A Study of 41 Canine Orthologues of Human Genes Involved in Monogenic Obesity Reveals Marker in the ADCY3 for Body Weight in Labrador Retrievers
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Phenotypes
2.2. Genotypes
2.3. Association Analysis
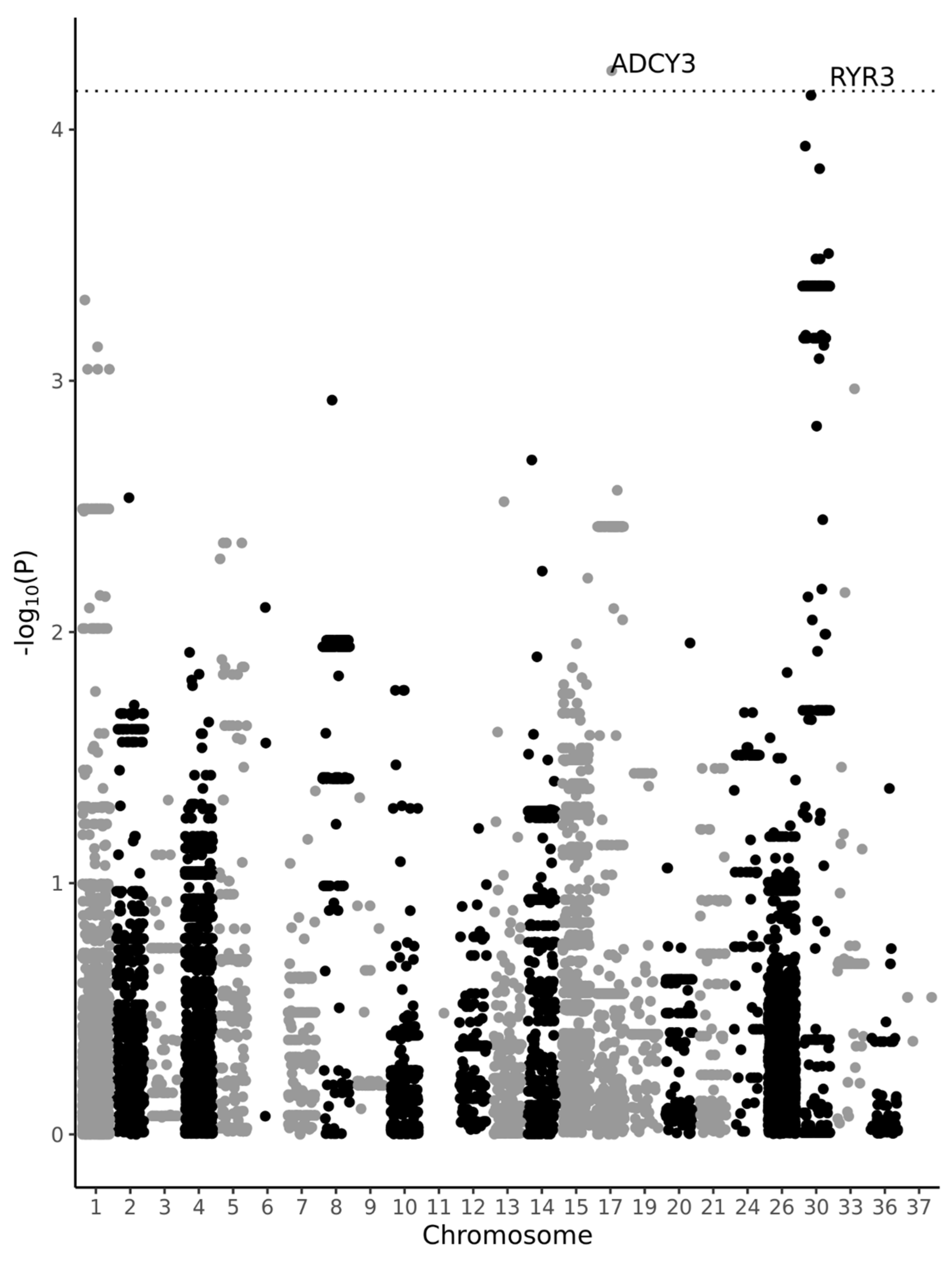
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mason, E. Obesity to Pet Dogs. Vet. Rec. 1970, 86, 612–616. [Google Scholar] [CrossRef]
- Edney, A.; Smith, P. Study of Obesity in Dogs Visiting Veterinary Practices in the United Kingdom. Vet. Rec. 1986, 118, 391–396. [Google Scholar] [CrossRef]
- Robertson, I. The Association of Exercise, Diet and Other Factors with Owner-Perceived Obesity in Privately Owned Dogs from Metropolitan Perth, WA. Prev. Vet. Med. 2003, 58, 75–83. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.; Thomson, P.; Pride, C.; Fawcett, A.; Grassi, T.; Jones, B. Prevalence of Obesity in Dogs Examined by Australian Veterinary Practices and the Risk Factors Involved. Vet. Rec. 2005, 156, 695–702. [Google Scholar] [CrossRef]
- Colliard, L.; Ancel, J.; Benet, J.-J.; Paragon, B.-M.; Blanchard, G. Risk Factors for Obesity in Dogs in France. J. Nutr. 2006, 136, 1951S–1954S. [Google Scholar] [CrossRef]
- Bjørnvad, C.R.; Gloor, S.; Johansen, S.S.; Sandøe, P.; Lund, T.B. Neutering Increases the Risk of Obesity in Male Dogs but Not in Bitches—A Cross-Sectional Study of Dog- and Owner-Related Risk Factors for Obesity in Danish Companion Dogs. Prev. Vet. Med. 2019, 170, 104730. [Google Scholar] [CrossRef] [PubMed]
- Nikpay, M.; Ravati, S.; Dent, R.; McPherson, R. Epigenome-Wide Study Identified Methylation Sites Associated with the Risk of Obesity. Nutrients 2021, 13, 1984. [Google Scholar] [CrossRef] [PubMed]
- Chami, N.; Preuss, M.; Walker, R.W.; Moscati, A.; Loos, R.J. The Role of Polygenic Susceptibility to Obesity among Carriers of Pathogenic Mutations in MC4R in the UK Biobank Population. PLoS Med. 2020, 17, e1003196. [Google Scholar] [CrossRef]
- Lund, E.M.; Armstrong, P.J.; Kirk, C.A.; Klausner, J.S. Prevalence and Risk Factors for Obesity in Adult Dogs from Private US Veterinary Practices. Int. J. Appl. Res. Vet. Med. 2006, 4, 177. [Google Scholar]
- Raffan, E.; Dennis, R.J.; O’Donovan, C.J.; Becker, J.M.; Scott, R.A.; Smith, S.P.; Withers, D.J.; Wood, C.J.; Conci, E.; Clements, D.N.; et al. A Deletion in the Canine POMC Gene Is Associated with Weight and Appetite in Obesity-Prone Labrador Retriever Dogs. Cell Metab. 2016, 23, 893–900. [Google Scholar] [CrossRef]
