Evaluation of a Porcine Endogenous Reference Gene (Internal Sample Control) in a Porcine Reproductive and Respiratory Syndrome Virus RT-qPCR
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Study 1—ISC Porcine Specificity
2.3. Study 2—ISC over Time in Individual Pigs
2.4. Study 3—ISC in Field Samples
2.5. Nucleic Acid Extraction and Amplification
2.6. Data Analysis
3. Results
3.1. Study 1—ISC Porcine Specificity
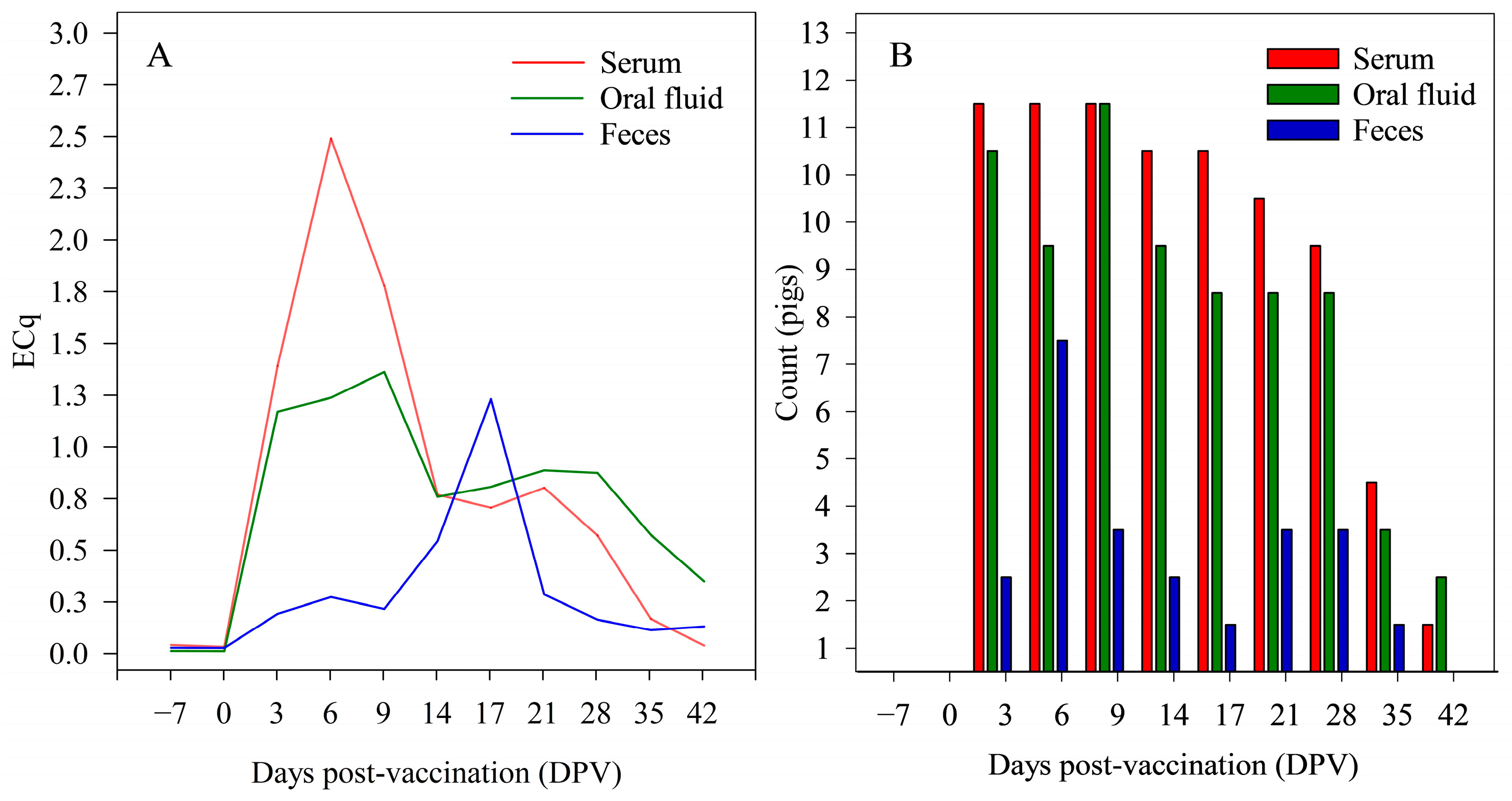
3.2. Study 2—ISC over Time in Individual Pigs
3.3. Study 3—ISC in Field Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Vallejo, J.J.; Van Het Hof, B.; Robben, J.; Van Wijk, J.A.E.; Van Die, I.; Joziasse, D.H.; Van Dijk, W. Approach for defining endogenous reference genes in gene expression experiments. Anal. Biochem. 2004, 329, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.F.; Brasseur, F.; Hulsbergen-van de Kaa, C.A.; van de Rakt, M.W.; Figdor, C.G.; Adema, G.J.; Hoogerbrugge, P.M.; Coulie, P.G.; de Vries, I.J.M. Cancer-germline gene expression in pediatric solid tumors using quantitative real-time PCR. Int. J. Cancer 2007, 120, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tulotta, C.; Lefley, D.V.; Freeman, K.; Gregory, W.M.; Hanby, A.M.; Heath, P.R.; Nutter, F.; Wilkinson, J.M.; Spicer-Hadlington, A.R.; Liu, X.; et al. Endogenous Production of IL1B by Breast Cancer Cells Drives Metastasis and Colonization of the Bone MicroenvironmentIL1B Promotes Breast Cancer Bone Metastasis. Clin. Cancer Res. 2019, 25, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, T.; Miao, Q.; Xie, S.; Chen, X.; Tang, J.; Peng, C.; Xu, X.; Wei, W.; You, Z.; et al. Detection of transgenic rice line TT51-1 in processed foods using conventional PCR, real-time PCR, and droplet digital PCR. Food Control 2019, 98, 380–388. [Google Scholar] [CrossRef]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Borm, S.V.; Steensels, M.; Ferreira, H.L.; Boschmans, M.; Vriese, J.D.; Lambrecht, B.; Berg, T.D. A universal avian endogenous real-time reverse transcriptase–polymerase chain reaction control and its application to avian influenza diagnosis and quantification. Avian Dis. 2007, 51, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Nygard, A.B.; Jørgensen, C.B.; Cirera, S.; Fredholm, M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol. 2007, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Higgins, P.J.; Crawford, D.R. Control selection for RNA quantitation. Biotechniques 2000, 29, 332–337. [Google Scholar] [CrossRef]
