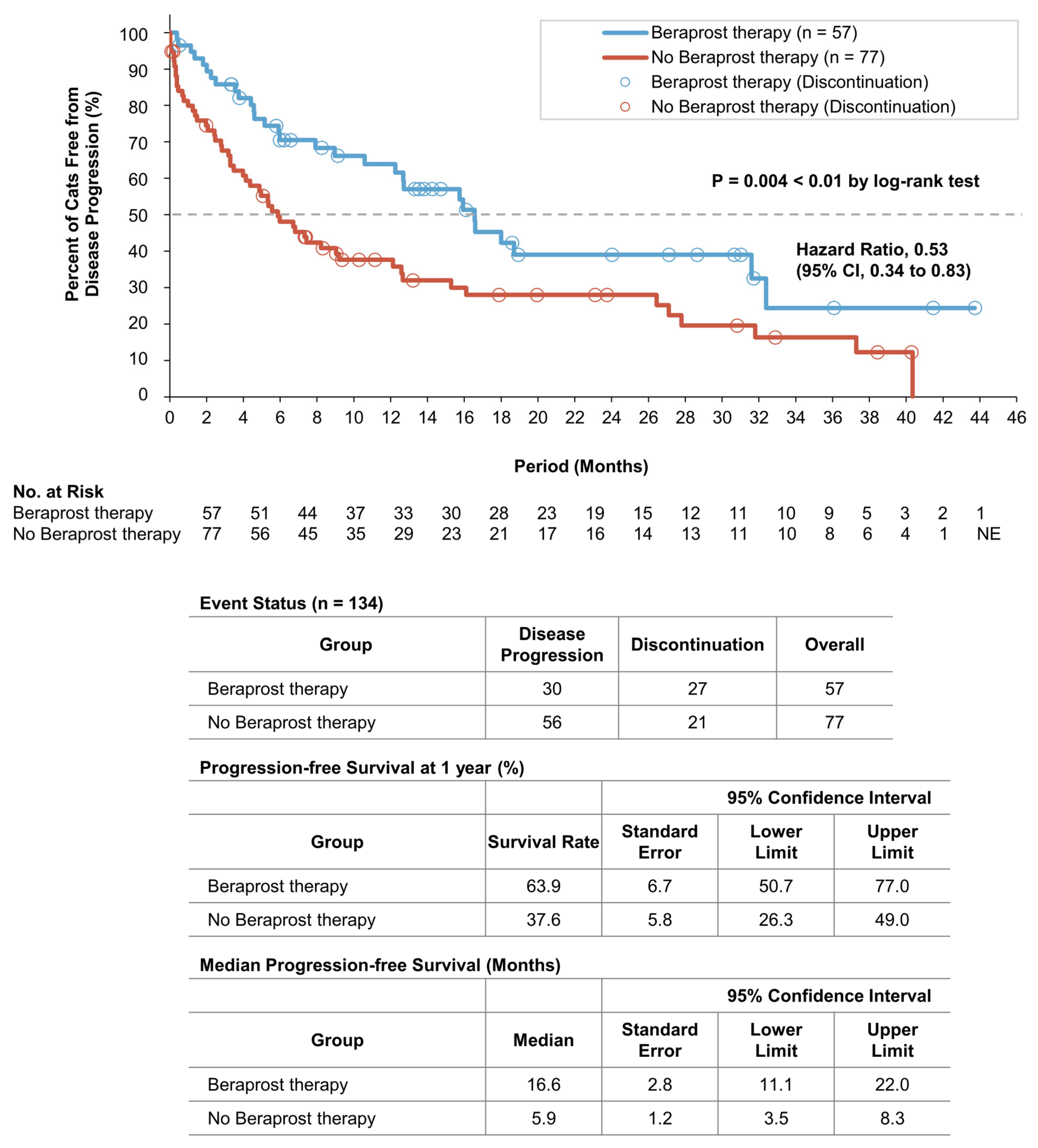
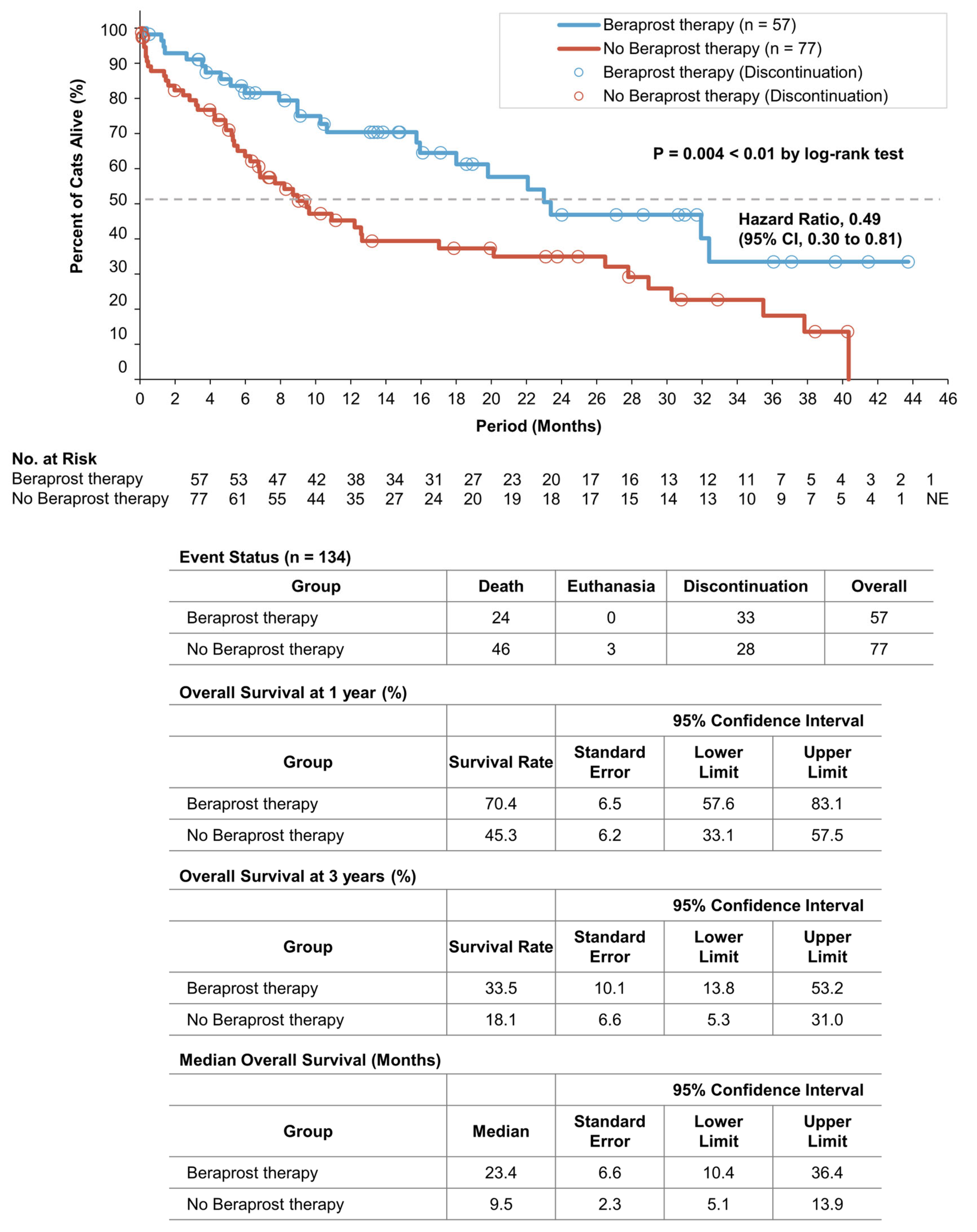
3.3. Survival Analyses of the Two Groups in Cohort A
3.4. Baseline Characteristics of All Cats in Cohort B
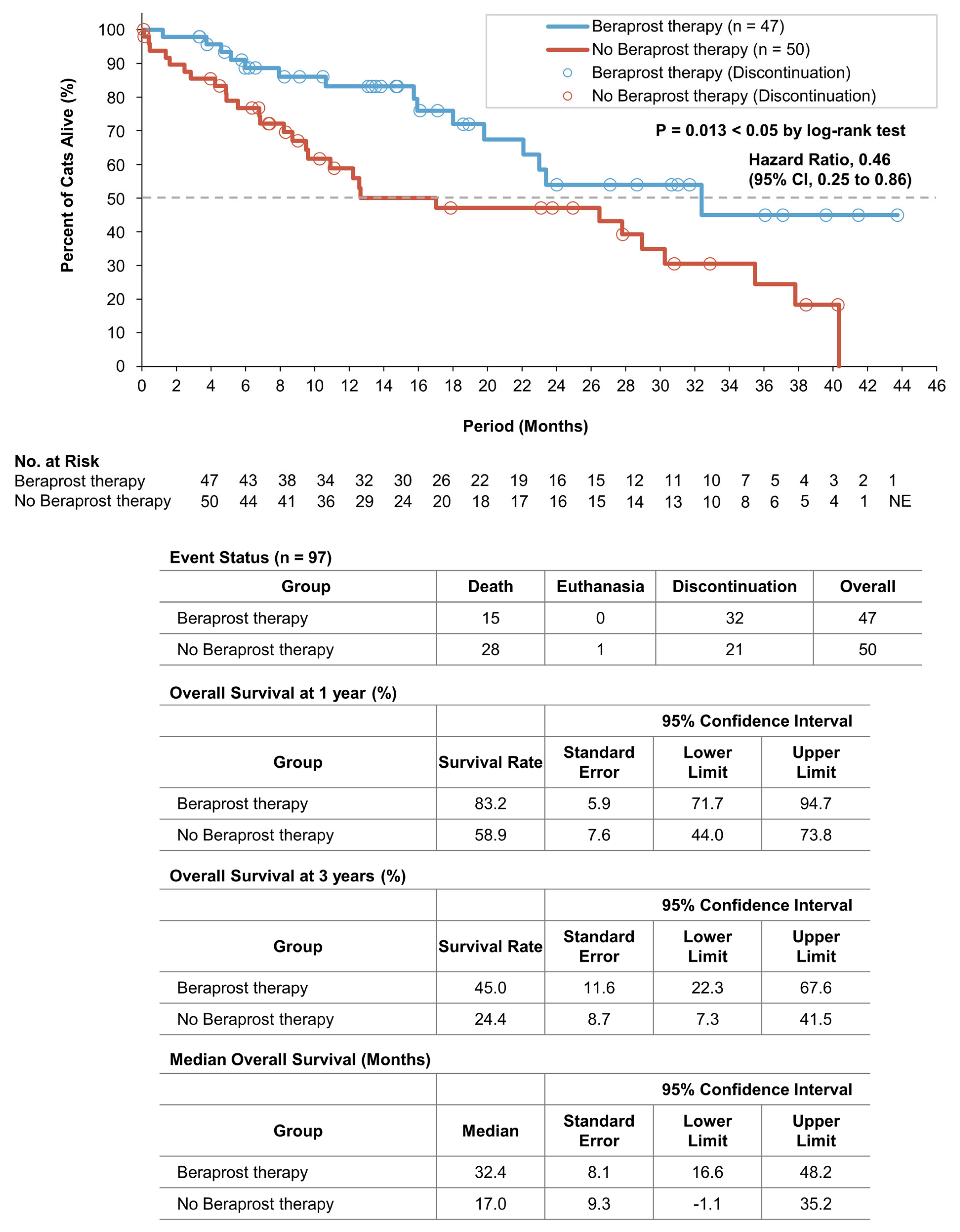
3.5. Survival Analyses of the Two Groups in Cohort B
3.6. Onset of Chronic Disorders of the Two Groups in Cohort A
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sparkes, A.H.; Caney, S.; Chalhoub, S.; Elliott, J.; Finch, N.; Gajanayake, I.; Langston, C.; Lefebvre, H.P.; White, J.; Quimby, J. ISFM Consensus Guidelines on the Diagnosis and Management of Feline Chronic Kidney Disease. J. Feline Med. Surg. 2016, 18, 219–239. [Google Scholar] [CrossRef] [PubMed]
- International Renal Interest Society. IRIS Treatment Recommendations for CKD. 2019. Available online: http://iris-kidney.com/guidelines/recommendations.html (accessed on 28 March 2021).
