Age-Related Differences in Testosterone Concentration and Its Relation to Testicular Biometrics, Hemodynamics, and Fertility in Alpacas (Vicugna pacos)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Reagents and Media
2.3. Animals and Management
2.4. Blood Sampling and Hormone Analysis
2.5. Testicular Analysis Using B-Mode Ultrasonography
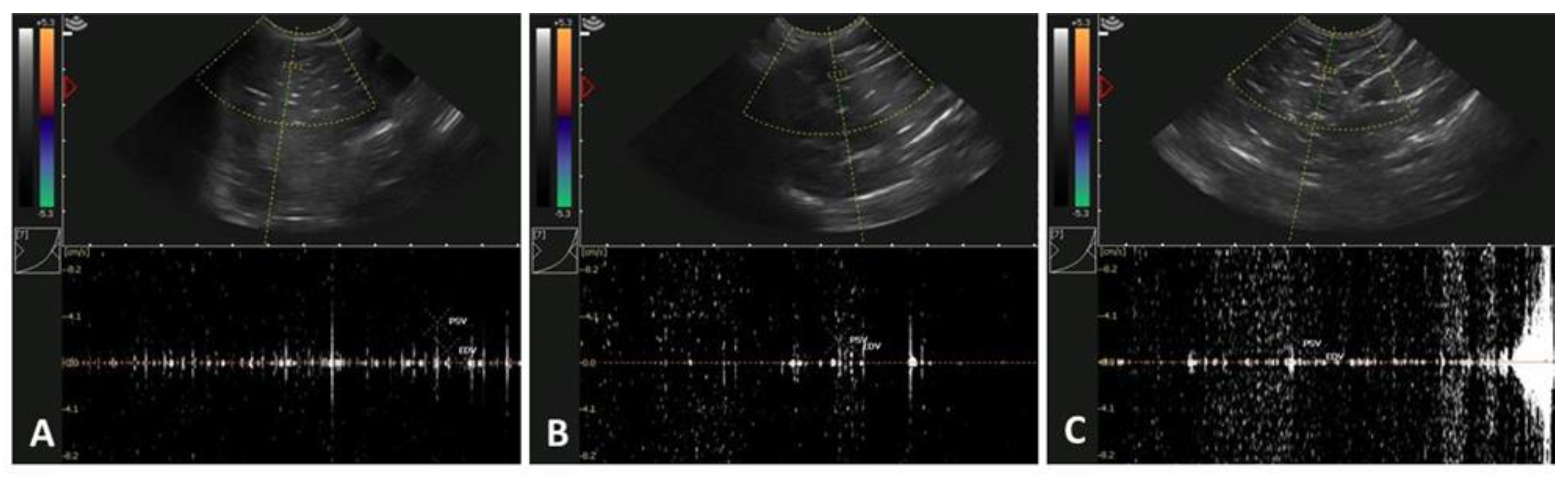
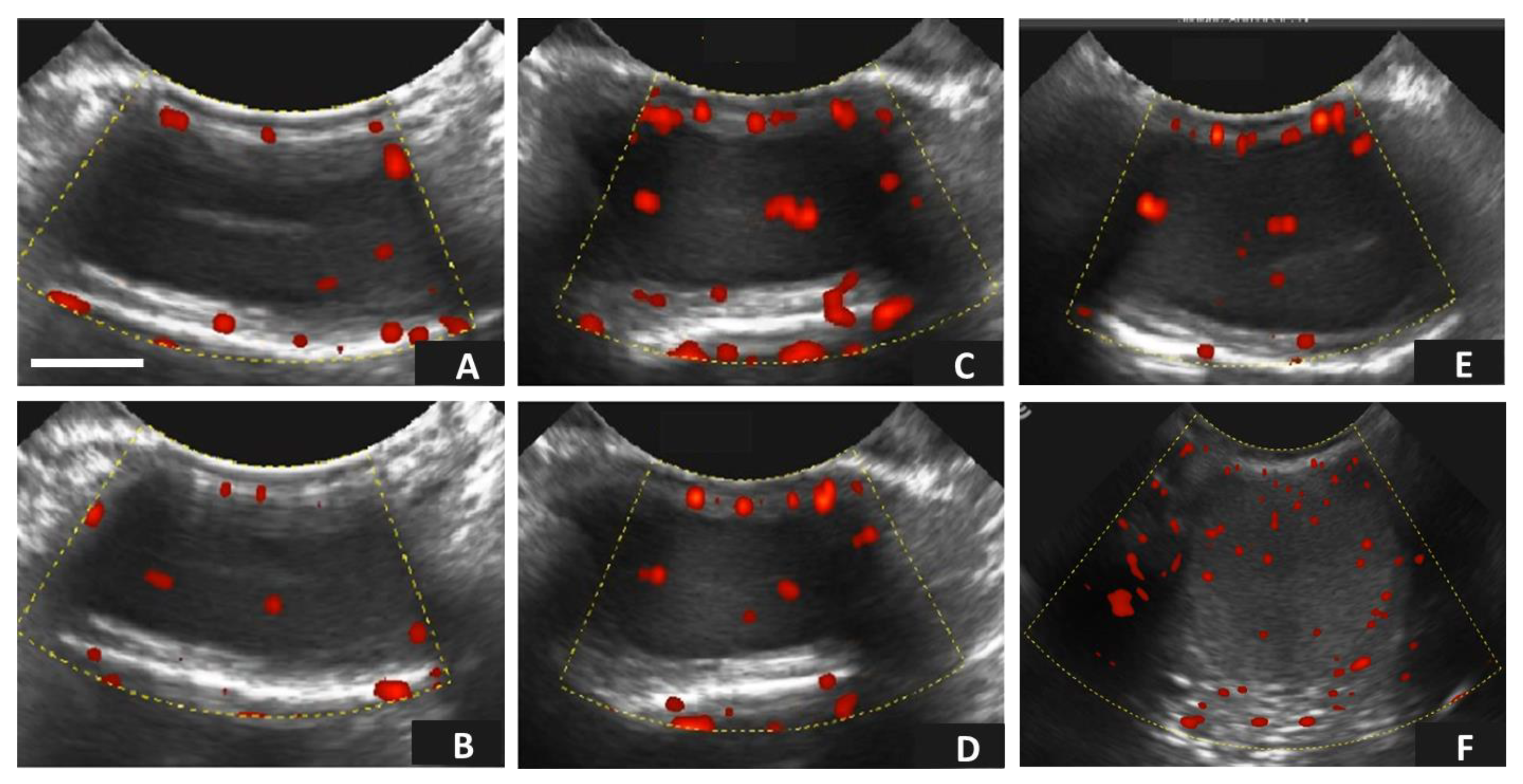
2.6. Testicular Assessment Using Doppler Ultrasonography
2.7. Male Fertility Assessment by Evaluation of Pregnancy Rate
2.8. Statistical Analysis
3. Results
3.1. Age-Related Effects on Testosterone Concentration, Testicular Morphometric Parameters, and Pregnancy Rate in Alpacas
3.2. Age-Related Effects on Testicular Circulatory Dynamics in Alpacas
3.3. Testosterone Concentration and Its Relation to Testicular Morphometry, Hemodynamics, and Pregnancy Rate in Alpacas
3.4. Correlation between Testosterone Concentration, Testicular Morphometry, Testicular Hemodynamics, and Pregnancy Rate in Alpacas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INEI. Censo Nacional agropecuario. In Resultados Definitivos IV Censo Nacional Agropecuario—2012; INEI: Jesús María, Lima, 2012; p. 1. [Google Scholar]
- Bravo, P.W.; Johnson, L.W. Reproductive Physiology of the Male Camelid. Vet. Clin. North Am. Food Anim. Pract. 1994, 10, 259–264. [Google Scholar] [CrossRef] [PubMed]
- FAO. Manual de Prácticas de Manejo de Alpacas y Llamas; FAI, Ed.; FAO: Rome, Italy, 1996; ISBN 9253039035. [Google Scholar]
- Bustinza, V. La Alpaca, Conocimiento del Gran Potencial; Andino Editorial de La Universidad Nacional del Altiplano: Puno, Perú, 2001; pp. 113–126. [Google Scholar]
- Renieri, C.; Pacheco, C.; Valbonesi, A. Selection Program in South American Domestic Camelids. Prod. Anim. 2007, 15, 205–210. [Google Scholar]
- Brown, B.W. A Review on Reproduction in South American Camelids. Anim. Reprod. Sci. 2000, 58, 169–195. [Google Scholar] [CrossRef]
- Hahn, J.; Foote, R.H.; Seidel, G.E. Testicular Growth and Related Sperm Output in Dairy Bulls. J. Anim. Sci. 1969, 29, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, U.B.; Gulavane, S.U.; Gaikwad, S.M.; Shelar, R.R.; Deshpande, V.P.; Rebeiro, R.; Yadav, K. Correlation of Testicular Ultrasonography, Testicular Biometry, Serum Testosterone Levels and Seminal Attributes in Pre and Post-Pubertal Age for Breeding Soundness Evaluation in Osmanabadi Bucks. Trop. Anim. Health Prod. 2019, 51, 1467–1480. [Google Scholar] [CrossRef]
