Sphingomyelin-Rich Lipid Extract Collar for Canine Atopic Dermatitis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Lipid Extract Collar
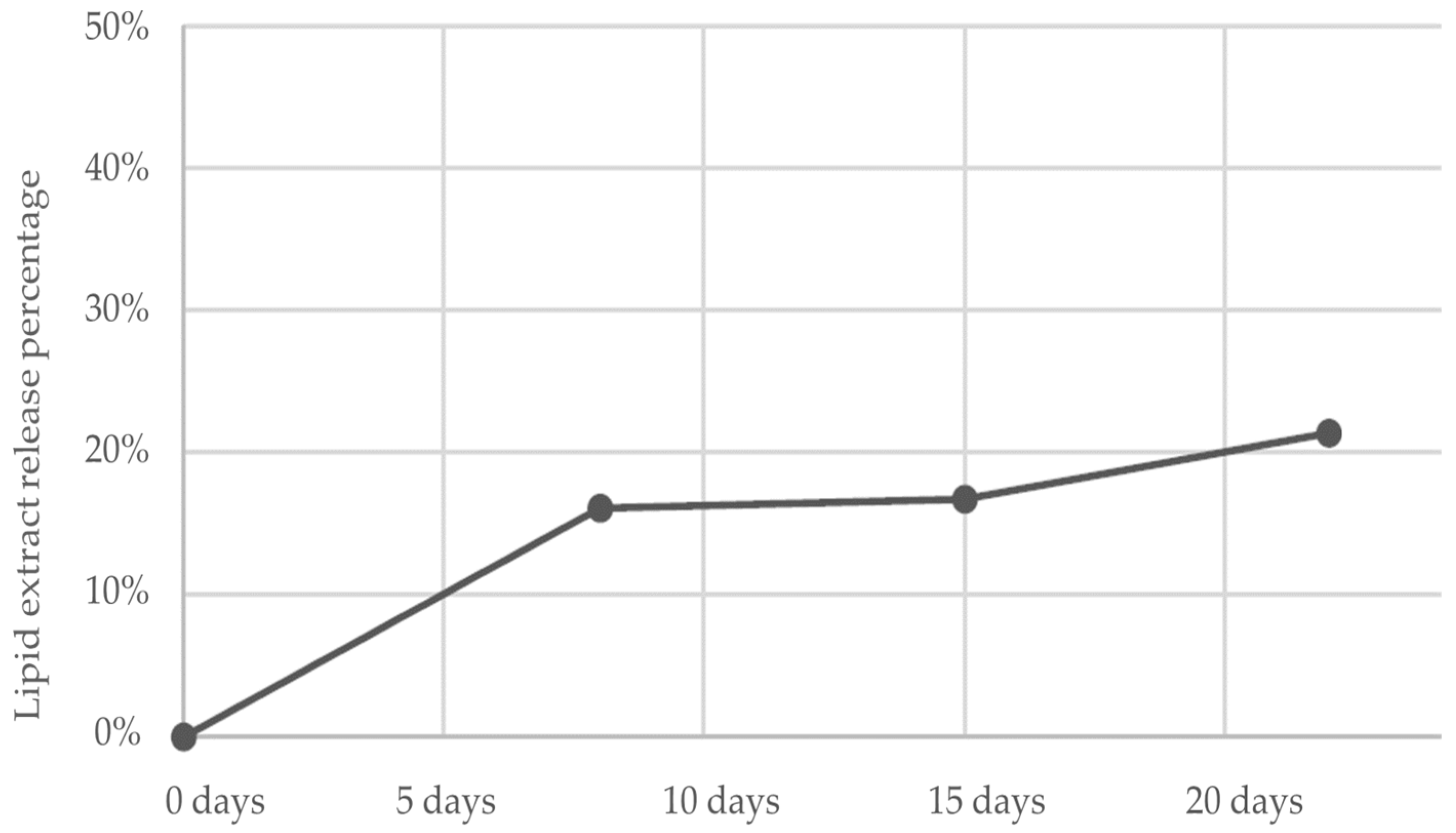
2.2. Evaluation of In Vitro Release Kinetic Pattern
2.3. Efficacy and Safety in Dogs with Atopic Dermatitis
2.4. Analyses of Used Collars
2.5. Compatibility Studies
3. Results
3.1. In Vitro Release
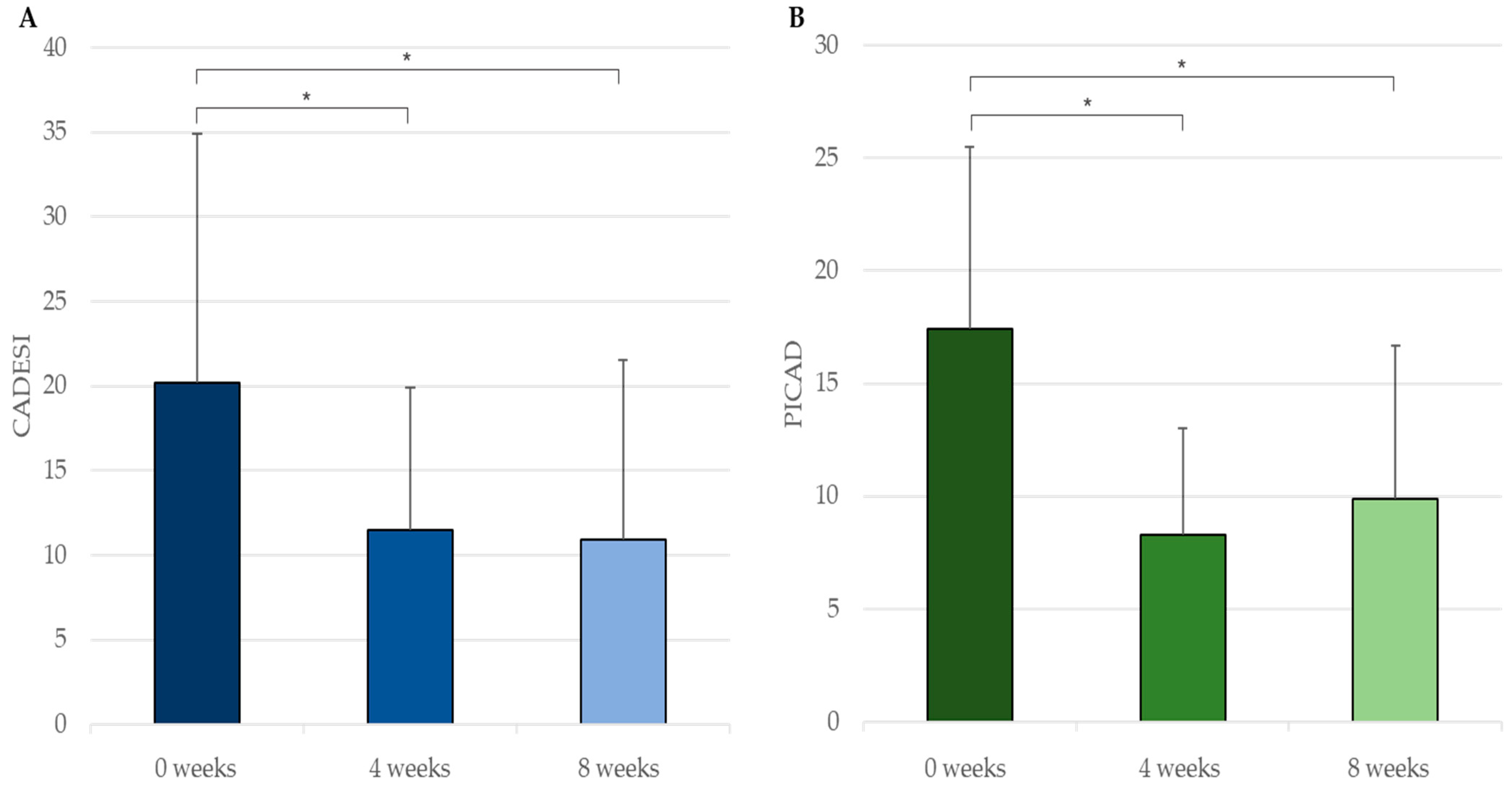
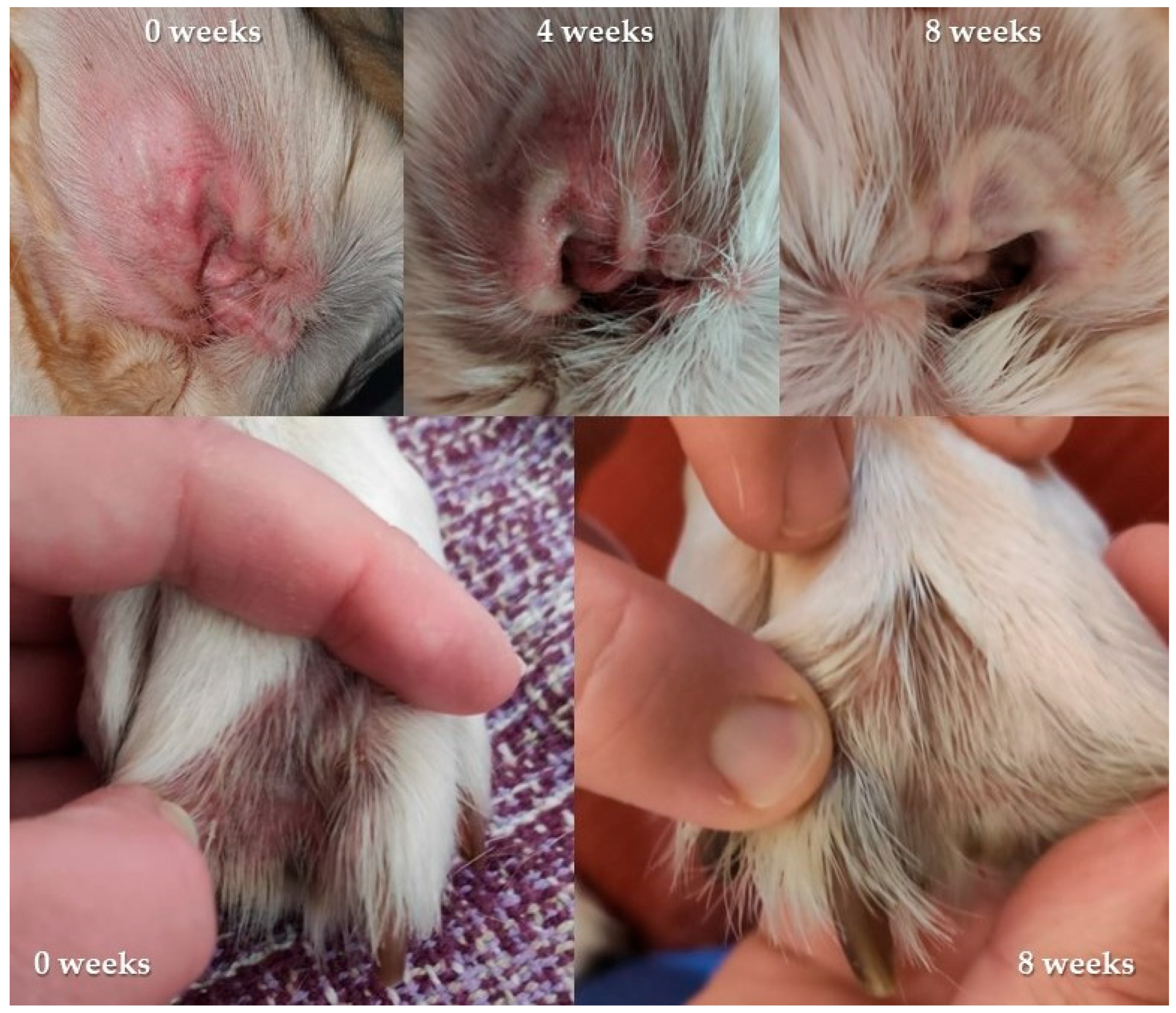
3.2. In Vivo Efficacy and Safety
3.3. Analysis of Used Collars
3.4. Compatibility with Imidacloprid, Flumethrin and Deltamethrin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R. Atopic Dermatitis in Domestic Animals: What Our Current Understanding Is and How This Applies to Clinical Practice. Vet. Sci. 2021, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzales, A.J.; Fadok, V.A. Update on Pathogenesis, Diagnosis, and Treatment of Atopic Dermatitis in Dogs. J. Am. Vet. Med. Assoc. 2019, 254, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Bruet, V.; Mosca, M.; Briand, A.; Bourdeau, P.; Pin, D.; Cochet-faivre, N.; Cadiergues, M. Clinical Guidelines for the Use of Antipruritic Drugs in the Control of the Most Frequent Pruritic Skin Diseases in Dogs. Vet. Sci. 2022, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S. Supplementary Therapy in Canine Atopic Dermatitis. Companion Anim. 2019, 24, 400–407. [Google Scholar] [CrossRef]
- Marsella, R.; Friberg, C. Topical Therapy for Atopic Dermatitis. A Practical Option? Are Expectations Met? Adv. Vet. Dermatol. 2017, 8, 220–223. [Google Scholar] [CrossRef]
- Olivry, T.; DeBoer, D.J.; Favrot, C.; Jackson, H.A.; Mueller, R.S.; Nuttall, T.; Prélaud, P. Treatment of Canine Atopic Dermatitis: 2015 Updated Guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet. Res. 2015, 11, 210. [Google Scholar] [CrossRef]
- Marsella, R.; Ahrens, K.; Wilkes, R. Differences in Behavior between Normal and Atopic Keratinocytes in Culture: Pilot Studies. Vet. Sci. 2022, 9, 329. [Google Scholar] [CrossRef]