- Grzemski, A.; Stachowiak, M.; Flisikowski, K.; Mankowska, M.; Krzeminska, P.; Gogulski, M.; Aleksiewicz, R.; Szydlowski, M.; Switonski, M.; Nowacka-Woszuk, J. FTO and IRX3 Genes Are Not Promising Markers for Obesity in Labrador Retriever Dogs. Ann. Anim. Sci. 2019, 19, 343–357. [Google Scholar] [CrossRef]
- Sheet, S.; Krishnamoorthy, S.; Cha, J.; Choi, S.; Choi, B.-H. Identification of Candidate Genes and Pathways Associated with Obesity-Related Traits in Canines via Gene-Set Enrichment and Pathway-Based GWAS Analysis. Animals 2020, 10, 2071. [Google Scholar] [CrossRef]
- Mankowska, M.; Krzeminska, P.; Graczyk, M.; Switonski, M. Confirmation That a Deletion in the POMC Gene Is Associated with Body Weight of Labrador Retriever Dogs. Res. Vet. Sci. 2017, 112, 116–118. [Google Scholar] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Bult, C.J.; Blake, J.A.; Smith, C.L.; Kadin, J.A.; Richardson, J.E. Mouse Genome Database (MGD) 2019. Nucleic Acids Res. 2019, 47, D801–D806. [Google Scholar] [CrossRef]
- Szydlowski, M.; Antkowiak, M. No Evidence That Long Runs of Homozygosity Tend to Harbor Risk Variants for Polygenic Obesity in Labrador Retriever Dogs. J. Appl. Genet. 2022, 63, 557–561. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. GigaScience 2015, 4, 7. [Google Scholar] [CrossRef]
- Voight, B.F.; Pritchard, J.K. Confounding from Cryptic Relatedness in Case-Control Association Studies. PLoS Genet. 2005, 1, e32. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.K.; Loh, P.-R.; Finucane, H.; Ripke, S.; Yang, J.; Patterson, N.; Daly, M.J.; Price, A.L.; Neale, B.M. LD Score Regression Distinguishes Confounding from Polygenicity in Genome-Wide Association Studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar] [CrossRef]
- Westfall, P.H.; Young, S.S. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment; John Wiley & Sons: Hoboken, NJ, USA, 1993; Volume 279, ISBN 0-471-55761-7. [Google Scholar]
- John, M.; Ankenbrand, M.J.; Artmann, C.; Freudenthal, J.A.; Korte, A.; Grimm, D.G. Efficient Permutation-Based Genome-Wide Association Studies for Normal and Skewed Phenotypic Distributions. Bioinformatics 2022, 38, ii5–ii12. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://Www.R-Project.Org/ (accessed on 21 September 2022).
- Xu, T.-R.; Yang, Y.; Ward, R.; Gao, L.; Liu, Y. Orexin Receptors: Multi-Functional Therapeutic Targets for Sleeping Disorders, Eating Disorders, Drug Addiction, Cancers and Other Physiological Disorders. Cell. Signal. 2013, 25, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, L. Targeting CAMP/PKA Pathway for Glycemic Control and Type 2 Diabetes Therapy. J. Mol. Endocrinol. 2016, 57, R93–R108. [Google Scholar] [CrossRef]