- Sumar, J.B. Llamas and Alpacas. In Reproduction in Farm Animals; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 218–236. [Google Scholar] [CrossRef]
- Brito, L.F.C.; Barth, A.D.; Wilde, R.E.; Kastelic, J.P. Testicular Ultrasonogram Pixel Intensity during Sexual Development and Its Relationship with Semen Quality, Sperm Production, and Quantitative Testicular Histology in Beef Bulls. Theriogenology 2012, 78, 69–76. [Google Scholar] [CrossRef]
- De Magalhães, F.F.; De Souza, M.B.; Da Silva, L.D.M. Testicular Ultrasound Evaluation in Small Animal Practice. Med. Veterinária 2019, 13, 126–135. [Google Scholar] [CrossRef]
- Da Silva-Álvarez, E.; Gaitskell-Phillips, G.; Ortiz-Rodríguez, J.M.; Serres, C.; García-Rodríguez, B.; Gutiérrez-Cepeda, L.; Martín- Cano, F.E.; Echegaray, A.; Escartin-Casas, N.; Requena, F.; et al. Evaluation of Testicular Echotexture with Ecotext as a Diagnostic Method of Testicular Dysfunction in Stallions. Theriogenology 2022, 185, 50–60. [Google Scholar] [CrossRef]
- Gloria, A.; Carluccio, A.; Wegher, L.; Robbe, D.; Valorz, C.; Contri, A. Pulse Wave Doppler Ultrasound of Testicular Arteries and Their Relationship with Semen Characteristics in Healthy Bulls. J. Anim. Sci. Biotechnol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Ginther, O.J. How Ultrasound Technologies Have Expanded and Revolutionized Research in Reproduction in Large Animals. Theriogenology 2014, 81, 112–125. [Google Scholar] [CrossRef]
- Velasco, A.; Ruiz, S. New Approaches to Assess Fertility in Domestic Animals: Relationship between Arterial Blood Flow to the Testicles and Seminal Quality. Animals 2020, 11, 12. [Google Scholar] [CrossRef]
- Perez-Guerra, U.H.; Quispe, Y.M.; Gonzáles, H.I.; Luque, N.; Ruelas, D.A.; Carretero, M.I.; Gutiérrez-Reinoso, M.A.; Pérez-Durand, M.G.; García-Herreros, M. Ovarian Follicular Dynamics and Its Functional Significance in Relation with Follicle Deviation, Vaginal Cytology, and Hormone Profiles in Llamas (Lama glama). Animals 2022, 12, 3299. [Google Scholar] [CrossRef]
- Auer, M.; Wagner, H.; Failing, K.; Wehrend, A. Epididymis incision as a method to collect epididymal sperm cells in alpacas. Vet. Med. Sci. 2022, 8, 157–163. [Google Scholar] [CrossRef]