- Marsella, R.; Olivry, T.; Carlotti, D.-N. Current Evidence of Skin Barrier Dysfunction in Human and Canine Atopic Dermatitis. Vet. Dermatol. 2011, 22, 239–248. [Google Scholar] [CrossRef]
- Olivry, T. Is the Skin Barrier Abnormal in Dogs with Atopic Dermatitis? Vet. Immunol. Immunopathol. 2011, 144, 11–16. [Google Scholar] [CrossRef]
- Combarros, D.; Goudounèche, D.; Cadiergues, M.C.; Simon, M. The Upper Epidermis of Atopic Dogs Is Altered at the Functional and Structural Levels. Vet. Dermatol. 2021, 32, 620. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.; Rajwa, B.; Gomes, P.; Hogenesch, H. Local and Systemic Changes in Lipid Profile as Potential Biomarkers for Canine Atopic Dermatitis. Metabolites 2021, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Banovic, F. Treatment of Canine Atopic Dermatitis: Time to Revise Our Strategy? Vet. Dermatol. 2019, 30, 87–90. [Google Scholar] [CrossRef]
- Marsella, R.; De Benedetto, A. Atopic Dermatitis in Animals and People: An Update and Comparative Review. Vet. Sci. 2017, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Gedon, N.K.Y.; Mueller, R.S. Atopic Dermatitis in Cats and Dogs: A Difficult Disease for Animals and Owners. Clin. Transl. Allergy 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Mitsutake, S.; Tsuji, K.; Kihara, A.; Igarashi, Y. Ceramide Biosynthesis in Keratinocyte and Its Role in Skin Function. Biochimie 2009, 91, 784–790. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier Function of the Skin: “La Raison d’être” of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Borodzicz, S.; Rudnicka, L.; Mirowska-Guzel, D.; Cudnoch-Jedrzejewska, A. The Role of Epidermal Sphingolipids in Dermatologic Diseases. Lipids Health Dis. 2016, 15, 13. [Google Scholar] [CrossRef]
