Investigating Polymorphisms and Expression Profile of Immune, Antioxidant, and Erythritol-Related Genes for Limiting Postparturient Endometritis in Holstein Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Dairy Cows and Research Samples
2.2. Isolation and Amplifying DNA
2.3. Finding Polymorphism
2.4. Transcript Levels of Immune, Antioxidant and Erythritol Related Genes
2.5. Statistical Analysis
3. Results
3.1. Genetic Polymorphisms of Immune, Antioxidant, and Erythritol-Related Genes
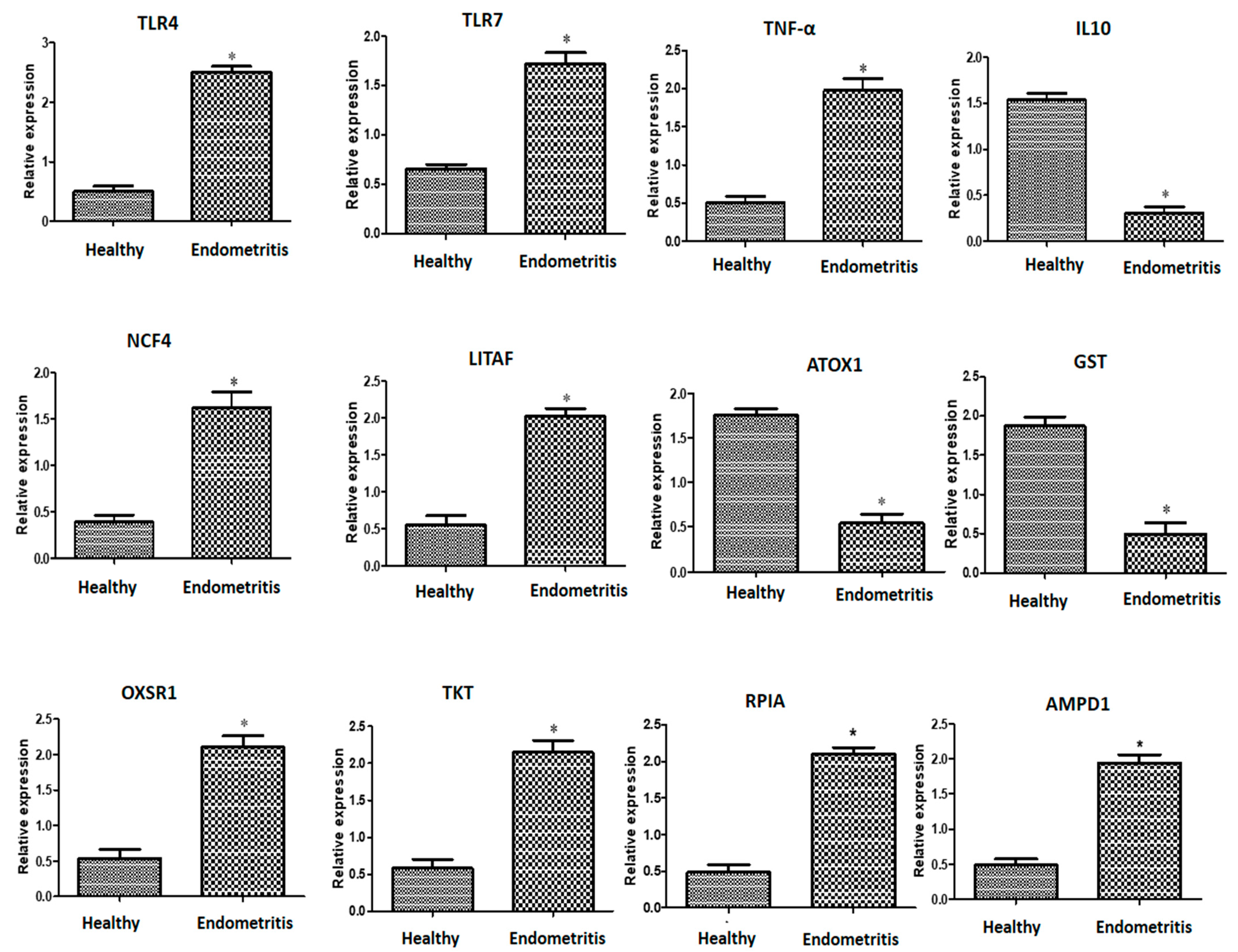
3.2. Patterns for Transcript Levels of Immune, Antioxidant, and Erythritol-Related Indicators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgante, M.; Gianesella, M.; Casella, S.; Stelletta, C.; Cannizzo, C.; Giudice, E.; Piccione, G. Response to glucose infusion in pregnant and nonpregnant ewes: Changes in plasma glucose and insulin concentrations. Comp. Clin. Pathol. 2012, 21, 961–965. [Google Scholar] [CrossRef]
- Grummer, R.R.; Mashek, D.G.; Hayirli, A. Dry matter intake and energy balance in the transition period. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Elshahawy, I.E.; Abdullaziz, I.A. Hemato-biochemical profiling in relation to metabolic disorders in transition dairy cows. AJVS 2017, 55, 25–33. [Google Scholar]
- LeBlanc, S.J.; Lissemore, K.D.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E. Major advances in disease prevention in dairy cattle. J. Dairy Sci. 2006, 89, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Ingvartsen, K.L.; Andersen, J.B. Symposium: Dry matter intake of lactating dairy cattle. Integration of metabolism and intake regulation: A review focusing on periparturient animals. J. Dairy Sci. 2000, 83, 1573–1597. [Google Scholar] [CrossRef]
- Riveros, J.L.; Urquieta, B.; Bonacic, C.; Hoffmann, B.; Bas, F.; Schuler, G. Endocrine changes during pregnancy, parturition and post-partum in Guanacos (Lama guanicoe). Anim. Reprod. Sci. 2009, 116, 318–325. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.; Nash, D.M.; Herath, S. Uterine diseases in cattle after parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef]
- Del Vecchio, R.P.; Matsas, D.J.; Fortin, S.; Sponenberg, D.P.; Lewis, G.S. Spontaneous uterine infections are associated with elevated prostaglandin F2α metabolite concentrations in postpartum dairy cows. Theriogenology 1994, 41, 413–421. [Google Scholar] [CrossRef]
- Dohmen, M.J.; Joop, K.; Sturk, A.; Bols, P.E.; Lohuis, J.A. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology 2000, 54, 1019–1032. [Google Scholar] [CrossRef]
- Donnez, J.; Cacciottola, L. Endometriosis: An Inflammatory Disease That Requires New Therapeutic Options. Int. J. Mol. Sci. 2022, 23, 1518. [Google Scholar] [CrossRef]