- Kutzler, M.; Tyson, R.; Grimes, M.; Timm, K. Determination of Testicular Blood Flow in Camelids Using Vascular Casting and Color Pulsed-Wave Doppler Ultrasonography. Vet. Med. Int. 2011, 2011, 638602. [Google Scholar] [CrossRef]
- Siqueira, L.G.; Arashiro, E.K.; Ghetti, A.M.; Souza, E.D.; Feres, L.F.; Pfeifer, L.F.; Fonseca, J.F.; Viana, J.H. Vascular and Morphological Features of the Corpus Luteum 12 to 20 Days after Timed Artificial Insemination in Dairy Cattle. J. Dairy Sci. 2019, 102, 5612–5622. [Google Scholar] [CrossRef]
- Perez Guerra, U.H.; Bustamante Quispe, C.W.; Luque Mamani, N.; Huayta Arizaca, R.F.; Condori Chuchi, E.A.; Catacora Flores, N.L.; Pérez Durand, M.G. Caracterización Ultrasonográfica Modo-B y Doppler Del Cuerpo Lúteo En Llamas. J. Selva Andin. Anim. Sci. 2021, 8, 3–11. [Google Scholar] [CrossRef]
- Abalos, M.C.; Acuña, F.; Cancino, A.K.; Aller, J.F. Effect of GnRH Analogue Administration on Day 7 after Natural Mating on Formation Accessory Corpus Luteum, Progesterone Concentration and Conception Rate in Llamas (Lama glama). Anim. Reprod. Sci. 2018, 190, 47–52. [Google Scholar] [CrossRef]
- Huanca Mamani, T. Empadre Controlado En Alpacas Sistema INIA; Instituto Nacional de Innovación Agraria: Lima, Perú, 2020. [Google Scholar]
- Parraguez, V.H.; Cortéz, S.; Gazitúa, F.J.; Ferrando, G.; MacNiven, V.; Raggi, L.A. Early Pregnancy Diagnosis in Alpaca (Lama pacos) and Llama (Lama glama) by Ultrasound. Anim. Reprod. Sci. 1997, 47, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.E. Pregnancy Diagnosis in Camelids. Livestock 2017, 22, 330–334. [Google Scholar] [CrossRef]
- Team, R.C. European Environment Agency. Choice Rev. Online 1999, 36, 194. [Google Scholar] [CrossRef]
- Aba, M.A. Chapter 14—Anatomy and Physiology of Reproduction in the Female Llama and Alpaca. In Llama and Alpaca Care: Medicine, Surgery, Reproduction, Nutrition, and Herd Health; WB Saunders: Philadelphia, PA, USA, 2014; pp. 140–150. [Google Scholar] [CrossRef]
- Bravo, W.P. Reproductive Anatomy and Physiology in the Male. In Llama and Alpaca Care: Medicine, Surgery, Reproduction, Nutrition, and Herd Health; WB Saunders: Philadelphia, PA, USA, 2014; pp. 150–161. [Google Scholar] [CrossRef]
