Oviduct Histopathology of Internal Laying and Egg-Bound Syndrome in Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
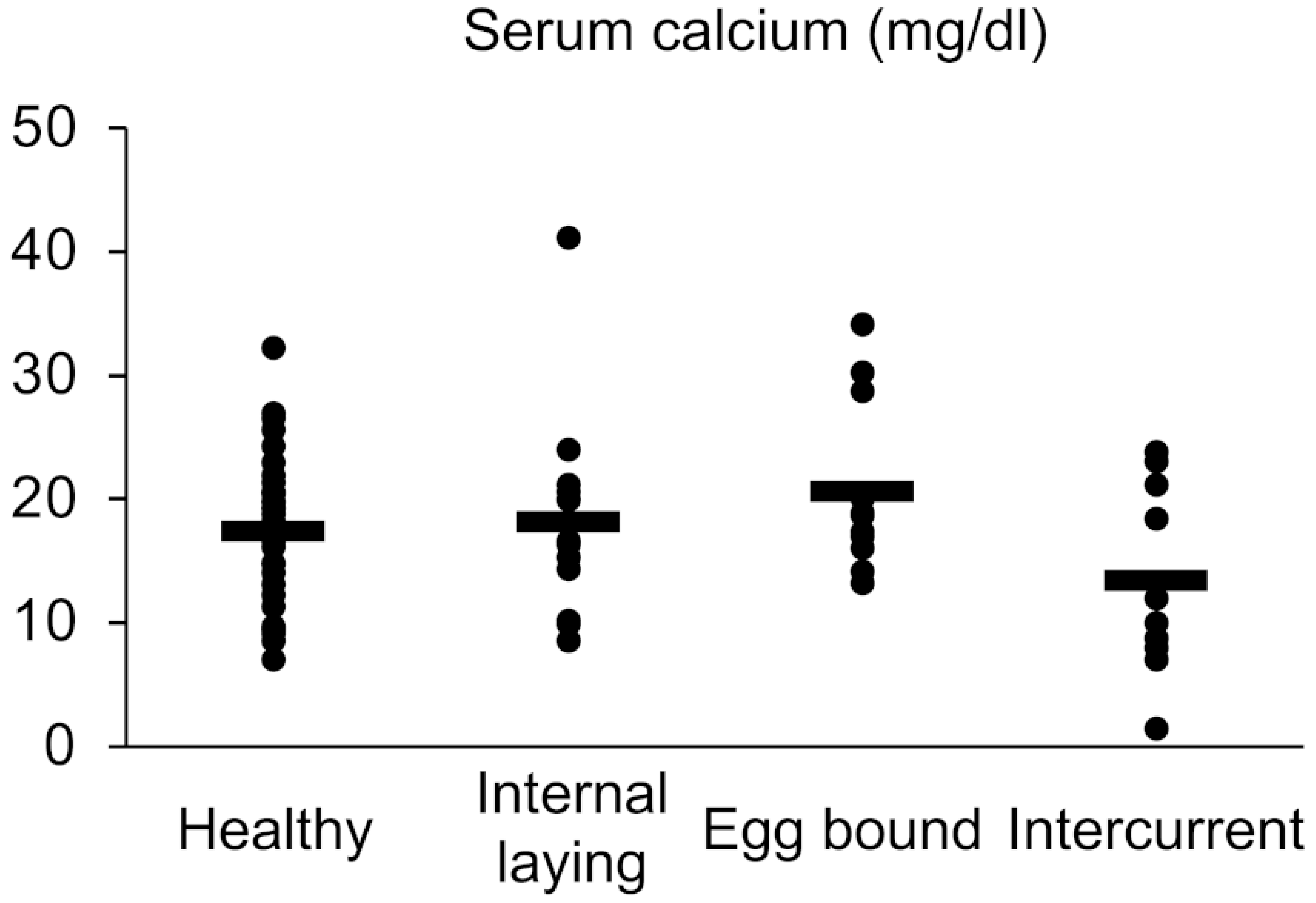
2.2. Measurement of Serum Calcium Level
2.3. Histology
2.4. Histoplanimetry
2.5. Statistical Analysis
3. Results
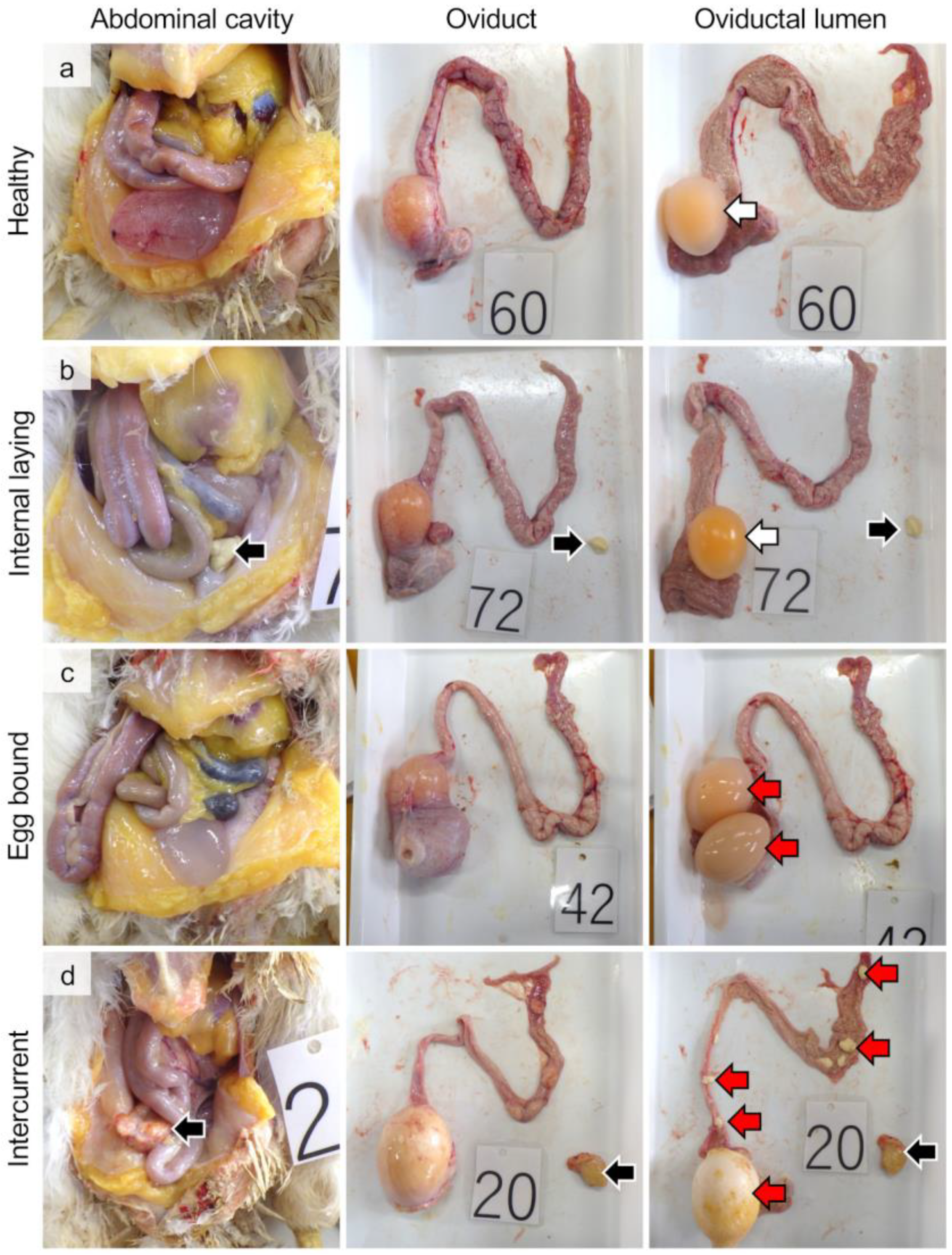
3.1. Gross Pathology and Incident Rate of Internal Laying and Egg-Bound Syndrome
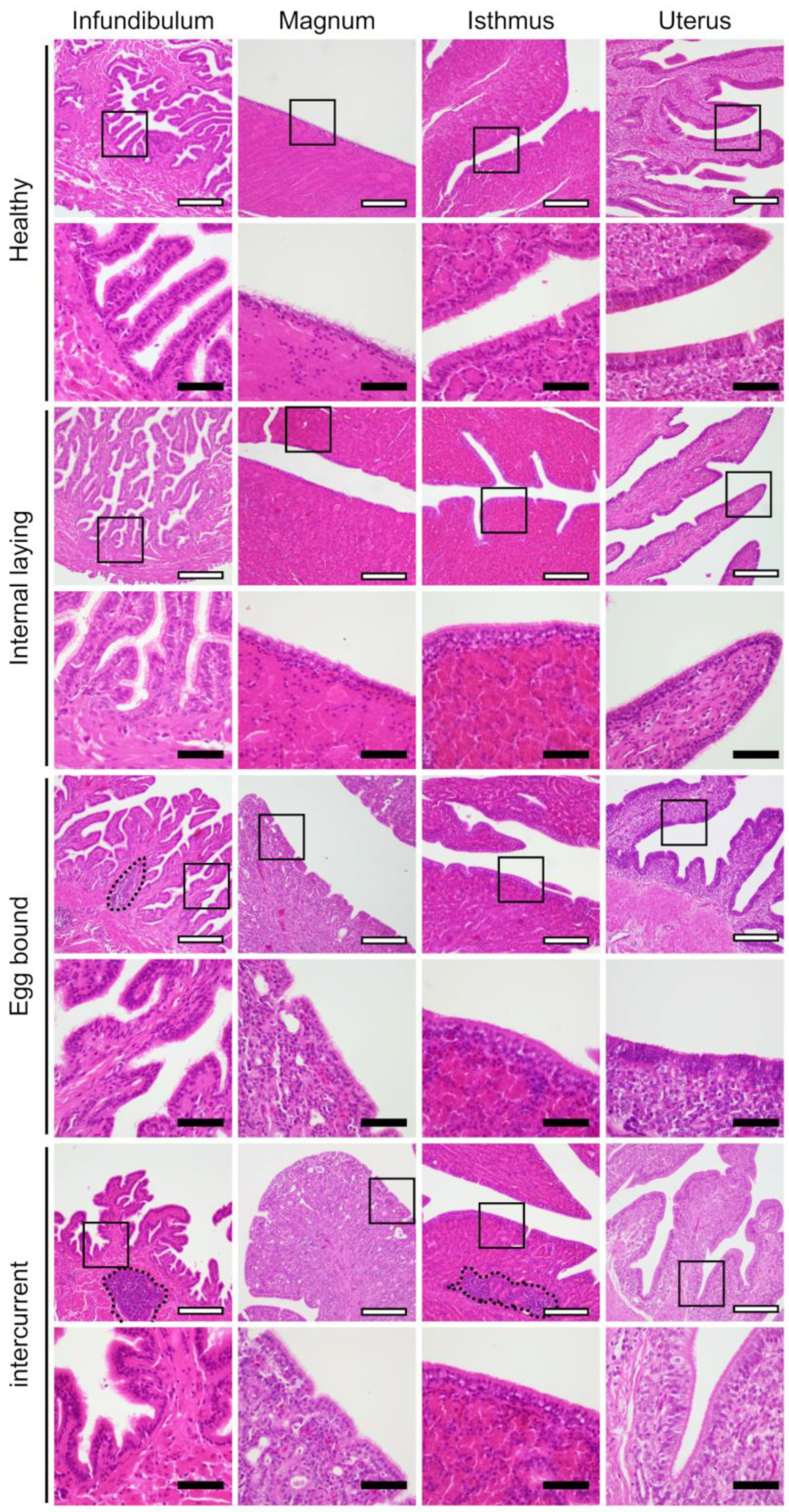
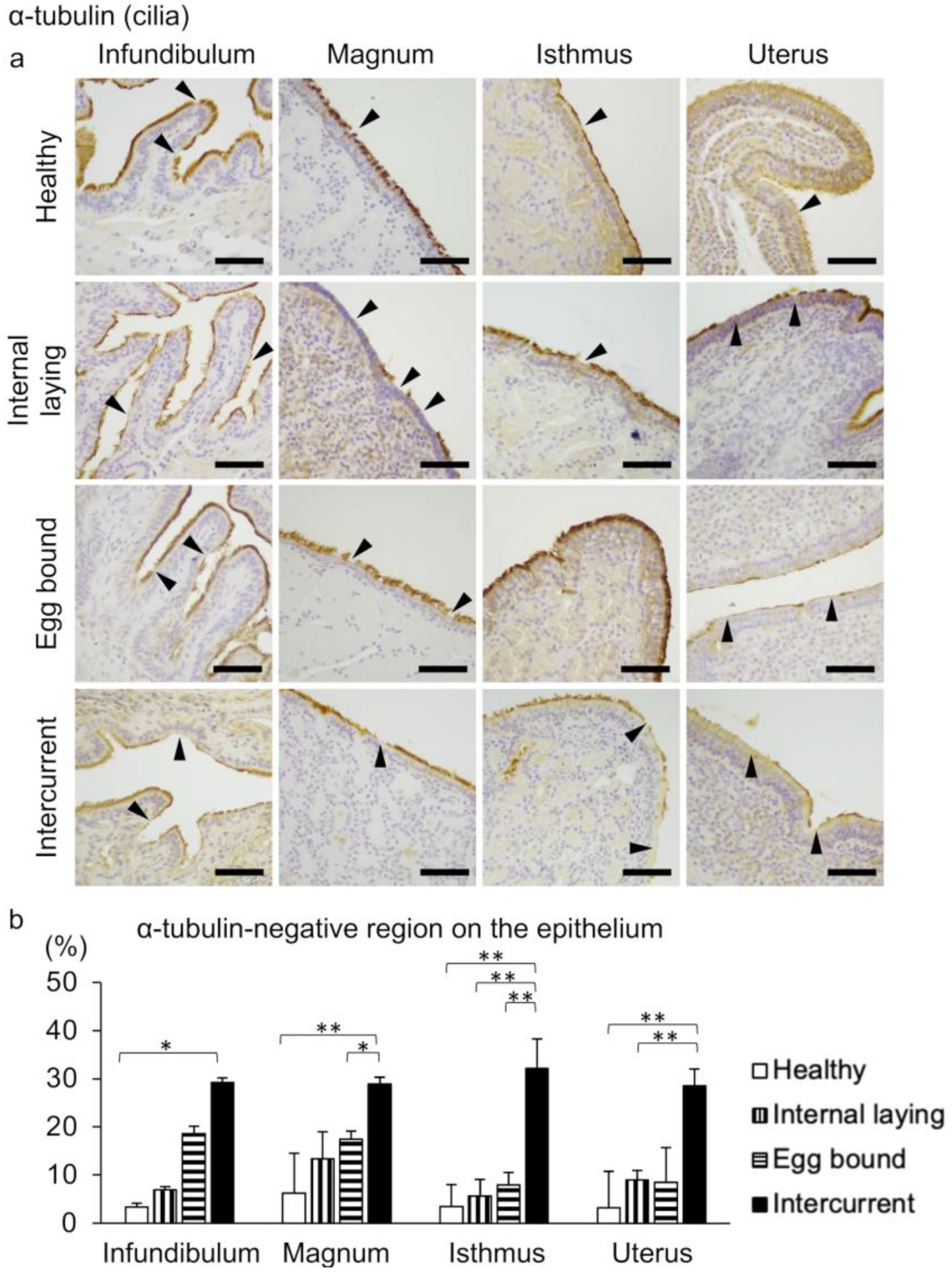
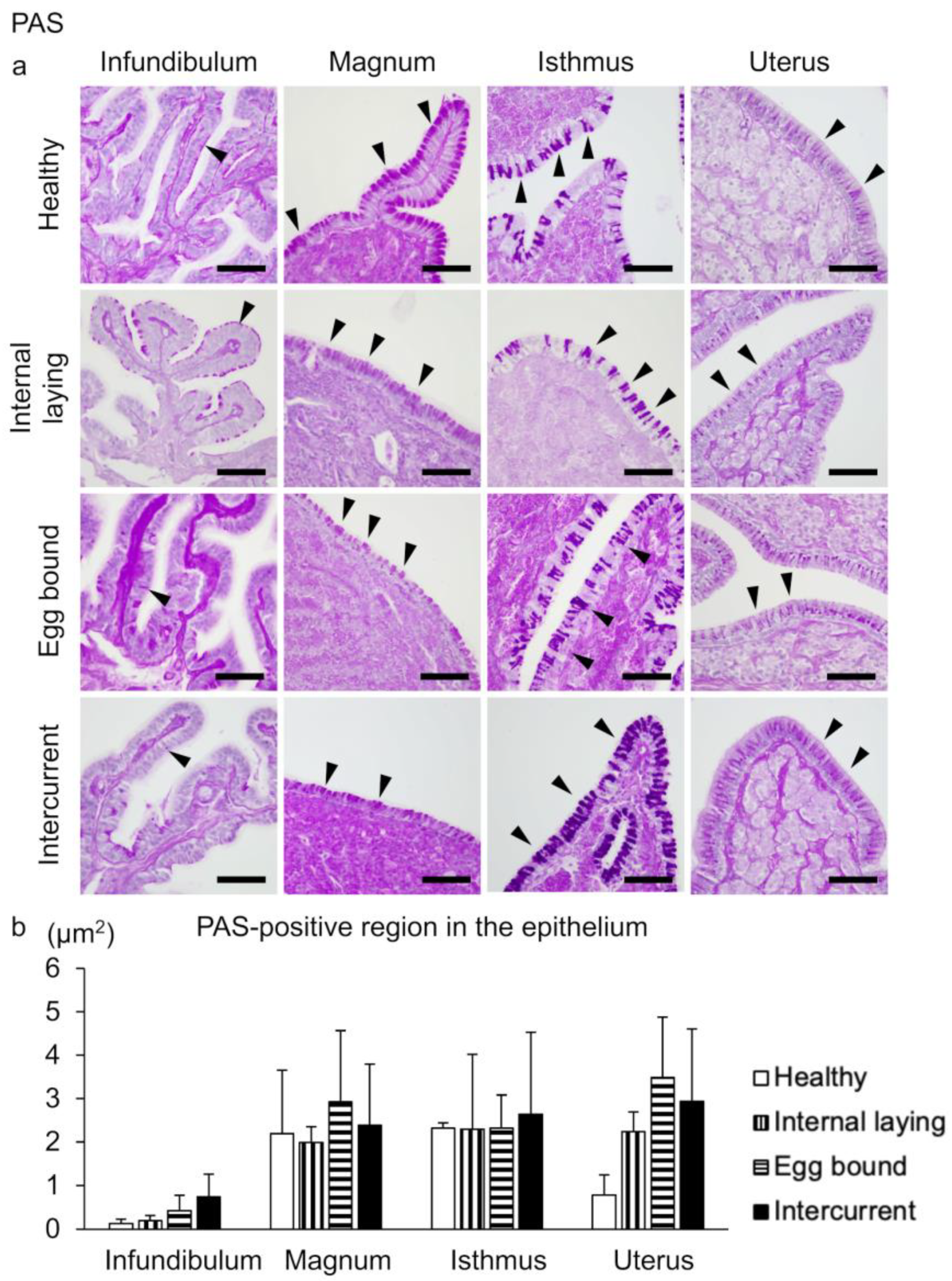
3.2. Oviduct Histopathology Focusing on the Ciliated Epithelium
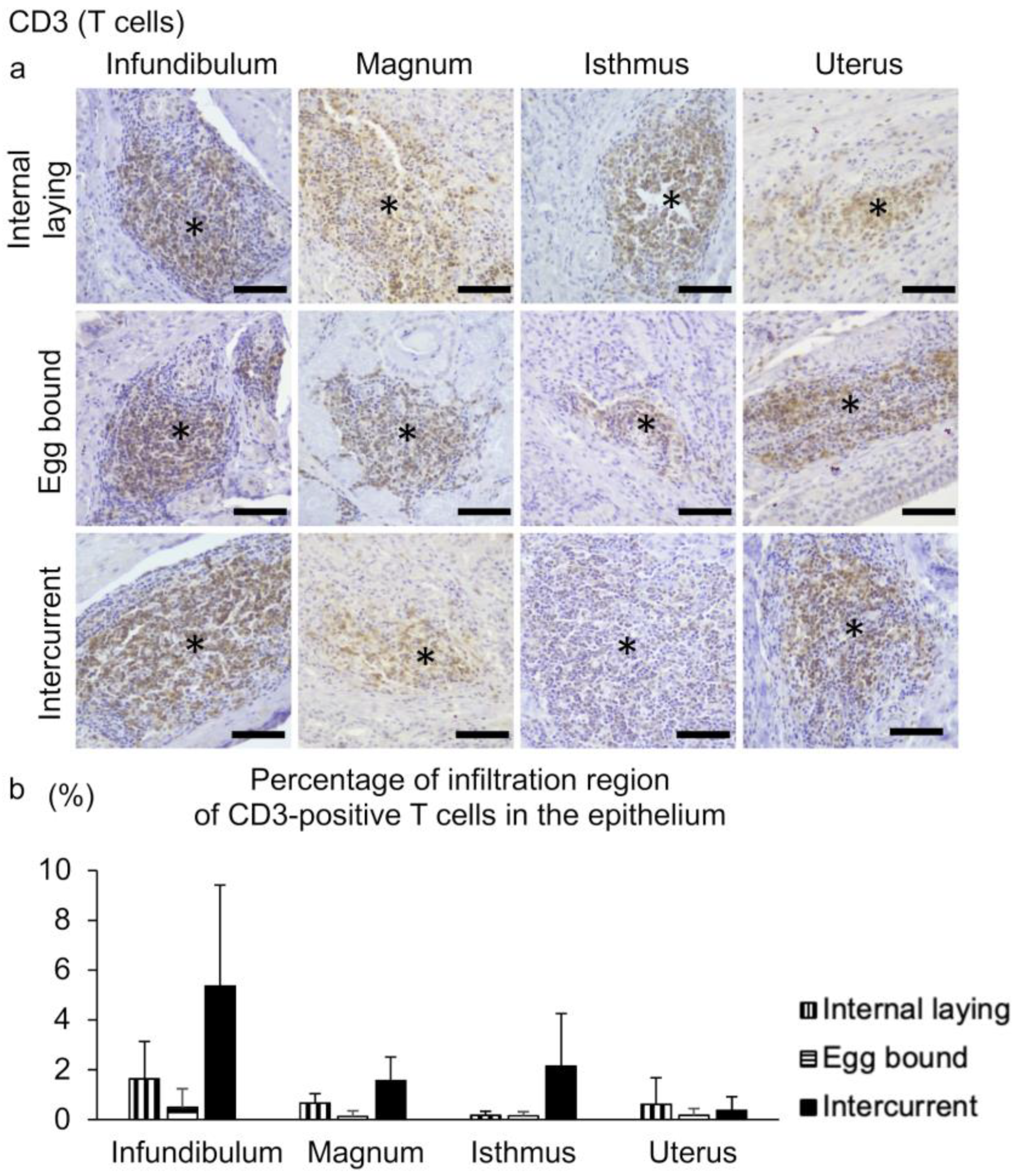
3.3. Inflammation in the Oviducts of the Laying Hens with Internal Laying and Egg-Bound Syndrome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dudde, A.; Krause, E.T.; Matthews, L.R.; Schrader, L. More than eggs—Relationship between productivity and learning in laying hens. Front. Psychol. 2018, 9, 2000. [Google Scholar] [CrossRef] [PubMed]