- Pouchelon, J.L.; Atkins, C.E.; Bussadori, C.; Oyama, M.A.; Vaden, S.L.; Bonagura, J.D.; Chetboul, V.; Cowgill, L.D.; Elliot, J.; Francey, T.; et al. Cardiovascular-renal axis disorders in the domestic dog and cat: A veterinary consensus statement. J. Small Anim. Pract. 2015, 56, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.L.; Peak, K.J.; Brodbelt, D.; Elliott, J.; Syme, H.M. Survival and the development of azotemia after treatment of hyperthyroid cats. J. Vet. Intern. Med. 2010, 24, 863–869. [Google Scholar] [CrossRef]
- Perez-Lopez, L.; Boronat, M.; Melian, C.; Saavedra, P.; Brito-Casillas, Y.; Wagner, A.M. Assessment of the association between diabetes mellitus and chronic kidney disease in adult cats. J. Vet. Intern. Med. 2019, 33, 1921–1925. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kanellis, J.; Hugo, C.; Truong, L.; Anderson, S.; Kerjaschki, D.; Schreiner, G.F.; Johnson, R.J. Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 2002, 13, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Jepson, R.; Syme, H.; Vallance, C.; Elliott, J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J. Vet. Intern. Med. 2008, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kainoh, M.N.S.; Nakadate, T. Cytoprotective Action of Beraprost Sodium against Peroxide-Induced Damage in Vascular Endothelial Cells. Pharmacology 1992, 45, 61–70. [Google Scholar] [CrossRef]
- Niwano, K.; Arai, M.; Tomaru, K.; Uchiyama, T.; Ohyama, Y.; Kurabayashi, M. Transcriptional stimulation of the eNOS gene by the stable prostacyclin analogue beraprost is mediated through cAMP-responsive element in vascular endothelial cells: Close link between PGI2 signal and NO pathways. Circ. Res. 2003, 93, 523–530. [Google Scholar] [CrossRef]
- Matsumoto, K.; Morishita, R.; Tomita, N.; Moriguchi, A.; Yamasaki, K.; Aoki, M.; Nakamura, T.; Higaki, J.; Ogihara, T. Impaired endothelial dysfunction in diabetes mellitus rats was restored by oral administration of prostaglandin I2 analogue. J. Endocrinol. 2002, 175, 217–223. [Google Scholar] [CrossRef]
- Goto, Y.; Yamaguchi, S.; Tamura, M.; Mochizuki, H.; Kurumatani, H.; Okano, K.; Miyamoto, M. A prostacyclin analog prevents the regression of renal microvascular network by inhibiting mitochondria-dependent apoptosis in the kidney of rat progressive glomerulonephritis. Prostaglandins Other Lipid Mediat. 2014, 112, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Inada, C.; Tamura, M.; Sato, N.; Yamada, M.; Itaba, S.; Okazaki, S.; Matsuura, H.; Fujii, S.; Matsuda, F.; et al. Beraprost sodium improves survival rates in anti-glomerular basement membrane glomerulonephritis and 5/6 nephrectomized chronic kidney disease rats. Eur. J. Pharmacol. 2013, 714, 325–331. [Google Scholar] [CrossRef]
- Takenaka, M.; Iio, A.; Sato, R.; Sakamoto, T.; Kurumatani, H.; KT-140 Clinical Study Group. A Double-blind, Placebo-controlled, Multicenter, Prospective, Randomized Study of Beraprost Sodium Treatment for Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2018, 32, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Syme, H.M.; Elliott, J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J. Vet. Intern. Med. 2012, 26, 275–281. [Google Scholar] [CrossRef] [PubMed]
- King, J.N.; Tasker, S.; Gunn-Moore, D.A.; Strehlau, G.; BENRIC (benazepril in renal insufficiency in cats) Study Group. Prognostic factors in cats with chronic kidney disease. J. Vet. Intern. Med. 2007, 21, 906–916. [Google Scholar] [CrossRef]
- Feinstein, A.R. An additional basic science for clinical medicine: II. The limitations of randomized trials. Ann. Intern. Med. 1983, 99, 544–550. [Google Scholar] [CrossRef]