- Cerrato, S.; Ramió-Lluch, L.; Brazís, P.; Fondevila, D.; Segarra, S.; Puigdemont, A. Effects of Sphingolipid Extracts on the Morphological Structure and Lipid Profile in an in Vitro Model of Canine Skin. Vet. J. 2016, 212, 58–64. [Google Scholar] [CrossRef]
- Segarra, S.; Naiken, T.; Garnier, J.; Hamon, V.; Coussay, N.; Bernard, F.X. Enhanced In Vitro Expression of Filaggrin and Antimicrobial Peptides Following Application of Glycosaminoglycans and a Sphingomyelin-Rich Lipid Extract. Vet. Sci. 2022, 9, 323. [Google Scholar] [CrossRef]
- Marsella, R.; Segarra, S.; Ahrens, K.; Alonso, C.; Ferrer, L. Topical Treatment with Sphingolipids and Glycosaminoglycans for Canine Atopic Dermatitis. BMC Vet. Res. 2020, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C.; Steffan, J.; Seewald, W.; Picco, F. A Prospective Study on the Clinical Features of Chronic Canine Atopic Dermatitis and Its Diagnosis. Vet. Dermatol. 2010, 21, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B. Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a Simplified Severity Scale for Assessing Skin Lesions of Atopic Dermatitis in Dogs. Vet. Dermatol. 2014, 25, 77-e25. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, D.N.; Boulet, M.; Ducret, J.; Machicote, G.; Jasmin, P.; Rème, C.A.; Albouy, M. The Use of Recombinant Omega Interferon Therapy in Canine Atopic Dermatitis: A Double-Blind Controlled Study. Vet. Dermatol. 2009, 20, 405–411. [Google Scholar] [CrossRef]
- Huggins, L.G.; Stevenson, M.; Baydoun, Z.; Mab, R.; Khouri, Y.; Schunack, B.; Traub, R.J. Field Trial Investigating the Efficacy of a Long-Acting Imidacloprid 10%/Flumethrin 4.5% Polymer Matrix Collar (Seresto®, Elanco) Compared to Monthly Topical Fipronil for the Chemoprevention of Canine Tick-Borne Pathogens in Cambodia. Curr. Res. Parasitol. Vector Borne Dis. 2022, 2, 100095. [Google Scholar] [CrossRef]
- Stanneck, D.; Kruedewagen, E.M.; Fourie, J.J.; Horak, I.G.; Davis, W.; Krieger, K.J. Efficacy of an Imidacloprid/Flumethrin Collar against Fleas and Ticks on Cats. Parasites Vectors 2012, 5, 1–17. [Google Scholar] [CrossRef]