- Donofrio, G.; Ravaneti, L.; Cavirani, S.; Herath, S.; Capocefalo, A.; Sheldon, I.M. Bacterial infection of endometrial stromal cells influences bovine herpesvirus 4 immediate early gene activation: A new insight into bacterial and viral interaction for uterine disease. Reproduction 2008, 136, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Fischer, D.P.; Noakes, D.E.; England, G.C.; Rycroft, A.; Dobson, H.; Sheldon, I.M. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007, 68, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Buzzaccarini, G.; Vitagliano, A.; Andrisani, A.; Santarsiero, C.M.; Cicinelli, R.; Nardelli, C.; Ambrosini, G.; Cicinelli, E. Chronic endometritis and altered embryo implantation: A unified pathophysiological theory from a literature systematic review. J. Assist. Reprod. Genet. 2020, 37, 2897–2911. [Google Scholar] [CrossRef]
- Liang, D.; Arnold, L.M.; Stowe, C.J.; Harmon, R.J.; Bewley, J.M. Estimating US dairy clinical disease costs with a stochastic simulation model. J. Dairy Sci. 2017, 100, 1472–1486. [Google Scholar] [CrossRef] [PubMed]
- Overton, M.; Fetrow, J. Economics of Postpartum Uterine Health; Proc Dairy Cattle Reproduction Council: Omaha, NE, USA, 2008; pp. 39–44. [Google Scholar]
- Ashwell, M.S.; Rexroad Jr, C.E.; Miller, R.H.; Van Raden, P.M.; Da, Y. Detection of loci affecting milk production and health traits in an Elite US Holstein population using microsatellite markers. Anim. Genet. 1997, 28, 216–222. [Google Scholar] [CrossRef]
- Bishop, M.D.; Hawkins, G.A.; Keeler, C.L. Use of DNA markers in animal selection. Theriogenology 1995, 43, 61–70. [Google Scholar] [CrossRef]
- Buch, L.H.; Kargo, M.; Berg, P.; Lassen, J.; Sørensen, A.C. The value of cows in reference populations for genomic selection of new functional traits. Animal. 2012, 6, 880–886. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 13, 951–969. [Google Scholar] [CrossRef]
- Tang, F.; Lao, K.; Surani, M.A. Development and applications of single-cell transcriptome analysis. Nat. Methods 2011, 8, 6–11. [Google Scholar] [CrossRef]
- Banchereau, R.; Cepika, A.M.; Banchereau, J.; Pascual, V. Understanding human autoimmunity and autoinflammation through transcriptomics. Annu. Rev. Immunol. 2017, 35, 337–370. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Chakravarty, A.K. Disease resistance for different livestock species. In Genetics and Breeding for Disease Resistance of Livestock; Academic Press: Cambridge, MA, USA, 2020; pp. 271–296. [Google Scholar] [CrossRef]
- Bishop, S.C.; Woolliams, J.A. Genomics and disease resistance studies in livestock. Livest. Sci. 2014, 166, 190–198. [Google Scholar] [CrossRef] [PubMed]
- van Harten, S.; Brito, R.; Almeida, A.M.; Scanlon, T.; Kilminster, T.; Milton, J.; Greeff, J.; Oldham, C.; Cardoso, L.A. Gene expression of regulatory enzymes involved in the intermediate metabolism of sheep subjected to feed restriction. Animals 2013, 7, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.C.; Viadas, C.; Seoane, A.; Sangari, F.J.; López-Goñi, I.; García-Lobo, J.M. Evaluation of the effects of erythritol on gene expression in Brucella abortus. PLoS ONE 2012, 7, e50876. [Google Scholar] [CrossRef]