- Black, N. Why we need observational studies to evaluate the effectiveness of health care. BMJ 1996, 312, 1215–1218. [Google Scholar] [CrossRef]
- Elliott, J.; Barber, P.J. Feline CKD: Clinical findings in 80 cases diagnosed between 1992 and 1995. J. Small Anim. Pract. 1998, 39, 78–85. [Google Scholar] [CrossRef]
- Syme, H.M.; Markwell, P.J.; Pfeiffer, D.; Elliott, J. Survival of cats with naturally occurring CKD is related to severity of proteinuria. J. Vet. Intern. Med. 2006, 20, 528–535. [Google Scholar] [CrossRef]
- Freeman, L.M.; Lachaud, M.P.; Matthews, S.; Rhodes, L.; Zollers, B. Evaluation of Weight Loss Over Time in Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2016, 30, 1661–1666. [Google Scholar] [CrossRef]
- Sassnau, R. Epidemiological investigation on the prevalence of feline hyperthyroidism in an urban population in Germany. Tierärztliche Praxis Kleintiere 2006, 34, 450–457. [Google Scholar]
- Miyamoto, Y.; Miyata, I.; Kurobane, K. Prevalence of feline hyperthyroidism in Osaka and the Chugoku region. J. Jpn. Vet. Med. Assoc. 2002, 55, 289–292. [Google Scholar] [CrossRef]
- Wakeling, J.; Elliott, J.; Syme, H. Evaluation of predictors for the diagnosis of hyperthyroidism in cats. J. Vet. Intern. Med. 2011, 25, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Paige, C.F.; Abbott, J.A.; Elvinger, F.; Pyle, R.L. Prevalence of cardiomyopathy in apparently healthy cats. J. Am. Vet. Med. Assoc. 2009, 234, 1398–1403. [Google Scholar] [CrossRef]
- Payne, J.R.; Brodbelt, D.C.; Luis Fuentes, V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J. Vet. Cardiol. 2015, 17 (Suppl. 1), S244–S257. [Google Scholar] [CrossRef]
- Gouni, V.; Chetboul, V.; Pouchelon, J.L.; Carlos Sampedrano, C.; Maurey, C.; Lefebvre, H.P. Azotemia in cats with feline hypertrophic cardiomyopathy: Prevalence and relationships with echocardiographic variables. J. Vet. Cardiol. 2008, 10, 117–123. [Google Scholar] [CrossRef]
- Wormser, C.; Mariano, A.; Holmes, E.S.; Aronson, L.R.; Volk, S.W. Post-transplant malignant neoplasia associated with cyclosporine-based immunotherapy: Prevalence, risk factors and survival in feline renal transplant recipients. Vet. Comp. Oncol. 2016, 14, e126–e134. [Google Scholar] [CrossRef]
- Owens, J.M.D.F.; Gilbertson, S.R. Pancreatic disease in the cat. JAAHA 1975, 11, 83–89. [Google Scholar]
- Geddes, R.F.; Elliott, J.; Syme, H.M. Relationship between plasma fibroblast growth factor-23 concentration and survival time in cats with chronic kidney disease. J. Vet. Intern. Med. 2015, 29, 1494–1501. [Google Scholar] [CrossRef]
- Boyd, L.M.; Langston, C.; Thompson, K.; Zivin, K.; Imanishi, M. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J. Vet. Intern. Med. 2008, 22, 1111–1117. [Google Scholar] [CrossRef]
- Jepson, R.E.; Elliott, J.; Brodbelt, D.; Syme, H.M. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J. Vet. Intern. Med. 2007, 21, 402–409. [Google Scholar] [CrossRef]
- Syme, H.M. Proteinuria in cats. Prognostic marker or mediator? J. Feline Med. Surg. 2009, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Koyama, H.; Watanabe, T.; Kitagawa, H.; Nakano, M.; Kajiwara, K.; King, J.N. Evaluation of the clinical efficacy of benazepril in the treatment of chronic renal insufficiency in cats. J. Vet. Intern. Med. 2006, 20, 1074–1079. [Google Scholar] [CrossRef]
- King, J.N.; Gunn-Moore, D.A.; Tasker, S.; Gleadhill, A.; Strehlau, G.; Benazepril in Renal Insufficiency in Cats Study Group. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J. Vet. Intern. Med. 2006, 20, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Mishina, M. Effects of benazepril hydrochloride in cats with experimentally induced or spontaneously occurring CKD. J. Vet. Med. Sci. 2007, 69, 1015–1023. [Google Scholar] [CrossRef]