- Stanneck, D.; Rass, J.; Radeloff, I.; Kruedewagen, E.; Le Sueur, C.; Hellmann, K.; Krieger, K. Evaluation of the Long-Term Efficacy and Safety of an Imidacloprid 10%/Flumethrin 4.5% Polymer Matrix Collar (Seresto®) in Dogs and Cats Naturally Infested with Fleas and/or Ticks in Multicentre Clinical Field Studies in Europe. Parasites Vectors 2012, 5, 1–11. [Google Scholar] [CrossRef]
- Brianti, E.; Falsone, L.; Napoli, E.; Prudente, C.; Gaglio, G.; Giannetto, S. Efficacy of a Combination of 10% Imidacloprid and 4.5% Flumethrin (Seresto®) in Slow Release Collars to Control Ticks and Fleas in Highly Infested Dog Communities. Parasites Vectors 2013, 6, 1–8. [Google Scholar] [CrossRef]
- Evans, A.; Bongiorno, G.; Fourie, J.J.; Lekouch, N.; Bianchi, R.; Khoury, C.; Thomas, E.; Chiummo, R.; Gradoni, L. Elevated and Sustained Anti-Feeding Effect of Scalibor® Deltamethrin Collar against the Sand Fly Phlebotomus Perniciosus in Dogs Confirmed for 1 Year Following Treatment. Med. Vet. Entomol. 2022, 36, 14–19. [Google Scholar] [CrossRef]
- Foglia Manzillo, V.; Oliva, G.; Pagano, A.; Manna, L.; Maroli, M.; Gradoni, L. Deltamethrin-Impregnated Collars for the Control of Canine Leishmaniasis: Evaluation of the Protective Effect and Influence on the Clinical Outcome of Leishmania Infection in Kennelled Stray Dogs. Vet. Parasitol. 2006, 142, 142–145. [Google Scholar] [CrossRef]
- Ligda, P.; Gizzarelli, M.; Kostopoulou, D.; Manzillo, V.F.; Pollmeier, M.; Kontrafouris, M.; Tsatsaki, O.; Oliva, G.; Sotiraki, S. Determination of the Effect of Collars Containing 10% w/w Imidacloprid and 4.5% w/w Flumethrin (Seresto®) on the Incidence of Leishmania and Other Canine Vector—Borne Pathogen Infections in Greece. Parasites Vectors 2023, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R.; Genovese, D.; Gilmer, L.; Ahrens, K.; Gatto, H.; Navarro, C. Investigations on the Effects of a Topical Ceramides-Containing Emulsion (Allerderm Spot on) on Clinical Signs and Skin Barrier Function in Dogs with Atopic Dermatitis: A Double-Blinded Randomized Controlled Study. Int. J. Appl. Res. Vet. Med. 2013, 11, 110–116. [Google Scholar] [CrossRef]