- Pérez, S.; Royo, L.J.; Astudillo, A.; Escudero, D.; Álvarez, F.; Rodríguez, A.; Gómez, E.; Otero, J. Identifying the most suitable endogenous control for determining gene expression in hearts from organ donors. BMC Mol. Biol. 2007, 8, 1819–1824. [Google Scholar] [CrossRef]
- Bai, J.; Trinetta, V.; Shi, X.; Noll, L.W.; Magossi, G.; Zheng, W.; Porter, E.P.; Cernicchiaro, N.; Renter, D.G.; Nagaraja, T.G. Comparison data of a two-target real-time PCR assay with and without an internal control in detecting Salmonella enterica from cattle lymph nodes. Data Br. 2018, 18, 1819–1824. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Noll, L.; Stoy, C.; Porter, E.; Fu, J.; Feng, Y.; Peddireddi, L.; Liu, X.; Dodd, K.A.; et al. Development of a real-time PCR assay for detection of African swine fever virus with an endogenous internal control. Transbound. Emerg. Dis. 2020, 67, 2446–2454. [Google Scholar] [CrossRef]
- Kuchipudi, S.V.; Tellabati, M.; Nelli, R.K.; White, G.A.; Perez, B.B.; Sebastian, S.; Slomka, M.J.; Brookes, S.M.; Brown, I.H.; Dunham, S.P.; et al. 18S rRNAis a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol. J. 2012, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cao, Y.; Köhler, J.; Lu, A.; Xu, S.; Wang, H. Unbiased RNA-Seq-driven identification and validation of reference genes for quantitative RT-PCR analyses of pooled cancer exosomes. BMC Genom. 2021, 22, 27. [Google Scholar] [CrossRef]
- Lee, P.D.; Sladek, R.; Greenwood, C.M.; Hudson, T.J. Control genes and variability: Absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 2002, 12, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Henao-Díaz, A.; Poonsuk, K.; Buckley, A.; van Geelen, A.; Lager, K.; Harmon, K.; Gauger, P.; Wang, C.; Ambagala, A.; et al. Pseudorabies (Aujeszky’s disease) virus DNA detection in swine nasal swab and oral fluid specimens using a gB-based real-time quantitative PCR. Prev. Vet. Med. 2021, 189, 105308. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.; Barnhart, K.; Blanco, J.; Freeman, L.; Harr, K.; Szaldovits, B.; Walton, R. Guidelines for the Determination of Reference Intervals (RI) in Veterinary Species. 2013. Available online: https://www.asvcp.org/page/QALS_Guidelines (accessed on 26 October 2022).