- Sawada, K.; Taki, A.; Yamakawa, T.; Seki, M. Key role for transketolase activity in erythritol production by Trichosporonoides megachiliensis SN-G42. J. Biosci Bioeng. 2009, 108, 385–390. [Google Scholar] [CrossRef]
- Zhang, L.; Nie, M.Y.; Liu, F.; Chen, J.; Wei, L.J.; Hua, Q. Multiple gene integration to promote erythritol production on glycerol in Yarrowia lipolytica. Biotechnol. Lett. 2021, 43, 1277–1287. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Kgwatalala, P.; Ibeagha, A.E.; Zhao, X. A critical analysis of disease-associated DNA polymorphisms in the genes of cattle, goat, sheep, and pig. Mamm. Genome. 2008, 19, 226–245. [Google Scholar] [CrossRef]
- Islam, M.A.; Rony, S.A.; Rahman, M.B.; Cinar, M.U.; Villena, J.; Uddin, M.J.; Kitazawa, H. Improvement of disease resistance in livestock: Application of immunogenomics and CRISPR/Cas9 technology. Animals 2020, 10, 2236. [Google Scholar] [CrossRef]
- Davies, D.; Meade, K.G.; Herath, S.; Eckersall, P.D.; Gonzalez, D.; White, J.O.; Conlan, R.S.; O’Farrelly, C.; Sheldon, I.M. Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod. Biol. Endocrinol. 2008, 6, 53. [Google Scholar] [CrossRef]
- Pereira, G.; Guo, Y.; Silva, E.; Silva, M.F.; Bevilacqua, C.; Charpigny, G.; Lopes-da-Costa, L.; Humblot, P. Subclinical endometritis differentially affects the transcriptomic profiles of endometrial glandular, luminal, and stromal cells of postpartum dairy cows. J. Dairy Sci. 2022, 105, 6125–6143. [Google Scholar] [CrossRef]
- Okawa, H.; Fujikura, A.; Wijayagunawardane, M.M.P.; Vos, P.L.A.M.; Taniguchi, M.; Takagi, M. Effect of diagnosis and treatment of clinical endometritis based on vaginal discharge score grading system in postpartum Holstein cows. J. Vet. Med. Sci. 2017, 79, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Boom, R.; Sol, C.J.; Salimans, M.M.; Jansen, C.L.; Wertheim-van Dillen, P.M.; Noordaa, J.V.D. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Boesenberg-Smith, K.A.; Pessarakli, M.M.; Wolk, D.M. Assessment of DNA yield and purity: An overlooked detail of PCR troubleshooting. Clin. Microbiol. Newsl. 2012, 34, 1+3–6. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- May, K.; Sames, L.; Scheper, C.; König, S. Genomic loci and genetic parameters for uterine diseases in first-parity Holstein cows and associations with milk production and fertility. J. Dairy Sci. 2021, 105, 509–524. [Google Scholar] [CrossRef]
- Asadpour, R.; Feyzi, L.; Jafari-Joozani, R.; Hamali, H.; Abolfazl Hajibemani, A.; Firouzamandi, M. The relationship between polymorphism of the CXCR1 gene and the risk of endometritis in Holstein dairy cows. Vet. Arh. 2020, 90, 557–563. [Google Scholar] [CrossRef]