- Incahuanaco, L.M.; Ayala, R.D.; Hinojosa, R.L.; Torres, E.Y.; Huanca, T.; Nina, A.; Málaga, J. Eficiencia Reproductiva de Alpacas Machos en Relación al Tamaño Testicular y Niveles Hormonales Durante Época Reproductiva en Puna Seca. Rev. Investig. Agropecu. Sci. Biotechnol. 2021, 1, 56–62. [Google Scholar] [CrossRef]
- Gandarillas, D.; Torres, E.A.; Quispe, A.E.; Rios, R.M.; Puma, A. Relación de Los Niveles de Testosterona Sérica y el Tamaño Testicular en Alpacas Según Grupo Etario y Mes del Año En Tacna, Perú. Rev. Investig. Agropecu. Sci. Biotechnol. 2022, 2, 14–20. [Google Scholar] [CrossRef]
- Ortega-Ferrusola, C.; Gracia-Calvo, L.A.; Ezquerra, J.; Pena, F.J. Use of Colour and Spectral Doppler Ultrasonography in Stallion Andrology. Reprod. Domest. Anim. 2014, 49, 88–96. [Google Scholar] [CrossRef]
- Schulze, M.; Beyer, S.; Beyer, F.; Bortfeldt, R.; Riesenbeck, A.; Leiding, C.; Jung, M.; Kleve-Feld, M. Relationship between Pubertal Testicular Ultrasonographic Evaluation and Future Reproductive Performance Potential in Piétrain Boars. Theriogenology 2020, 158, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Bott; Rodriguez, J.; Sandoval, S.; Tibary, A. Relationship between Testicular Measurements Using Calipers or Ultrasonography with Testicular Weight in Alpacas (Vicugna pacos). Theriogenology 2008, 70, 576. [Google Scholar] [CrossRef]
- Hedia, M.G.; El-Belely, M.S. Testicular Morphometric and Echotextural Parameters and Their Correlation with Intratesticular Blood Flow in Ossimi Ram Lambs. Large Anim. Rev. 2021, 27, 77–82. [Google Scholar]
- Giuliano, S.M.; Spirito, S.E.; Miragaya, M.H.; Rutter, B.; Agüero, A.; Capdevielle, E.F.; Boquet, M.D.; Ferrari, M.R. Aparato Reproductor y Parámetros Seminales de Vicuña (Vicugna vicugna vicugna). InVet 2013, 15, 47–55. [Google Scholar]
- Abraham, M.C.; Puhakka, J.; Ruete, A.; Al-Essawe, E.M.; Verdier, K.; Morrell, J.M.; Båge, R. Testicular Length as an Indicator of the Onset of Sperm Production in Alpacas under Swedish Conditions. Acta Vet. Scand. 2016, 58, 10. [Google Scholar] [CrossRef]
- Fernández-Baca, S. Manipulation of Reproductive Functions in Male and Female New World Camelids. Anim. Reprod. Sci. 1993, 33, 307–323. [Google Scholar] [CrossRef]