- Henao-Díaz, A.; Giménez-Lirola, L.; Magtoto, R.; Ji, J.; Zimmerman, J. Evaluation of three commercial porcine reproductive and respiratory syndrome virus (PRRSV) oral fluid antibody ELISAs using samples of known status. Res. Vet. Sci. 2019, 125, 113–118. [Google Scholar] [CrossRef]
- Molina-Barrios, R.M. Porcine Reproductive and Respiratory Syndrome Virus: Understanding and Managing Persistent Infection. Doctoral Dissertation, Iowa State University, Ames, IA, USA, 2008. [Google Scholar] [CrossRef]
- Wills, R.W.; Zimmerman, J.J.; Yoon, K.J.; Swenson, S.L.; McGinley, M.J.; Hill, H.T.; Platt, K.B.; Christopher-Hennings, J.; Nelson, E.A. Porcine reproductive and respiratory syndrome virus: A persistent infection. Vet. Microbiol. 1997, 55, 231–240. [Google Scholar] [CrossRef]
- Prieto, C.; Castro, J.M. Porcine reproductive and respiratory syndrome virus infection in the boar: A review. Theriogenology 2005, 63, 1–16. [Google Scholar] [CrossRef]
- Martínez-Lobo, F.J.; De Lome, L.C.; Díez-Fuertes, F.; Segalés, J.; García-Artiga, C.; Simarro, I.; Castro, J.M.; Prieto, C. Safety of porcine reproductive and respiratory syndrome modified live virus (MLV) vaccine strains in a young pig infection model. Vet. Res. 2013, 44, 115. [Google Scholar] [CrossRef]
- Toman, M.; Celer, V.; Kavanová, L.; Levá, L.; Frolichova, J.; Ondráčková, P.; Kudláčková, H.; Nechvátalová, K.; Salat, J.; Faldyna, M. Dynamics and differences in systemic and local immune responses after vaccination with inactivated and live commercial vaccines and subsequent subclinical infection with PRRS virus. Front. Immunol. 2019, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Pugnale, P.; Latorre, P.; Rossi, C.; Crovatto, K.; Pazienza, V.; De Gottardi, A.; Negro, F. Real-time multiplex PCR assay to quantify hepatitis C virus RNA in peripheral blood mononuclear cells. J. Virol. Methods 2006, 133, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Obuekwe, J.; Baun, T.; Rogers, J.; Patel, T.; Snow, L. Prompt detection of influenza A and B viruses using the BD Veritor™ System Flu A+ B, Quidel® Sofia® Influenza A+ B FIA, and Alere BinaxNOW® Influenza A&B compared to real-time reverse transcription-polymerase chain reaction (RT-PCR). Diagn. Microbiol. Infect. Dis. 2014, 79, 10–13. [Google Scholar] [CrossRef]
- de Cassia-Pires, R.; de Melo, M.D.F.A.D.; Barbosa, R.D.H.; Roque, A.L.R. Multiplex PCR as a tool for the diagnosis of Leishmania spp. kDNA and the gapdh housekeeping gene of mammal hosts. PLoS ONE 2017, 12, e0173922. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Cheng, W.C.; Chen, C.R.; Shu, W.Y.; Tsai, M.L.; Huang, C.L.; Hsu, I.C. Identification of human housekeeping genes and tissue-selective genes by microarray meta-analysis. PLoS ONE 2011, 6, e22859. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Brattelid, T.; Winer, L.H.; Levy, F.O.; Liestøl, K.; Sejersted, O.M.; Andersson, K.B. Reference gene alternatives to Gapdh in rodent and human heart failure gene expression studies. BMC Mol. Biol. 2010, 11, 22. [Google Scholar] [CrossRef]