- Hajibemani, A.; Sharifiyazdi, H.; Mirzaei, A.; Ghasrodashti, A.R. Characterization of single nucleotide polymorphism in the 5’-untranslated region (5’-UTR) of Lactoferrin gene and its association with reproductive parameters and uterine infection in dairy cattle. Vet. Res. Forum. 2012, 3, 37–43. [Google Scholar] [PubMed]
- Goroohi, Z.; Sharifiyazdi, H.; Mirzaei, A. Association between beta defensin gene polymorphism and clinical endometritis in dairy cows. Comp. Clin. Pathol. 2019, 28, 377–382. [Google Scholar] [CrossRef]
- Osman, N.M.; Mossallam, A.A.; El Seedy, F.R.; Mahfouz, E.R. Single nucleotide polymorphisms in TLR4 gene and endometritis resistance in river buffalo (Bubalus bubalis). JJBS 2018, 11, 577–583. [Google Scholar]
- Abou Mossallam, A.A.; Osman, N.M.; Othman, O.E.; Mahfouz, E.R. Polymorphism evaluation of TLR2 gene associated with endometritis infection in buffalo reared in Egypt. BJI 2022, 26, 45–55. [Google Scholar] [CrossRef]
- Burel, J.G.; Babor, M.; Pomaznoy, M.; Lindestam Arlehamn, C.S.; Khan, N.; Sette, A.; Peters, B. Host rranscriptomics as a tool to identify diagnostic and mechanistic immune signatures of tuberculosis. Front. Immunol. 2019, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.; Torres, C.G.; Carvallo, F.; Duchens, M.; Peralta, O.A. Endometrial expression of selected transcripts in postpartum of primiparous Holstein cows with clinical and subclinical endometritis. Anim. Reprod. Sci. 2015, 156, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Raliou, M.; Dembélé, D.; Düvel, A.; Bolifraud, P.; Aubert, J.; Mary-Huard, T.; Rocha, D.; Piumi, F.; Mockly, S.; Heppelmann, M.; et al. Subclinical endometritis in dairy cattle is associated with distinct mRNA expression patterns in blood and endometrium. PLoS ONE 2019, 14, e0220244. [Google Scholar] [CrossRef]
- Pothmann, H.; Flick, P.; Tichy, A.; Gabler, C.; Drillich, M. Messenger RNA expression of selected factors at different sites of the bovine endometrium associated with uterine health. Front. Vet. Sci. 2021, 8, 649758. [Google Scholar] [CrossRef]
- Janowski, T.; Barański, W.; Łukasik, K.; Skarżyński, D.; Rudowska, M.; Zduńczyk, S. Prevalence of subclinical endometritis in repeat breeding cows and mRNA expression of tumor necrosis factor α and inducible nitric oxide synthase in the endometrium of repeat breeding cows with and without subclinical endometritis. Pol. J. Vet. Sci. 2013, 16, 693–699. [Google Scholar] [CrossRef]
- Taha, A.A.; Mahfouz, E.R.; Bibars, M.A.; Hassan, N.A.; Othman, O.E. Cytokine genes expression in uteri of Bubalus bubalis associated with endometritis infection. Jordan J. Biol. Sci. 2021, 14, 245–251. [Google Scholar]