- Osada, T.; Murase, N.; Kime, R.; Shiroishi, K.; Shimomura, K.; Nagata, H.; Katsumura, T. Arterial Blood Flow of All Abdominal-Pelvic Organs Using Doppler Ultrasound: Range, Variability and Physiological Impact. Physiol. Meas. 2007, 28, 1303–1316. [Google Scholar] [CrossRef]
- Gacem, S.; Papas, M.; Catalan, J.; Miró, J. Examination of Jackass (Equus asinus) Accessory Sex Glands by B-Mode Ultrasound and of Testicular Artery Blood Flow by Colour Pulsed-Wave Doppler Ultrasound: Correlations with Semen Production. Reprod. Domest. Anim. 2020, 55, 181–188. [Google Scholar] [CrossRef]
- Lemos, H.; Dorado, J.; Hidalgo, M.; Gaivão, I.; Martins-Bessa, A. Assessment of Dog Testis Perfusion by Colour and Pulsed-Doppler Ultrasonography and Correlation with Sperm Oxidative DNA Damage. Top. Companion Anim. Med. 2020, 41, 100452. [Google Scholar] [CrossRef]
- Pozor, M.A.; McDonnell, S.M. Color Doppler Ultrasound Evaluation of Testicular Blood Flow in Stallions. Theriogenology 2004, 61, 799–810. [Google Scholar] [CrossRef]
- Ginther, O.J. Ultrasonic Imaging and Animal Reproduction: Book 4, Color-Doppler Ultrasonography. In Color Doppler Ultrason; Equiservices Publishing: Cross Plains, WI, USA, 2007; pp. 1–258. [Google Scholar]
- Zelli, R.; Troisi, A.; Elad Ngonput, A.; Cardinali, L.; Polisca, A. Evaluation of Testicular Artery Blood Flow by Doppler Ultrasonography as a Predictor of Spermatogenesis in the Dog. Res. Vet. Sci. 2013, 95, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Hedia, M.; El-Shalofy, A. Ageing Affects Plasma Steroid Concentrations and Testicular Volume, Echotexture and Haemodynamics in Rams. Andrologia 2022, 54, e14309. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, E.A.; Emam, I.A.; Fadl, A.M. Assessment of the Accuracy of Testicular Dysfunction Detection in Male Donkey (Equus asinus) with the Aid of Colour-Spectral Doppler in Relation to Plasma Testosterone and Serum Nitric Oxide Levels. Reprod. Domest. Anim. 2021, 56, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Olvera, S.F.; Rajmil, O.; Sanchez-Curbelo, J.R.; Vinay, J.; Rodriguez-Espinosa, J.; Ruiz-Castañé, E. Association of Serum Testosterone Levels and Testicular Volume in Adult Patients. Andrologia 2018, 50, e12933. [Google Scholar] [CrossRef]
- El Zawam, A.; Tibary, A.; Patino, C. Basal Levels and HCG Responses of Serum Testosterone and Estrogen in Male Alpacas. Front. Vet. Sci. 2020, 7, 595856. [Google Scholar] [CrossRef]