- Sent, U.; Gossl, R.; Elliott, J.; Syme, H.M.; Zimmering, T. Comparison of Efficacy of Long-term Oral Treatment with Telmisartan and Benazepril in Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2015, 29, 1479–1487. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Hoppe, S.; Pomianowski, A.; Tipold, A. Reactive seizures in cats: A retrospective study of 64 cases. Vet. J. 2019, 244, 1–6. [Google Scholar] [CrossRef]
- Hatamizadeh, P.; Fonarow, G.C.; Budoff, M.J.; Darabian, S.; Kovesdy, C.P.; Kalantar-Zadeh, K. Cardiorenal syndrome: Pathophysiology and potential targets for clinical management. Nat. Rev. Nephrol. 2013, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 730) | Beraprost Prescription | ||||
|---|---|---|---|---|---|
| Yes (n = 124) | No (n = 606) | ||||
| IRIS | Stage 1 | number | 143 | 13 | 130 |
| Stage 2 | number | 369 | 37 | 332 | |
| Stage 3 | number | 134 | 57 | 77 | |
| Stage 4 | number | 84 | 17 | 67 | |
| Total (n = 134) | Beraprost Therapy (n = 57) | No Beraprost Therapy (n = 77) | p Value | ||
|---|---|---|---|---|---|
| Age (years) | median (25th, 75th percentile) | 15.3 (10.5, 17.3) | 15.9 (13.0, 17.3) | 15.1 (8.6, 17.4) | 0.254 |
| Weight (kg) | median (25th, 75th percentile) | 3.6 (3.0, 4.4) | 3.8 (3.1, 4.4) | 3.4 (2.8, 4.4) | 0.142 |
| Sex | |||||
| Female | number (%) | 62 (46.3) | 30 (52.6) | 32 (41.6) | 0.224 |
| Male | number (%) | 72 (53.7) | 27 (47.4) | 45 (58.4) | |
| Neutered | number (%) | 115 (85.8) | 46 (80.7) | 69 (89.6) | 0.210 |
| Breeds | |||||
| Domestic Shorthair | number (%) | 91 (67.9) | 37 (64.9) | 54 (70.1) | 0.577 |
| Japanese Bobtail | number (%) | 7 (5.2) | 3 (5.3) | 4 (5.2) | |
| American Shorthair | number (%) | 6 (4.5) | 3 (5.3) | 3 (3.9) | |
| Other | number (%) | 30 (22.4) | 14 (24.5) | 16 (20.8) | |
| Biochemistry | |||||
| Creatinine (mg/dL) | median (25th, 75th percentile) | 3.3 (3.0, 3.7) | 3.3 (3.0, 3.7) | 3.3 (3.0, 3.7) | 0.767 |
| Urea (mg/dL) | median (25th, 75th percentile) | 42.0 (35.0, 60.0) | 38.0 (31.0, 52.5) | 46.0 (37.0, 70.0) ** | 0.001 |
| Phosphate (mg/dL) | median (25th, 75th percentile) | 4.9 (4.0, 6.1) | 4.6 (3.9, 5.3) | 5.3 (4.2, 6.8) ** | 0.006 |
| Calcium (mg/dL) | median (25th, 75th percentile) | 9.7 (9.2, 10.1) | 9.8 (9.4, 10.4) * | 9.6 (9.0, 10.0) | 0.016 |
| Potassium (mmol/L) | median (25th, 75th percentile) | 3.9 (3.5, 4.2) | 3.9 (3.6, 4.2) | 3.9 (3.5, 4.3) | 0.445 |
| Hematology | |||||
| Packed cell volume (%) | median (25th, 75th percentile) | 35.0 (30.0, 40.0) | 36.0 (31.0, 41.0) | 33.5 (29.5, 40.0) | 0.075 |
| Urinalysis | |||||
| Urine specific gravity | median (25th, 75th percentile) | 1.014 (1.012, 1.018) | 1.014 (1.012, 1.018) | 1.014 (1.012, 1.018) | 0.800 |
| Urine protein (mg/dL) | mean (SD) | 59.6 (167.9) | 27.5 (60.1) | 90.0 (223.6) * | 0.048 |
| Blood pressure measurement | |||||
| Systolic blood pressure (mmHg) | median (25th, 75th percentile) | 153.0 (132.0, 165.0) | 153.0 (132.0, 165.0) | 151.5 (132.8, 160.3) | 0.976 |
| Treatment | |||||
| Beraprost (RAPROS) | number (%) | 57 (42.5%) | 57 (100.0%) ** | 0 (0.0%) | <0.001 |
| Dose (μg/kg twice daily) | mean (SD, range) | 15.0 (4.0, 7.1–26.2) | 15.0 (4.0, 7.1–26.2) ** | 0 (0, 0–0) | <0.001 |
| Subcutaneous fluid therapy (total) | number (%) | 106 (79.1%) | 42 (73.7%) | 64 (83.1%) | 0.203 |
| At clinic | number (%) | 87 (64.9%) | 32 (56.1%) | 55 (71.4%) | 0.071 |
| At home | number (%) | 72 (53.7%) | 28 (49.1%) | 44 (57.1%) | 0.385 |