- Botos, I.; Segal, D.M.; Davies, D.R. The structural biology of Toll-like receptors. Structure 2011, 13, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Into, T.; Yasuda, M.; Okusawa, T.; Hamahira, S.; Kuroki, Y.; Eto, A.; Nisizawa, T.; Morita, M.; Shibata, K.-I. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and Staphylococcus aureus Peptidoglycans. J. Immunol. 2003, 171, 3675–3683. [Google Scholar] [CrossRef]
- White, S.N.; Taylor, K.H.; Abbey, C.A.; Gill, C.A.; Womack, J.E. Haplotype variation in bovine Toll-like receptor 4 and computational prediction of a positively selected ligand-binding domain. Proc. Natl. Acad. Sci. USA 2003, 100, 10364–10369. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Martinez-Espinosa, I.; Serrato, J.A.; Ortiz-Quintero, B. Role of IL-10-producing natural killer cells in the regulatory mechanisms of inflammation during systemic infection. Biomolecules 2021, 21, 4. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, D.; Huo, J.; Biswas, S.K.; Huang, Y.; Yang, Y.; Xu, S.; Lam, K.P. IL-10 enhances human natural killer cell effector functions via metabolic reprogramming regulated by mTORC1 signaling. Front. Immunol. 2021, 23, 619195. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Krakowski, L.; Marczuk, J. Inflammatory cytokine and acute phase protein concentrations in the peripheral blood and uterine washings of cows with subclinical endometritis in the late postpartum period. Vet. Res. Commun. 2015, 39, 143–149. [Google Scholar] [CrossRef]
- Ju, Z.; Wang, C.; Wang, X.; Yang, C.; Sun, Y.; Jiang, Q.; Wang, F.; Li, M.; Zhong, J.; Huang, J. Role of an SNP in alternative splicing of bovine NCF4 and mastitis susceptibility. PLoS ONE 2015, 24, e0143705. [Google Scholar] [CrossRef]
- Ju, Z.; Wang, C.; Wang, X.; Yang, C.; Zhang, Y.; Sun, Y.; Jiang, Q.; Li, R.; Li, J.; Zhong, J.; et al. The effect of the SNP g.18475 A>G in the 3’UTR of NCF4 on mastitis susceptibility in dairy cattle. Cell Stress Chaperones. 2018, 23, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, C.P.; Pant, S.D.; Schenkel, F.S.; Sharma, B.S.; Karrow, N.A. SNPs in the bovine IL-10 receptor are associated with somatic cell score in Canadian dairy bulls. Mamm. Genome 2009, 20, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Takashiba, S.; Van Dyke, T.E.; Shapira, L.; Amar, S. Lipopolysaccharide-inducible and salicylate-sensitive nuclear factor (s) on human tumor necrosis factor alpha promoter. Infect. Immun. 1995, 63, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Hasenstein, J.; Lamont, S. Analysis of chicken TLR4, CD28, MIF, MD-2, and LITAF genes in a Salmonella enteritidis resource population. Poult. Sci. 2004, 83, 544–549. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 2002, 85, 2173–2179. [Google Scholar] [CrossRef]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The antioxidant properties of selenium and vitamin E; their role in periparturient dairy cattle health regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Flores, A.G.; Unger, V.M. Atox1 contains positive residues that mediate membrane association and aid subsequent copper loading. J. Membr. Biol. 2013, 246, 903–913. [Google Scholar] [CrossRef]