- Holm, M.; De Meyts, E.R.; Andersson, A.M.; Skakkebæk, N.E. Leydig Cell Micronodules Are a Common Finding in Testicular Biopsies from Men with Impaired Spermatogenesis and Are Associated with Decreased Testosterone/LH Ratio. J. Pathol. 2003, 199, 378–386. [Google Scholar] [CrossRef]
- Hedia, M.G.; El-Belely, M.S.; Ismail, S.T.; El-Maaty, A.M. View of Evaluation of Testicular Blood Flow and Ultrasonographic Measurements in Rams with Emphasis on Laterality. J. Adn. Vet. Res. 2020, 10, 17–20. [Google Scholar]
- El-Shalofy, A.S.; Hedia, M.G. Exogenous Oxytocin Administration Improves the Testicular Blood Flow in Rams. Andrologia 2021, 53, e14193. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.B.; England, G.C.W.; Mota Filho, A.C.; Ackermann, C.L.; Sousa, C.V.S.; de Carvalho, G.G.; Silva, H.V.R.; Pinto, J.N.; Linhares, J.C.S.; Oba, E.; et al. Semen Quality, Testicular B-Mode and Doppler Ultrasound, and Serum Testosterone Concentrations in Dogs with Established Infertility. Theriogenology 2015, 84, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Nguyen, D.N.; Tam Nguyen, T.T.; Nguyen, V.Q.H.; Pham, C.K.; Le, D.D.; Cao, N.T. Should Scrotal Color Doppler Ultrasound Be Routinely Indicated in Fertility Evaluation of Non-Azoospermic Men? Curr. Urol. 2020, 14, 211–218. [Google Scholar] [CrossRef]
- Sumar, J.B. Reproduction in Female South Americann Domestic Camelids. J. Reprod. Fertil. Suppl. 1999, 54, 169–178. [Google Scholar] [PubMed]
- Argañaraz, M.E.; Apichela, S.A.; Zampini, R.; Vencato, J.; Stelletta, C. Biochemical and Protein Profile of Alpaca (Vicugna pacos) Uterine Horn Fluid during Early Pregnancy. Reprod. Domest. Anim. 2015, 50, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.W.; Solis, P.; Ordoñez, C.; Alarcon, V. Fertility of the Male Alpaca: Effect of Daily Consecutive Breeding. Anim. Reprod. Sci. 1997, 46, 305–312. [Google Scholar] [CrossRef]
- Barraza, D.E.; Zampini, R.; Apichela, S.A.; Pacheco, J.I.; Argañaraz, M.E. Changes in Mucins and Matrix Metalloproteases in the Endometrium of Early Pregnant Alpacas (Vicugna pacos). Acta Histochem. 2018, 120, 438–445. [Google Scholar] [CrossRef]
- Tan, R.H.H.; Dascanio, J.J. Infertility Associated with Persistent Hymen in an Alpaca and a Llama. Can. Vet. J. La Rev. Vet. Can. 2008, 49, 1113–1117. [Google Scholar]