- Höhne, A.; Petow, S.; Bessei, W.; Schrader, L. Contrafreeloading and foraging-related behavior in hens differing in laying performance and phylogenetic origin. Poult. Sci. 2023, 102, 102489. [Google Scholar] [CrossRef] [PubMed]
- Greenacre, C.B. Reproductive diseases of the backyard hen. J. Exot. Pet Med. 2015, 24, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Palani, S.; Gurusamipalayam, A.B.; Thippichettipalayam, R.G.K.M.; Perumal, B. Clinical and gross pathological investigations of internal laying in commercial layer chicken. Adv. Anim. Vet. Sci. 2015, 3, 71–78. [Google Scholar] [CrossRef]
- Paris, E.A.; Bahr, J.M.; Bitterman, P.; Basu, S.; Abramowicz, J.S.; Barua, A. Incidence of malignant transformation in the oviductal fimbria in laying hens, a preclinical model of spontaneous ovarian cancer. PLoS ONE 2021, 16, e0255007. [Google Scholar] [CrossRef] [PubMed]
- Palani, S.; Gurusamypalayam, A.B.; Thippichettipalayam, R.G.K.M.; Balachandran, A. Prevalence and pathology of egg bound syndrome in commercial White leghorn chicken. J. Worlds Poult. Res. 2014, 4, 30–36. [Google Scholar]