- Narindrasorasak, S.; Zhang, X.; Roberts, E.A.; Sarkar, B. Comparative analysis of metal binding characteristics of copper chaperone proteins, Atx1 and ATOX1. Bioinorg. Chem. Appl. 2004, 2, 105–123. [Google Scholar] [CrossRef]
- Trevisan, M.; Browne, R.; Ram, P.; Muti, J.; Freudenheim, A.; Carosella, M.; Armstrong, D. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ateya, A.; El-Sayed, A.; Mohamed, R. Gene expression and serum profile of antioxidant markers discriminate periparturient period time in dromedary camels. Mammal Res. 2021, 66, 603–613. [Google Scholar] [CrossRef]
- Sangari, F.J.; Agüero, J. Identification of Brucella abortus B19 vaccine strain by the detection of DNA polymorphism at the ery locus. Vaccine 1994, 12, 435–438. [Google Scholar] [CrossRef]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine immunity and microbiota: A shifting paradigm. Front. Immunol. 2019, 17, 2387. [Google Scholar] [CrossRef] [PubMed]
- Yockey, L.J.; Iwasaki, A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef]
- Pisani, L.F.; Lecchi, C.; Invernizzi, G.; Sartorelli, P.; Savoini, G.; Ceciliani, F. In vitro modulatory effect of omega-3 polyunsaturated fatty acid (EPA and DHA) on phagocytosis and ROS production of goat neutrophils. Vet. Immunol. Immunopathol. 2009, 131, 79–85. [Google Scholar] [CrossRef]
- Shi, H.R.; Huang, X.Y.; Yan, Z.Q.; Yang, Q.L.; Wang, P.F.; Li, S.G.; Sun, W.; Gun, S. Effect of Clostridium perfringens type C on TLR4/MyD88/NF-κB signaling pathway in piglet small intestines. Microb. Pathog. 2019, 135, 7. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
| Investigated Marker | Sense | Antisense | Annealing Temperature (°C) | Size of PCR Product (bp) | Reference |
|---|---|---|---|---|---|
| TLR4 | 5′-AGAGACGACACTACAGTGCCTCG-3′ | 5′-GAAGTCATTTAGAGAGACTAG-3′ | 60 | 528 | Current study |
| TLR7 | 5′-TTTTCCACAGCTCATCTCTTCA-3′ | 5′-AAGGAGGCTGGAGAGATGCCTG-3′ | 60 | 420 | Current study |
| TNF-α | 5′-ACCAGCCAGGAGAGAGACAAGC-3′ | 5′-GTCAGCAGGCACCACCAGCTGGT-3′ | 62 | 551 | Current study |
| IL10 | 5′-ATGCATAGCTCAGCACTACTCTG-3′ | 5′-TCACCATCCTGGAGGTCTTCT-3′ | 60 | 571 | Current study |
| NCF4 | 5′-CCTGGGACCACAGCCTAAACGA-3′ | 5′-CTTGATGGTGCTGATGGTGTCC-3′ | 58 | 865 | Current study |
| LITAF | 5′-CTTTCTATGAAGGGCTTTTTTC-3′ | 5′-CACCCAATATACAGATTTTTGA-3′ | 62 | 644 | Current study |
| ATOX1 | 5′-GCTCCTGTGGCGTGCACACCCG-3′ | 5′-TGGTGTGCAGGCCAAGACTTGG-3′ | 60 | 450 | Current study |
| GST | 5′-CGGCTCAGGCCGCCGCCGAGC-3′ | 5′-TGGGACAGCAGGGTCTCGAAAG-3′ | 58 | 480 | Current study |
| OXSR1 | 5′-AGCGCCAGGCGCCGTCCGACC-3′ | 5′-GAGTATTGTAGCGATGGTAGC-3′ | 60 | 525 | Current study |
| TKT | 5′-GCTGCTTGCAGCTCCGCAGCC-3′ | 5′-GAGCCAGTGGCCACATCGGTGA-3′ | 62 | 456 | Current study |