- Blaskovic, M.; Rosenkrantz, W.; Neuber, A.; Sauter-Louis, C.; Mueller, R.S. The Effect of a Spot-on Formulation Containing Polyunsaturated Fatty Acids and Essential Oils on Dogs with Atopic Dermatitis. Vet. J. 2014, 199, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Idée, A.; Mosca, M.; Pin, D. Skin Barrier Reinforcement Effect Assessment of a Spot-on Based on Natural Ingredients in a Dog Model of Tape Stripping. Vet. Sci. 2022, 9, 390. [Google Scholar] [CrossRef]
- Panzuti, P.; Vidémont, E.; Fantini, O.; Fardouet, L.; Noël, G.; Cappelle, J.; Pin, D. A Moisturizer Formulated with Glycerol and Propylene Glycol Accelerates the Recovery of Skin Barrier Function after Experimental Disruption in Dogs. Vet. Dermatol. 2020, 31, 344-e89. [Google Scholar] [CrossRef]
- Tretter, S.; Mueller, R. The Influence of Topical Unsaturated Fatty Acids and Essential Oils on Normal and Atopic Dogs. J. Am. Anim. Hosp. Assoc. 2011, 47, 236–240. [Google Scholar] [CrossRef]
- Bourdeau, P.; Bruet, V.; Gremillet, C. Evaluation of Phytosphingosine-Containing Shampoo and Microemulsion Spray in the Clinical Control of Allergic Dermatoses in Dogs: Preliminary Results of a Multicentre Study. In Proceedings of the Selected Abstracts from the North American Veterinary Dermatology Forum, Kauai, HI, USA, 18–22 April 2007; pp. 175–195. [Google Scholar]
- Fujimura, M.; Nakatsuji, Y.; Fujiwara, S.; Rème, C.; Gatto, H. Spot-on Skin Lipid Complex as an Adjunct Therapy in Dogs with Atopic Dermatitis: An Open Pilot Study. Vet. Med. Int. 2011, 2011, 281846. [Google Scholar] [CrossRef]
- Hobi, S.; Klinger, C.; Classen, J.; Mueller, R.S. The Effects of a Topical Lipid Complex Therapy on Dogs with Atopic Dermatitis: A Double Blind, Randomized, Placebo-Controlled Study. Vet. Dermatol. 2017, 28, 369-e84. [Google Scholar] [CrossRef]