| Kidney Diet (total) | number (%) | 75 (56.0%) | 35 (61.4%) | 40 (51.9%) | 0.296 |
| Royal Canin (Renal Support) | number (%) | 53 (39.6%) | 22 (38.6%) | 31 (40.3%) | 0.860 |
| Hill’s (k/d) | number (%) | 34 (25.4%) | 16 (28.1%) | 18 (23.4%) | 0.553 |
| Other | number (%) | 33 (24.6%) | 20 (35.1%) * | 13 (16.9%) | 0.025 |
| Phosphate binder | number (%) | 40 (29.9%) | 16 (28.1%) | 24 (31.2%) | 0.849 |
| Ferric chloride (Lenziaren) | |||||
| Oral activated charcoal | number (%) | 37 (27.6%) | 15 (26.3%) | 22 (28.6%) | 0.846 |
| ACEI/ARB (total) | number (%) | 34 (25.4%) | 15 (26.3%) | 19 (24.7%) | 0.843 |
| Benazepril (Fortekor) | number (%) | 27 (20.1%) | 10 (17.5%) | 17 (22.1%) | 0.664 |
| Telmisartan (Semintra) | number (%) | 10 (7.5%) | 5 (8.8%) | 5 (6.5%) | 0.743 |
| Calcium channel blocker | number (%) | 11 (8.2%) | 7 (12.3%) | 4 (5.2%) | 0.203 |
| Amlodipine | |||||
| Erythrocyte-stimulating agents | number (%) | 34 (25.4%) | 11 (19.3%) | 23 (29.9%) | 0.228 |
| Darbepoetin alfa | |||||
| Managing inappetence, nausea and vomiting (total) | number (%) | 102 (76.1%) | 41 (71.9%) | 61 (79.2%) | 0.413 |
| Maropitant (Cerenia) | number (%) | 88 (65.7%) | 34 (59.6%) | 54 (70.1%) | 0.270 |
| Mirtazapine | number (%) | 44 (32.8%) | 21 (36.8%) | 23 (29.9%) | 0.458 |
| Famotidine | number (%) | 27 (20.1%) | 16 (28.1%) | 11 (14.3%) | 0.054 |
| Omeprazole | number (%) | 8 (6.0%) | 2 (3.5%) | 6 (7.8%) | 0.466 |
| Metoclopramide | number (%) | 11 (8.2%) | 5 (8.8%) | 6 (7.8%) | 1.000 |
| Coexisting Disorders | |||||
| Any | number (%) | 38 (28.4%) | 18 (31.6%) | 20 (26.0%) | 0.562 |
| Hyperthyroidism | number (%) | 13 (9.7%) | 7 (12.3%) | 6 (7.8%) | 0.395 |
| Congestive heart failure | number (%) | 10 (7.5%) | 2 (3.5%) | 8 (10.4%) | 0.189 |
| Neoplasia † | number (%) | 8 (6.0%) | 4 (7.0%) | 4 (5.2%) | 0.723 |
| Diabetes mellitus | number (%) | 4 (3.0%) | 3 (5.3%) | 1 (1.3%) | 0.312 |
| Pancreatitis | number (%) | 3 (2.2%) | 2 (3.5%) | 1 (1.3%) | 0.575 |
| Progression-Free Survival | ||||||
| Covariate | Coefficient | Standard Error | Hazard Ratio for Disease Progression | 95% Confidence Interval | p Value | |
| Lower Limit | Upper Limit | |||||
| Beraprost | −0.52 | 0.24 | 0.59 | 0.37 | 0.96 | 0.032 * |
| Urea (mg/dL) ≥ 60.0 | 1.06 | 0.27 | 2.90 | 1.72 | 4.89 | <0.001 ** |
| Overall Survival | ||||||
| Covariate | Coefficient | Standard Error | Hazard Ratio for Death | 95% Confidence Interval | p Value | |
| Lower Limit | Upper Limit | |||||
| Beraprost | −0.54 | 0.27 | 0.58 | 0.34 | 0.99 | 0.047 * |
| Urea (mg/dL) ≥ 60.0 | 0.96 | 0.34 | 2.61 | 1.35 | 5.06 | 0.004 ** |
| Phosphate (mg/dL) ≥ 7.0 | 0.88 | 0.46 | 2.41 | 0.98 | 5.94 | 0.056 |
| Progression-Free Survival | |||||
| Beraprost Therapy versus No Beraprost Therapy | |||||
| Subgroups | Number of Cats | Hazard Ratio for Disease Progression | 95% Confidence Interval | p Value | |
| Lower Limit | Upper Limit | ||||
| All | 134 | 0.53 | 0.34 | 0.83 | 0.005 ** |
| Age (years) ≤ 15.0 | 61 | 0.39 | 0.18 | 0.83 | 0.015 * |
| Weight (kg) ≥ 3.0 | 99 | 0.53 | 0.31 | 0.90 | 0.018 * |
| Creatinine (mg/dL) < 4.0 | 109 | 0.53 | 0.32 | 0.88 | 0.015 * |
| Urea (mg/dL) < 120.0 | 129 | 0.57 | 0.36 | 0.89 | 0.014 * |
| Phosphate (mg/dL) < 6.0 | 97 | 0.51 | 0.30 | 0.88 | 0.015 * |
| Calcium (mg/dL) < 10.6 | 116 | 0.48 | 0.30 | 0.78 | 0.003 ** |
| Potassium (mmol/L) ≥ 3.5 | 104 | 0.59 | 0.35 | 0.98 | 0.042 * |
| Packed cell volume (%) ≥ 30.0 | 105 | 0.47 | 0.28 | 0.79 | 0.005 ** |
| Urine protein (mg/dL) ≤ 300 | 112 | 0.60 | 0.37 | 0.97 | 0.037 * |
| Overall Survival | |||||
| Beraprost Therapy versus No Beraprost Therapy | |||||