- Saeed, S.; Bonnefond, A.; Tamanini, F.; Mirza, M.U.; Manzoor, J.; Janjua, Q.M.; Din, S.M.; Gaitan, J.; Milochau, A.; Durand, E.; et al. Loss-of-Function Mutations in ADCY3 Cause Monogenic Severe Obesity. Nat. Genet. 2018, 50, 175–179. [Google Scholar] [CrossRef]
- Toumba, M.; Fanis, P.; Vlachakis, D.; Neocleous, V.; Phylactou, L.A.; Skordis, N.; Mantzoros, C.S.; Pantelidou, M. Molecular Modelling of Novel ADCY3 Variant Predicts a Molecular Target for Tackling Obesity. Int. J. Mol. Med. 2022, 49, 10. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Arslan, M.; Froguel, P. Genetics of Obesity in Consanguineous Populations: Toward Precision Medicine and the Discovery of Novel Obesity Genes. Obesity 2018, 26, 474–484. [Google Scholar] [CrossRef]
- Tong, T.; Shen, Y.; Lee, H.-W.; Yu, R.; Park, T. Adenylyl Cyclase 3 Haploinsufficiency Confers Susceptibility to Diet-Induced Obesity and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 34179. [Google Scholar] [CrossRef]
- Wang, Z.; Li, V.; Chan, G.C.; Phan, T.; Nudelman, A.S.; Xia, Z.; Storm, D.R. Adult Type 3 Adenylyl Cyclase–Deficient Mice Are Obese. PLoS ONE 2009, 4, e6979. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Leng, Y.; Yang, Y.; Zweifel, L.S.; Palmiter, R.D.; Storm, D.R. Ablation of Type III Adenylyl Cyclase in Mice Causes Reduced Neuronal Activity, Altered Sleep Pattern, and Depression-like Phenotypes. Biol. Psychiatry 2016, 80, 836–848. [Google Scholar] [CrossRef]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L. New Genetic Loci Implicated in Fasting Glucose Homeostasis and Their Impact on Type 2 Diabetes Risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef]
- Pitman, J.L.; Wheeler, M.C.; Lloyd, D.J.; Walker, J.R.; Glynne, R.J.; Gekakis, N. A Gain-of-Function Mutation in Adenylate Cyclase 3 Protects Mice from Diet-Induced Obesity. PLoS ONE 2014, 9, e110226. [Google Scholar] [CrossRef]
- Rearick, D.; Prakash, A.; McSweeny, A.; Shepard, S.S.; Fedorova, L.; Fedorov, A. Critical Association of NcRNA with Introns. Nucleic Acids Res. 2011, 39, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, A.A.; Cenik, C.; Chua, H.N.; Roth, F.P.; Moore, M.J. Introns in UTRs: Why We Should Stop Ignoring Them. BioEssays 2012, 34, 1025–1034. [Google Scholar] [CrossRef]
- Cenik, C.; Chua, H.N.; Zhang, H.; Tarnawsky, S.P.; Akef, A.; Derti, A.; Tasan, M.; Moore, M.J.; Palazzo, A.F.; Roth, F.P. Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear MRNA Export for Secretory and Mitochondrial Genes. PLoS Genet. 2011, 7, e1001366. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Pouliquin, P. What We Don’t Know about the Structure of Ryanodine Receptor Calcium Release Channels. Clin. Exp. Pharmacol. Physiol. 2003, 30, 713–723. [Google Scholar] [CrossRef]