- Vaughan, J.; Mihm, M.; Wittek, T. Factors Influencing Embryo Transfer Success in Alpacas: A Retrospective Study. Anim. Reprod. Sci. 2013, 136, 194–204. [Google Scholar] [CrossRef]
- Sumar, J. Reproductive physiology in South American camelids. In Genetics of Reproduction in Sheep; Land, R.B., Robinson, D.W., Eds.; Butterwotths: London, UK, 1985; pp. 81–95. [Google Scholar]
- Tibary, A.; Vaughan, J. Reproductive physiology and infertility in male South American camelids: A review and clinical observations. Small Rumin. Res. 2006, 61, 283–298. [Google Scholar] [CrossRef]
| Experimental Groups | n | Testosterone (ng/mL) | Testicular Length (mm) | Testicular Width (mm) | Testicular Area (cm2) | Testicular Volume(cm3) | Pregnancy Rate (%) |
|---|---|---|---|---|---|---|---|
| YM (~12–14 mo.) | 5 | 2.47 ± 0.31 a | 36.75 ± 3.05 a | 21.59 ± 1.91 a | 6.59 ± 1.09 a | 11.69 ± 2.69 a | 86.85 ± 3.06 a |
| IM (~24 mo.) | 5 | 8.45 ± 1.53 b | 34.1 ± 1.30 a | 19.69 ± 0.65 a | 5.14 ± 0.50 a | 8.77 ± 1.24 a | 89.06 ± 2.44 a |
| OM (≥36 mo.) | 5 | 22.66 ± 2.15 c | 38.01 ± 1.55 a | 21.01 ± 1.64 a | 6.48 ± 0.69 a | 10.93 ± 1.43 a | 84.01 ± 3.06 a |
| Experimental Groups | n | Resistive Index (RI) | Pulsatility Index (PI) | Test. Vascular Area (% TVA) |
|---|---|---|---|---|
| YM (~12–14 mo.) | 5 | 0.54 ± 0.03 | 0.53 ± 0.08 | 9.16 ± 4.83 |
| IM (~24 mo.) | 5 | 0.49 ± 0.06 | 0.31 ± 0.09 | 3.77 ± 0.75 |
| OM (≥36 mo.) | 5 | 0.57 ± 0.05 | 0.47 ± 013 | 3.84 ± 0.25 |
| Variable | Β | Standard Error | Exp (B)/ (OR) | Z | p |
|---|---|---|---|---|---|
| Intercept | 0.24 | 0.01 | 1.27 | 16.29 | <0.0001 * |
| Testicular Length | 0.02 | 0.01 | 1.02 | 1.93 | 0.102 |
| Testicular Width | −0.02 | 0.02 | 0.98 | −0.89 | 0.410 |
| Testicular Area | 0.09 | 0.05 | 1.09 | 1.74 | 0.133 |
| Testicular Volume | −0.05 | 0.02 | 0.95 | −2.36 | 0.046 * |
| Pregnancy Rate | 0.05 | 0.31 | 1.18 | 0.18 | 0.860 |
| Resistive Index | −0.22 | 0.18 | 0.8 | −1.23 | 0.266 |
| Pulsatility Index | 0.02 | 0.09 | 1.02 | 0.18 | 0.863 |
| Test. Vascular Area | −0.01 | 0.00 | 0.99 | −2.90 | 0.027 * |
| Testosterone | Testicular Length | Testicular Width | Testicular Area | Testicular Volume | Resistive Index | Pulsatility Index | Testicular Vascular Area | Pregnancy Rate | |
|---|---|---|---|---|---|---|---|---|---|
| Testosterone | - | ||||||||