- Wei, K.; Zhang, T.; Ma, L. Divergent and convergent evolution of housekeeping genes in human–pig lineage. PeerJ 2018, 6, e4840. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Zimmerman, J.J.; Giménez-Lirola, L.G. Internal reference genes with the potential for normalizing quantitative PCR results for oral fluid specimens. Anim. Health Res. Rev. 2022, 23, 147–156. [Google Scholar] [CrossRef]
- Nyström, K.; Biller, M.; Grahn, A.; Lindh, M.; Larson, G.; Olofsson, S. Real time PCR for monitoring regulation of host gene expression in herpes simplex virus type 1-infected human diploid cells. J. Virol. Methods 2004, 118, 83–94. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Zakrajsek, B.A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 2000, 46, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Ramírez, B.; Armenta-Leyva, B.; Henao-Díaz, A.; Cheng, T.Y.; Zhang, J.; Rawal, G.; Ye, F.; Giménez-Lirola, L.; Zimmerman, J.J. Effect of extrinsic factors on the detection of PRRSV and a porcine-specific internal sample control in diagnostic specimens tested by RT-qPCR. J. Vet. Diagn. 2023, 10406387231174556. [Google Scholar] [CrossRef]
| Specimen | Intercept 1 | Slope 2 (95% CI) | p-Value |
|---|---|---|---|
| Serum | 1.80 | 0.00 (−0.00, 0.00) | >0.05 |
| Oral fluid | 2.13 | 0.00 (0.00, 0.01) | >0.05 |
| Feces | 1.31 | −0.00 (−0.00, 0.00) | >0.05 |
| ECqs (95% CI) 1 | |||
|---|---|---|---|
| Specimen | 5th Percentile | 2.5th Percentile | 1.25th Percentile |
| Serum | 1.26 (1.12, 1.40) | 1.16 (1.10, 1.27) | 1.11 (0.56, 1.23) |
| Oral fluid | 0.28 (0.22, 0.44) | 0.25 (0.09, 0.36) | 0.11 (0.03, 0.26) |
| Feces 2 | 0.49 (0.18, 0.60) | 0.37 (0.17, 0.55) | 0.18 (0.17, 0.52) |
| Fecal swab 2 | 0.27 (0.04, 0.44) | 0.14 (0.03, 0.39) | 0.04 (0.03, 0.34) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguía-Ramírez, B.; Armenta-Leyva, B.; Henao-Díaz, A.; Ye, F.; Baum, D.H.; Giménez-Lirola, L.G.; Zimmerman, J.J. Evaluation of a Porcine Endogenous Reference Gene (Internal Sample Control) in a Porcine Reproductive and Respiratory Syndrome Virus RT-qPCR. Vet. Sci. 2023, 10, 381. https://doi.org/10.3390/vetsci10060381
Munguía-Ramírez B, Armenta-Leyva B, Henao-Díaz A, Ye F, Baum DH, Giménez-Lirola LG, Zimmerman JJ. Evaluation of a Porcine Endogenous Reference Gene (Internal Sample Control) in a Porcine Reproductive and Respiratory Syndrome Virus RT-qPCR. Veterinary Sciences. 2023; 10(6):381. https://doi.org/10.3390/vetsci10060381
Chicago/Turabian StyleMunguía-Ramírez, Berenice, Betsy Armenta-Leyva, Alexandra Henao-Díaz, Fangshu Ye, David H. Baum, Luis G. Giménez-Lirola, and Jeffrey J. Zimmerman. 2023. "Evaluation of a Porcine Endogenous Reference Gene (Internal Sample Control) in a Porcine Reproductive and Respiratory Syndrome Virus RT-qPCR" Veterinary Sciences 10, no. 6: 381. https://doi.org/10.3390/vetsci10060381
APA StyleMunguía-Ramírez, B., Armenta-Leyva, B., Henao-Díaz, A., Ye, F., Baum, D. H., Giménez-Lirola, L. G., & Zimmerman, J. J. (2023). Evaluation of a Porcine Endogenous Reference Gene (Internal Sample Control) in a Porcine Reproductive and Respiratory Syndrome Virus RT-qPCR. Veterinary Sciences, 10(6), 381. https://doi.org/10.3390/vetsci10060381