| Subgroups | Number of Cats | Hazard Ratio for Death | 95% Confidence Interval | p Value | |
| Lower Limit | Upper Limit | ||||
| All | 134 | 0.49 | 0.30 | 0.81 | 0.005 ** |
| Age (years) ≤ 15.0 | 61 | 0.25 | 0.10 | 0.67 | 0.006 ** |
| Weight (kg) ≥ 3.0 | 99 | 0.45 | 0.24 | 0.81 | 0.008 ** |
| Creatinine (mg/dL) < 4.0 | 109 | 0.45 | 0.25 | 0.80 | 0.007 ** |
| Urea (mg/dL) < 120.0 | 129 | 0.53 | 0.32 | 0.88 | 0.014 * |
| Phosphate (mg/dL) < 6.0 | 97 | 0.46 | 0.25 | 0.86 | 0.015 * |
| Calcium (mg/dL) < 10.6 | 116 | 0.44 | 0.26 | 0.76 | 0.003 ** |
| Potassium (mmol/L) ≥ 3.5 | 104 | 0.55 | 0.31 | 0.97 | 0.039 * |
| Packed cell volume (%) ≥ 30.0 | 105 | 0.42 | 0.23 | 0.75 | 0.004 ** |
| Urine protein (mg/dL) ≤ 300 | 112 | 0.54 | 0.32 | 0.92 | 0.023 * |
| Total (n = 97) | Beraprost Therapy (n = 47) | No Beraprost Therapy (n = 50) | p Value | ||
|---|---|---|---|---|---|
| Age (years) | median (25th, 75th percentile) | 15.6 (12.0, 17.1) | 15.9 (13.8, 17.1) | 15.1 (9.5, 17.3) | 0.302 |
| Weight (kg) | median (25th, 75th percentile) | 3.7 (3.0, 4.7) | 3.9 (3.4, 4.7) | 3.7 (2.9, 4.8) | 0.337 |
| Sex | |||||
| Female | number (%) | 53 (54.6%) | 27 (57.4%) | 26 (52.0%) | 0.684 |
| Male | number (%) | 44 (45.4%) | 20 (42.6%) | 24 (48.0%) | |
| Neutered | number (%) | 85 (87.6%) | 38 (80.9%) | 47 (94.0%) | 0.066 |
| Breeds | |||||
| Domestic Shorthair | number (%) | 66 (68.0%) | 33 (70.2%) | 33 (66.0%) | 0.670 |
| Japanese Bobtail | number (%) | 5 (5.2%) | 3 (6.4%) | 2 (4.0%) | |
| American Shorthair | number (%) | 6 (6.2%) | 3 (6.4%) | 3 (6.0%) | |
| Other | number (%) | 20 (20.6%) | 8 (17.0%) | 12 (24.0%) | |
| Biochemistry | |||||
| Creatinine (mg/dL) | median (25th, 75th percentile) | 3.2 (3.0, 3.5) | 3.2 (3.0, 3.6) | 3.2 (3.0, 3.4) | 0.335 |
| Urea (mg/dL) | median (25th, 75th percentile) | 38.0 (32.0, 48.0) | 36.0 (30.5, 44.0) | 40.5 (35.3, 48.8) * | 0.045 |
| Phosphate (mg/dL) | median (25th, 75th percentile) | 4.3 (3.8, 5.1) | 4.3 (3.8, 5.0) | 4.4 (3.9, 5.2) | 0.280 |
| Calcium (mg/dL) | median (25th, 75th percentile) | 9.7 (9.2, 10.1) | 9.8 (9.4, 10.4) * | 9.5 (9.0, 10.0) | 0.032 |
| Potassium (mmol/L) | median (25th, 75th percentile) | 3.9 (3.5, 4.2) | 4.0 (3.7, 4.2) | 3.9 (3.5, 4.2) | 0.119 |
| Hematology | |||||
| Packed cell volume (%) | median (25th, 75th percentile) | 36.5 (32.0, 40.0) | 37.0 (32.5, 41.0) | 35.0 (31.0, 40.0) | 0.146 |
| Urinalysis | |||||
| Urine specific gravity | median (25th, 75th percentile) | 1.014 (1.013, 1.017) | 1.014 (1.013, 1.017) | 1.014 (1.012, 1.018) | 0.837 |
| Urine protein (mg/dL) | mean (SD) | 36.7 (116.6) | 22.8 (51.5) | 52.7 (161.3) | 0.141 |
| Blood pressure measurement | |||||
| Systolic blood pressure (mmHg) | median (25th, 75th percentile) | 155.0 (139.0, 165.0) | 158.0 (140.0, 166.0) | 155.0 (137.5, 160.5) | 0.456 |
| Treatment | |||||
| Beraprost (RAPROS) | number (%) | 47 (48.5%) | 47 (100.0%) ** | 0 (0.0%) | <0.001 |
| Dose (μg/kg twice daily) | mean (SD, range) | 14.4 (3.6, 7.1–22.2) | 14.4 (3.6, 7.1–22.2) ** | 0 (0, 0–0) | <0.001 |
| Subcutaneous fluid therapy (total) | number (%) | 76 (78.4%) | 33 (70.2%) | 43 (86.0%) | 0.084 |
| At clinic | number (%) | 65 (67.0%) | 27 (57.4%) | 38 (76.0%) | 0.083 |
| At home | number (%) | 48 (49.5%) | 20 (42.6%) | 28 (56.0%) | 0.225 |
| Kidney Diet (total) | number (%) | 53 (54.6%) | 28 (59.6%) | 25 (50.0%) | 0.416 |
| Royal Canin (Renal Support) | number (%) | 37 (38.1%) | 17 (36.2%) | 20 (40.0%) | 0.835 |
| Hill’s (k/d) | number (%) | 24 (24.7%) | 13 (27.7%) | 11 (22.0%) | 0.639 |
| Other | number (%) | 28 (28.9%) | 19 (40.4%) * | 9 (18.0%) | 0.024 |
| Phosphate binder | number (%) | 24 (24.7%) | 9 (19.1%) | 13 (26.0%) | 0.474 |