- Johnson, J.D.; Kuang, S.; Misler, S.; Polonsky, K.S. Ryanodine Receptors in Human Pancreatic β Cells: Localization and Effects on Insulin Secretion. FASEB J. 2004, 18, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J.; Pinton, P.; Varadi, A.; Tacchetti, C.; Ainscow, E.K.; Pozzan, T.; Rizzuto, R.; Rutter, G.A. Dense Core Secretory Vesicles Revealed as a Dynamic Ca2+ Store in Neuroendocrine Cells with a Vesicle-Associated Membrane Protein Aequorin Chimaera. J. Cell Biol. 2001, 155, 41–52. [Google Scholar] [CrossRef]
- Giannini, G.; Clementi, E.; Ceci, R.; Marziali, G.; Sorrentino, V. Expression of a Ryanodine Receptor-Ca2+ Channel That Is Regulated by TGF-β. Science 1992, 257, 91–94. [Google Scholar] [CrossRef]
- Giannini, G.; Conti, A.; Mammarella, S.; Scrobogna, M.; Sorrentino, V. The Ryanodine Receptor/Calcium Channel Genes Are Widely and Differentially Expressed in Murine Brain and Peripheral Tissues. J. Cell Biol. 1995, 128, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Kong, M.; Kim, T.M.; Suh, Y.H.; Kim, W.-H.; Lim, J.H.; Song, J.H.; Jung, M.H. NFATc4 and ATF3 Negatively Regulate Adiponectin Gene Expression in 3T3-L1 Adipocytes. Diabetes 2006, 55, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-H.; Chang, E.Y.-C.; Chang, Y.-C.; Hee, S.-W.; Tsai, Y.-C.; Chang, T.-J.; Chuang, L.-M. Knockdown of RyR3 Enhances Adiponectin Expression Through an Atf3-Dependent Pathway. Endocrinology 2013, 154, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sypniewski, M.; Szydlowski, M. A Study of 41 Canine Orthologues of Human Genes Involved in Monogenic Obesity Reveals Marker in the ADCY3 for Body Weight in Labrador Retrievers. Vet. Sci. 2023, 10, 390. https://doi.org/10.3390/vetsci10060390
Sypniewski M, Szydlowski M. A Study of 41 Canine Orthologues of Human Genes Involved in Monogenic Obesity Reveals Marker in the ADCY3 for Body Weight in Labrador Retrievers. Veterinary Sciences. 2023; 10(6):390. https://doi.org/10.3390/vetsci10060390
Chicago/Turabian StyleSypniewski, Mateusz, and Maciej Szydlowski. 2023. "A Study of 41 Canine Orthologues of Human Genes Involved in Monogenic Obesity Reveals Marker in the ADCY3 for Body Weight in Labrador Retrievers" Veterinary Sciences 10, no. 6: 390. https://doi.org/10.3390/vetsci10060390
APA StyleSypniewski, M., & Szydlowski, M. (2023). A Study of 41 Canine Orthologues of Human Genes Involved in Monogenic Obesity Reveals Marker in the ADCY3 for Body Weight in Labrador Retrievers. Veterinary Sciences, 10(6), 390. https://doi.org/10.3390/vetsci10060390