| Testicular Length | 0.20 | - | |||||||
| Testicular Width | 0.07 | 0.90 *** | - | ||||||
| Testicular Area | 0.07 | 0.91 *** | 0.97 *** | - | |||||
| TesticularVolume | 0.08 | 0.96 *** | 0.95 *** | 0.96 *** | - | ||||
| Resistive Index | −0.01 | −0.30 | −0.33 | −0.33 | −0.44 | - | |||
| Pulsatility Index | 0.02 | 0.68 * | 0.59 * | 0.61 * | 0.66 * | −0.10 | - | ||
| Test. VascularArea | −0.52 | 0.25 | 0.25 | 0.40 | 0.27 | −0.08 | 0.26 | - | |
| Pregnancy rate | 0.03 | −0.45 | −0.54 | −0.48 | −0.37 | −0.18 | 0.01 | −0.15 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Durand, M.G.; Massa-Guzmán, A.; Luque-Mamani, N.; Ruelas-Calloapaza, D.A.; Urviola-Sánchez, J.M.; Condori-Chuchi, E.A.; Gutiérrez-Reinoso, M.A.; Perez-Guerra, U.H.; García-Herreros, M. Age-Related Differences in Testosterone Concentration and Its Relation to Testicular Biometrics, Hemodynamics, and Fertility in Alpacas (Vicugna pacos). Vet. Sci. 2023, 10, 429. https://doi.org/10.3390/vetsci10070429
Pérez-Durand MG, Massa-Guzmán A, Luque-Mamani N, Ruelas-Calloapaza DA, Urviola-Sánchez JM, Condori-Chuchi EA, Gutiérrez-Reinoso MA, Perez-Guerra UH, García-Herreros M. Age-Related Differences in Testosterone Concentration and Its Relation to Testicular Biometrics, Hemodynamics, and Fertility in Alpacas (Vicugna pacos). Veterinary Sciences. 2023; 10(7):429. https://doi.org/10.3390/vetsci10070429
Chicago/Turabian StylePérez-Durand, Manuel G., Angela Massa-Guzmán, Natalio Luque-Mamani, Domingo A. Ruelas-Calloapaza, Jesús M. Urviola-Sánchez, Eloy A. Condori-Chuchi, Miguel A. Gutiérrez-Reinoso, Uri H. Perez-Guerra, and Manuel García-Herreros. 2023. "Age-Related Differences in Testosterone Concentration and Its Relation to Testicular Biometrics, Hemodynamics, and Fertility in Alpacas (Vicugna pacos)" Veterinary Sciences 10, no. 7: 429. https://doi.org/10.3390/vetsci10070429
APA StylePérez-Durand, M. G., Massa-Guzmán, A., Luque-Mamani, N., Ruelas-Calloapaza, D. A., Urviola-Sánchez, J. M., Condori-Chuchi, E. A., Gutiérrez-Reinoso, M. A., Perez-Guerra, U. H., & García-Herreros, M. (2023). Age-Related Differences in Testosterone Concentration and Its Relation to Testicular Biometrics, Hemodynamics, and Fertility in Alpacas (Vicugna pacos). Veterinary Sciences, 10(7), 429. https://doi.org/10.3390/vetsci10070429