- Jung, J.; Nam, E.; Park, S.; Han, S.; Hwang, C. Clinical Use of a Ceramide-Based Moisturizer for Treating Dogs with Atopic Dermatitis. J. Vet. Sci. 2013, 14, 199–205. [Google Scholar] [CrossRef]
- Piekutowska, A.; Pin, D.; Rème, C.A.; Gatto, H.; Haftek, M. Effects of a Topically Applied Preparation of Epidermal Lipids on the Stratum Corneum Barrier of Atopic Dogs. J. Comp. Pathol. 2008, 138, 197–203. [Google Scholar] [CrossRef]
- Popa, I.; Remoue, N.; Osta, B.; Pin, D.; Gatto, H.; Haftek, M.; Portoukalian, J. The Lipid Alterations in the Stratum Corneum of Dogs with Atopic Dermatitis Are Alleviated by Topical Application of a Sphingolipid-containing Emulsion. Clin. Exp. Dermatol. 2012, 37, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Reme, C.A.; Mondon, A.; Calmon, J.P.; Poisson, L.; Jasmin, P.; Carlotti, D.N. FC-40 Efficacy of Combined Topical Therapy with Antiallergic Shampoo and Lotion for the Control of Signs Associated with Atopic Dermatitis in Dogs. Vet. Dermatol. 2004, 15, 33. [Google Scholar] [CrossRef]
- Ferreira, T.C.; Fonseca, R.; Guedes, D.M. Epidermal Dysfunctions in Canine Atopic Dermatitis: Clinical Impacts and Therapies. Rev. Bras. Hig. e Sanidade Anim. 2018, 12, 396–406. [Google Scholar] [CrossRef]
- Halliwell, R.; Pucheu-Haston, C.M.; Olivry, T.; Prost, C.; Jackson, H.; Banovic, F.; Nuttall, T.; Santoro, D.; Bizikova, P.; Mueller, R.S. Feline Allergic Diseases: Introduction and Proposed Nomenclature. Vet. Dermatol. 2021, 32, 8-e2. [Google Scholar] [CrossRef]
- Vargo, C.; Banovic, F. Feline Atopic Skin Syndrome. Today’s Veterinary Practice, 10 February 2022; pp. 78–83. [Google Scholar]
- Mueller, R.S.; Nuttall, T.; Prost, C.; Schulz, B.; Bizikova, P. Treatment of the Feline Atopic Syndrome—A Systematic Review. Vet. Dermatol. 2021, 32, 43-e8. [Google Scholar] [CrossRef]