| Ferric chloride (Lenziaren) | |||||
| Oral activated charcoal | number (%) | 26 (26.8%) | 13 (27.7%) | 13 (26.0%) | 1.000 |
| ACEI/ARB (total) | number (%) | 26 (26.8%) | 12 (25.5%) | 14 (28.0%) | 0.822 |
| Benazepril (Fortekor) | number (%) | 19 (19.6%) | 7 (14.9%) | 12 (24.0%) | 0.312 |
| Telmisartan (Semintra) | number (%) | 10 (10.3%) | 5 (10.6%) | 5 (10.0%) | 1.000 |
| Calcium channel blocker | number (%) | 9 (9.3%) | 7 (14.9%) | 2 (4.0%) | 0.085 |
| Amlodipine | |||||
| Erythrocyte-stimulating agents | number (%) | 21 (21.6%) | 8 (17.0%) | 13 (26.0%) | 0.330 |
| Darbepoetin alfa | |||||
| Managing inappetence, nausea and vomiting (total) | number (%) | 77 (79.4%) | 36 (76.6%) | 41 (82.0%) | 0.618 |
| Maropitant (Cerenia) | number (%) | 66 (68.0%) | 29 (61.7%) | 37 (74.0%) | 0.276 |
| Mirtazapine | number (%) | 31 (32.0%) | 18 (38.3%) | 13 (26.0%) | 0.276 |
| Famotidine | number (%) | 22 (22.7%) | 14 (29.8%) | 8 (16.0%) | 0.146 |
| Omeprazole | number (%) | 6 (6.2%) | 2 (4.3%) | 4 (8.0%) | 0.678 |
| Metoclopramide | number (%) | 10 (10.3%) | 4 (8.5%) | 6 (12.0%) | 0.742 |
| Coexisting Disorders | |||||
| Any | number (%) | 24 (24.7%) | 11 (23.4%) | 13 (26.0%) | 0.817 |
| Hyperthyroidism | number (%) | 10 (10.3%) | 6 (12.8%) | 4 (8.0%) | 0.516 |
| Congestive heart failure | number (%) | 4 (4.1%) | 0 (0.0%) | 4 (8.0%) | 0.118 |
| Neoplasia † | number (%) | 6 (6.2%) | 3 (6.4%) | 3 (6.0%) | 1.000 |
| Diabetes mellitus | number (%) | 2 (2.1%) | 1 (2.1%) | 1 (2.0%) | 1.000 |
| Pancreatitis | number (%) | 2 (2.1%) | 1 (2.1%) | 1 (2.0%) | 1.000 |
| Total (n = 134) | Beraprost Therapy (n = 57) | No Beraprost Therapy (n = 77) | ||
|---|---|---|---|---|
| Any | number (%) | 21 (15.7%) | 5 (8.8%) | 16 (20.8%) |
| Cardiovascular disorders (total) | number (%) | 10 (7.5%) | 2 (3.5%) | 8 (10.4%) |
| Congestive heart failure | number (%) | 4 (3.0%) | 0 (0.0%) | 4 (5.2%) |
| Cardiomyopathy | number (%) | 6 (4.5%) | 2 (3.5%) | 4 (5.2%) |
| Endocrine disorders (total) | number (%) | 2 (1.5%) | 0 (0.0%) | 2 (2.6%) |
| Hyperthyroidism | number (%) | 1 (0.7%) | 0 (0.0%) | 1 (1.3%) |
| Diabetes mellitus | number (%) | 1 (0.7%) | 0 (0.0%) | 1 (1.3%) |
| Neuromuscular disorders (total) | number (%) | 3 (2.2%) | 0 (0.0%) | 3 (3.9%) |
| Epilepsy & Seizures | number (%) | 3 (2.2%) | 0 (0.0%) | 3 (3.9%) |
| Neoplasia (total) | number (%) | 6 (4.5%) | 3 (5.3%) | 3 (3.9%) |
| Mast cell tumor | number (%) | 1 (0.7%) | 1 (1.8%) | 0 (0.0%) |
| Lymphoma | number (%) | 1 (0.7%) | 0 (0.0%) | 1 (1.3%) |
| Squamous cell carcinoma | number (%) | 1 (0.7%) | 1 (1.8%) | 0 (0.0%) |
| Fibrosarcoma | number (%) | 1 (0.7%) | 0 (0.0%) | 1 (1.3%) |
| Pulmonary adenocarcinoma | number (%) | 1 (0.7%) | 1 (1.8%) | 0 (0.0%) |
| Mesothelioma | number (%) | 1 (0.7%) | 0 (0.0%) | 1 (1.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, H.; Matsuura, T.; Sano, T. Beraprost and Overall Survival in Cats with Chronic Kidney Disease. Vet. Sci. 2023, 10, 459. https://doi.org/10.3390/vetsci10070459
Ito H, Matsuura T, Sano T. Beraprost and Overall Survival in Cats with Chronic Kidney Disease. Veterinary Sciences. 2023; 10(7):459. https://doi.org/10.3390/vetsci10070459
Chicago/Turabian StyleIto, Hiroyuki, Takumi Matsuura, and Tadashi Sano. 2023. "Beraprost and Overall Survival in Cats with Chronic Kidney Disease" Veterinary Sciences 10, no. 7: 459. https://doi.org/10.3390/vetsci10070459
APA StyleIto, H., Matsuura, T., & Sano, T. (2023). Beraprost and Overall Survival in Cats with Chronic Kidney Disease. Veterinary Sciences, 10(7), 459. https://doi.org/10.3390/vetsci10070459