- Cornax, I.; Walzem, R.L.; Larner, C.; Macfarlane, R.D.; Klasing, K.C. Mobilization of ectopic yolk in Gallus gallus domesticus: A novel reverse lipid transport process. J. Exp. Biol. 2013, 216, 1949–1958. [Google Scholar] [CrossRef]
- Roberts, J.R. Factors affecting egg internal quality and egg shell quality in laying hens. Jpn. Poult. Sci. 2004, 41, 161–177. [Google Scholar] [CrossRef]
- Parto, P.; Khaksar, Z.; Akramifard, A.; Moghisi, B. The microstructure of oviduct in laying turkey hen as observed by light and scanning electron microscopies. World J. Zool. 2011, 6, 120–125. [Google Scholar]
- Sah, N.; Mishra, B. Regulation of egg formation in the oviduct of laying hen. World’s Poult. Sci. J. 2018, 74, 509–522. [Google Scholar] [CrossRef]
- Dixon, R.E.; Hwang, S.J.; Hennig, G.W.; Ramsey, K.H.; Schripsema, J.H.; Sanders, K.M.; Ward, S.M. Chlamydia infection causes loss of pacemaker cells and inhibits oocyte transport in the mouse oviduct. Biol. Reprod. 2009, 80, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, M.; Ichii, O.; Nakamura, T.; Kanazawa, S.O.; Elewa, Y.H.A.; Kon, Y. Autoimmune abnormality affects ovulation and oocyte-pick-up in MRL/MpJ-FasLpr/Lpr mice. Lupus 2018, 27, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Talbot, P.; Riveles, K. Smoking and reproduction: The oviduct as a target of cigarette smoke. Reprod. Biol. Endocrinol. 2005, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Komatsu, K.; Hirao, M.; Toyooka, Y.; Koyama, H.; Tissir, F.; Goffinet, A.M.; Uemura, T.; Fujimori, T. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development 2014, 141, 4558–4568. [Google Scholar] [CrossRef]
- Wilson, W.O.; Huang, R.H. A Comparison of the time of ovipositing for Coturnix and Chicken. Poult. Sci. 1962, 41, 1843–1845. [Google Scholar] [CrossRef]
- Sterling, K.G.; Bell, D.D.; Pesti, G.M.; Aggrey, S.E. Relationships among strain, performance, and environmental temperature in commercial laying hens. J. Appl. Poult. Res. 2003, 12, 85–91. [Google Scholar] [CrossRef]
- Joyner, C.J.; Peddie, M.J.; Taylor, T.G. The effect of Age on egg production in the domestic hen. Gen. Comp. Endocrinol. 1987, 65, 331–336. [Google Scholar] [CrossRef]