- Santoro, D.; Pucheu-Haston, C.M.; Prost, C.; Mueller, R.S.; Jackson, H. Clinical Signs and Diagnosis of Feline Atopic Syndrome: Detailed Guidelines for a Correct Diagnosis. Vet. Dermatol. 2021, 32, 26-e6. [Google Scholar] [CrossRef]
- Zuriaga, E.; Segarra, S. Clinical Efficacy of a Lipid Extract Collar in Feline Atopic Skin Syndrome: A Case Report. In Proceedings of the Southern European Veterinary Conference, Seville, Spain, 20–22 October 2022. [Google Scholar]


| Component | Mean % 0 Weeks | Mean % 8 Weeks | Variation |
|---|---|---|---|
| Ceramides | 0.14833 | 0.11250 | −24.16% |
| Dihydroceramides | 0.01000 | 0.01250 | +25.00% |
| Sphingomyelins | 2.64833 | 2.20000 | −16.93% |
| Dihydrosphingomyelins | 0.14833 | 0.13625 | −8.15% |
| Glycosylceramides | 0.06167 | 0.04875 | −20.95% |
| Lactosylceramides | 0.13667 | 0.10750 | −21.34% |
| Total sphingolipids | 3.15330 | 2.62130 | −16.87% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segarra, S.; Sanmiguel, D.; Zuriaga, E.; Leclerc, S.; Cabañas, J.; Seigneuric, E.; Miquel, A.; Vázquez, A.; Ferrer, L. Sphingomyelin-Rich Lipid Extract Collar for Canine Atopic Dermatitis. Vet. Sci. 2023, 10, 389. https://doi.org/10.3390/vetsci10060389
Segarra S, Sanmiguel D, Zuriaga E, Leclerc S, Cabañas J, Seigneuric E, Miquel A, Vázquez A, Ferrer L. Sphingomyelin-Rich Lipid Extract Collar for Canine Atopic Dermatitis. Veterinary Sciences. 2023; 10(6):389. https://doi.org/10.3390/vetsci10060389
Chicago/Turabian StyleSegarra, Sergi, David Sanmiguel, Eliseo Zuriaga, Sophie Leclerc, Jesús Cabañas, Estelle Seigneuric, Aurélie Miquel, Ana Vázquez, and Lluís Ferrer. 2023. "Sphingomyelin-Rich Lipid Extract Collar for Canine Atopic Dermatitis" Veterinary Sciences 10, no. 6: 389. https://doi.org/10.3390/vetsci10060389
APA StyleSegarra, S., Sanmiguel, D., Zuriaga, E., Leclerc, S., Cabañas, J., Seigneuric, E., Miquel, A., Vázquez, A., & Ferrer, L. (2023). Sphingomyelin-Rich Lipid Extract Collar for Canine Atopic Dermatitis. Veterinary Sciences, 10(6), 389. https://doi.org/10.3390/vetsci10060389