| RPIA | 5′-CCACGTGCAGTTGCCGGGACGT-3′ | 5′-TTGATGAGGTTGAGGTCAGCGTC-3′ | 58 | 390 | Current study |
| AMPD1 | 5′-CAGAGAGTGCAGATCACTGGC-3′ | 5′-ACTCATCCATCTCGTTGAGCAT-3′ | 60 | 502 | Current study |
| Investigated Marker | Primer | Product Size (bp) | Annealing Temperature (°C) | GenBank Isolate | Origin |
|---|---|---|---|---|---|
| TLR4 | F5′-CCTTGCGTACAGGTTGTTCC-3 R5′-GGCTGCCTAAATGTCTCAGGT-3′ | 133 | 59 | MT424003.1 | Current study |
| TLR7 | F5′-CCAAGGTGCTTTCCAGTTGC-3 R5′-ACCAGACAAACCACACAGCA-3′ | 161 | 58 | NM_001033761.1 | Current study |
| TNF-α | F5′-AGAGACAAGCAGCTGCAGAAC-3′ R5′-GCAGGGTATGTGAGAGAGAGC-3′ | 96 | 60 | NM_173966.3 | Current study |
| IL10 | F5′-GCACTACTCTGTTGCCTGGT-3′ R5′-AAGCTGTGCAGTTGGTCCTT-3′ | 179 | 60 | NM_174088.1 | Current study |
| NCF4 | F5′-ATGAGGCGGGAGTTCCAGA-3′ R5′-CACCATGAGCTTCACGTCCT-3′ | 102 | 58 | NM_001045983.1 | Current study |
| LITAF | F5′-GCGGCGGTAAAATGTCTGTT-3′ R5′-TTGACAGCCACCGTCTCTTC-3′ | 100 | 58 | NM_001046252.2 | Current study |
| ATOX1 | F5′-CAGGAAAGGCTGTCTCCTACC-3′ R5′-CCTAGATCTGTCTGGAGGGC-3′ | 116 | 59 | NM_001130758.1 | Current study |
| GST | F5′-ACCAGTCCAATGCCATCCTG-3′ R5′-CAGCGAAGGTCCTCTACACC-3′ | 115 | 60 | NM_177516.1 | Current study |
| OXSR1 | F5′-CGCAGAGTAGCAAAGAGGCG-3′ R5′-CGCAAACTCACTGACCTCTCT-3′ | 187 | 59 | NM_001075892.2 | Current study |
| TKT | F5′-TGCTGAGATCATGGCTGTCC-3′ R5′-CCGTCCAAGTCGGAGTTGAT-3′ | 195 | 58 | NM_001003906.1 | Current study |
| RPIA | F5′-GAAGTCGACGCTGACCTCAA-3′ R5′-GGCAATCACGATGAAGCGAC-3′ | 99 | 59 | NM_001035433.2 | Current study |
| AMPD1 | F5′-TTCGTCCAAAACCGCGTCTA-3′ R5′-TGAGGGTTGATGGTGGCTTC-3′ | 155 | 58 | NM_001100349.1 | Current study |
| ß. actin | F5′-TCGTGATGGACTCCGGTGA-3′ R5′-TGTCACGGACGATTTCCGCTC-3′ | 183 | 60 | AY141970.1 | Current study |
| Gene | SNPs | Healthy n = 65 | Endometritis n = 65 | Total n = 130 | Kind of Inherited Change | Amino Acid Order and Sort | Chi Score | p Value |
|---|---|---|---|---|---|---|---|---|
| TLR4 | T55C | 38 | - | 38/130 | Nonsynonymous | 19 Y to H | 60.95 | <0.0001 |
| T171C | 29 | - | 29/130 | Synonymous | 57 S | 46.51 | <0.0001 | |
| G213A | - | 41 | 41/130 | Synonymous | 71 Q | 65.76 | <0.0001 | |
| G285T | 48 | - | 48/130 | Nonsynonymous | 95 L to F | 76.98 | <0.0001 | |
| C381T | 35 | - | 36/130 | Synonymous | 127 D | 57.74 | <0.0001 | |
| G400A | 28 | - | 28/130 | Nonsynonymous | 134 D to N | 44.91 | <0.0001 | |
| C491T | - | 37 | 37/130 | Nonsynonymous | 164 A to V | 59.34 | <0.0001 | |
| TLR7 | G56A | 42 | - | 42/130 | Nonsynonymous | 19 C to Y | 67.37 | <0.0001 |
| TNF-α | T87C | 26 | - | 26/130 | Synonymous | 29 L | 41.70 | <0.0001 |
| T208C | - | 31 | 31/130 | Nonsynonymous | 70 C to R | 49.72 | <0.0001 | |
| A389G | - | 52 | 52/130 | Nonsynonymous | 130 K to R | 83.40 | <0.0001 | |
| IL10 | G148A | - | 30 | 30/130 | Nonsynonymous | 50 E to K | 48.12 | <0.0001 |