- Liu, X.T.; Lin, X.; Mi, Y.L.; Zeng, W.D.; Zhang, C.Q. Age-related changes of yolk precursor formation in the liver of laying hens. J. Zhejiang Univ. Sci. B. 2018, 19, 390–399. [Google Scholar] [CrossRef]
- Crespo, R.; Shivaprasad, H.L. Developmental, metabolic, and other noninfectious disorders. In Disease of Poultry, 13th ed.; Swayne, D.E., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 1233–1270. [Google Scholar]
- Navara, K.J.; Pinson, S.E.; Chary, P.; Taube, P.C. Higher rates of internal ovulations occur in broiler breeder hens treated with testosterone. Poult. Sci. 2015, 94, 1346–1352. [Google Scholar] [CrossRef]
- Ghosh, A.; Syed, S.M.; Tanwar, P.S. In vivo genetic cell lineage tracing reveals that oviductal secretory cells self-renew and give rise to ciliated cells. Development 2017, 144, 3031–3041. [Google Scholar] [CrossRef]
- Stadnicka, K.; Sławińska, A.; Dunisławska, A.; Pain, B.; Bednarczyk, M. Molecular signatures of epithelial oviduct cells of a laying hen (Gallus gallus domesticus) and quail (Coturnix japonica). BMC Dev. Biol. 2018, 18, 9. [Google Scholar] [CrossRef]