| C152T | 55 | - | 55/130 | Nonsynonymous | 51 A to V | 88.22 | <0.0001 | |
| G225A | 34 | - | 34/130 | Synonymous | 75 K | 54.53 | <0.0001 | |
| G321A | - | 28 | 28/130 | Synonymous | 107 E | 44.89 | <0.0001 | |
| G357C | 49 | - | 49/130 | Synonymous | 119 L | 78.59 | <0.0001 | |
| NCF4 | A744G | - | 37 | 37/130 | Synonymous | 248 P | 59.34 | <0.0001 |
| LITAF | A392G | 56 | - | 56/130 | Nonsynonymous | 131 D to G | 89.82 | <0.0001 |
| ATOX1 | A75G | - | 27 | 27/130 | Synonymous | 25 A | 43.31 | <0.0001 |
| C141T | - | 46 | 46/130 | Synonymous | 47 C | 73.78 | <0.0001 | |
| GST | A30G | 36 | - | 36/130 | Synonymous | 10 E | 57.74 | <0.0001 |
| OXSR1 | G35T | 48 | - | 48/130 | Nonsynonymous | 12 R to L | 76.98 | <0.0001 |
| C195T | 21 | - | 21/130 | Synonymous | 65Y | 33.68 | <0.0001 | |
| A270G | 30 | - | 30/130 | Synonymous | 90 K | 48.12 | <0.0001 | |
| C414A | - | 51 | 51/130 | Synonymous | 139 V | 81.80 | <0.0001 | |
| TKT | G76T | - | 39 | 39/130 | Nonsynonymous | 26 G to W | 62.55 | <0.0001 |
| A396G | 53 | - | 53/130 | Synonymous | 132 Q | 85.01 | <0.0001 | |
| RPIA | G56A | 33 | - | 33/130 | Nonsynonymous | 19 R to H | 52.93 | <0.0001 |
| T72C | 44 | - | 44/130 | Synonymous | 24 H | 70.57 | <0.0001 | |
| C202T | - | 57 | 57/130 | Nonsynonymous | 68 R to C | 91.43 | <0.0001 | |
| AMPD1 | T315C | 47 | - | 47/130 | Synonymous | 105 N | 75.39 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sharif, M.; Abdo, M.; Shabrawy, O.E.; El-Naga, E.M.A.; Fericean, L.; Banatean-Dunea, I.; Ateya, A. Investigating Polymorphisms and Expression Profile of Immune, Antioxidant, and Erythritol-Related Genes for Limiting Postparturient Endometritis in Holstein Cattle. Vet. Sci. 2023, 10, 370. https://doi.org/10.3390/vetsci10060370
Al-Sharif M, Abdo M, Shabrawy OE, El-Naga EMA, Fericean L, Banatean-Dunea I, Ateya A. Investigating Polymorphisms and Expression Profile of Immune, Antioxidant, and Erythritol-Related Genes for Limiting Postparturient Endometritis in Holstein Cattle. Veterinary Sciences. 2023; 10(6):370. https://doi.org/10.3390/vetsci10060370
Chicago/Turabian StyleAl-Sharif, Mona, Mohamed Abdo, Omnia El Shabrawy, Eman M. Abu El-Naga, Liana Fericean, Ioan Banatean-Dunea, and Ahmed Ateya. 2023. "Investigating Polymorphisms and Expression Profile of Immune, Antioxidant, and Erythritol-Related Genes for Limiting Postparturient Endometritis in Holstein Cattle" Veterinary Sciences 10, no. 6: 370. https://doi.org/10.3390/vetsci10060370
APA StyleAl-Sharif, M., Abdo, M., Shabrawy, O. E., El-Naga, E. M. A., Fericean, L., Banatean-Dunea, I., & Ateya, A. (2023). Investigating Polymorphisms and Expression Profile of Immune, Antioxidant, and Erythritol-Related Genes for Limiting Postparturient Endometritis in Holstein Cattle. Veterinary Sciences, 10(6), 370. https://doi.org/10.3390/vetsci10060370