- Hosotani, M.; Ichii, O.; Nakamura, T.; Masum, M.A.; Otani, Y.; Elewa, Y.H.A.; Kon, Y. Altered ciliary morphofunction in the oviductal infundibulum of systemic autoimmune disease-prone MRL/MpJ-Faslpr/lpr mice. Cell Tissue Res. 2020, 380, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, M.; Ichii, O.; Namba, T.; Masum, M.A.; Nakamura, T.; Hasegawa, Y.; Watanabe, T.; Kon, Y. Expression of Indian hedgehog signaling in murine oviductal infundibulum and its relationship with epithelial homeostasis. Cell Tissue Res. 2022, 391, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, A.; Djahanbakhch, O.; Saridogan, E.; Lyons, R.A. The effect of interleukin-6 on ciliary beat frequency in the human Fallopian tube. Fertil. Steril. 2008, 90, 391–394. [Google Scholar] [CrossRef]
- Grosse-Onnebrink, J.; Werner, C.; Loges, N.T.; Hormann, K.; Blum, A.; Schmidt, R.; Olbrich, H.; Omran, H. Effect of TH2 cytokines and interferon gamma on beat frequency of human respiratory cilia. Pediatr. Res. 2016, 79, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, S.; Saraiva, C.; Oliveira, I.; Stilwell, G.; Esteves, A. Effects of Age, weight, and housing system on prevalence of dead on arrival and carcass condemnation causes in laying hens. Poult. Sci. 2021, 100, 100910. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Ghanbarpour, R. Characterization of Escherichia Coli isolates from commercial layer hens with salpingitis. Am. J. Anim. Vet. Sci. 2010, 5, 208–214. [Google Scholar] [CrossRef]
- Poulsen, L.L.; Kudirkiene, E.; Jørgensen, S.L.; Djordjevic, S.P.; Cummins, M.L.; Christensen, J.P.; Christensen, H.; Bisgaard, M.; Thøfner, I. Whole genome sequence comparison of avian pathogenic Escherichia Coli from acute and chronic salpingitis of egg laying hens. BMC Vet. Res. 2020, 16, 148. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, K.; Chen, S.; Yan, K.; Du, X.; Zhang, C.; Guo, M.; Wu, Y. Evaluation of the reproductive system development and egg-laying performance of hens infected with TW I-type infectious bronchitis virus. Vet. Res. 2020, 51, 95. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosotani, M.; Hamano, S.; Iwasaki, T.; Hasegawa, Y.; Ueda, H.; Watanabe, T. Oviduct Histopathology of Internal Laying and Egg-Bound Syndrome in Laying Hens. Vet. Sci. 2023, 10, 260. https://doi.org/10.3390/vetsci10040260
Hosotani M, Hamano S, Iwasaki T, Hasegawa Y, Ueda H, Watanabe T. Oviduct Histopathology of Internal Laying and Egg-Bound Syndrome in Laying Hens. Veterinary Sciences. 2023; 10(4):260. https://doi.org/10.3390/vetsci10040260
Chicago/Turabian StyleHosotani, Marina, Sohei Hamano, Tomohito Iwasaki, Yasuhiro Hasegawa, Hiromi Ueda, and Takafumi Watanabe. 2023. "Oviduct Histopathology of Internal Laying and Egg-Bound Syndrome in Laying Hens" Veterinary Sciences 10, no. 4: 260. https://doi.org/10.3390/vetsci10040260
APA StyleHosotani, M., Hamano, S., Iwasaki, T., Hasegawa, Y., Ueda, H., & Watanabe, T. (2023). Oviduct Histopathology of Internal Laying and Egg-Bound Syndrome in Laying Hens. Veterinary Sciences, 10(4), 260. https://doi.org/10.3390/vetsci10040260





