Concomitant Campylobacteriosis in a Puppy and in Its Caregiver: A One Health Perspective Paradigm in Human-Pet Relationship
Abstract
Simple Summary
Abstract
1. Introduction
2. Case Presentation
2.1. Signalment, Anamnesis and Clinical Findings
2.2. Differential and Diagnostic Work-Up
2.3. Treatment and Follow Up
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Fernández, A.; Dionisi, A.M.; Arena, S.; Iglesias-Torrens, Y.; Carattoli, A.; Luzzi, I. Human Campylobacteriosis in Italy: Emergence of Multi-Drug Resistance to Ciprofloxacin, Tetracycline, and Erythromycin. Front. Microbiol. 2018, 9, 1906. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2016. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2018. EFSA J. 2019, 16. [Google Scholar]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; Heikkilä, T.; Pernu, N.; Kovanen, S.; Hielm-Björkman, A.; Kivistö, R. Raw Meat-Based Diets in Dogs and Cats. Vet. Sci. 2017, 4, 33. [Google Scholar] [CrossRef]
- Vinassa, M.; Vergnano, D.; Valle, E.; Giribaldi, M.; Nery, J.; Prola, L.; Bergero, D.; Schiavone, A. Profiling Italian Cat and Dog Owners’ Perceptions of Pet Food Quality Traits. BMC Vet. Res. 2020, 16, 131. [Google Scholar] [CrossRef]
- Hoummady, S.; Fantinati, M.; Maso, D.; Bynens, A.; Banuls, D.; Santos, N.R.; Roche, M.; Priymenko, N. Comparison of Canine Owner Profile According to Food Choice: An Online Preliminary Survey in France. BMC Vet. Res. 2022, 18, 163. [Google Scholar] [CrossRef]
- Empert-Gallegos, A.; Hill, S.; Yam, P.S. Insights into Dog Owner Perspectives on Risks, Benefits, and Nutritional Value of Raw Diets Compared to Commercial Cooked Diets. PeerJ 2020, 8, e10383. [Google Scholar] [CrossRef]
- Morelli, G.; Bastianello, S.; Catellani, P.; Ricci, R. Raw Meat-Based Diets for Dogs: Survey of Owners’ Motivations, Attitudes and Practices. BMC Vet. Res. 2019, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Chandler, M.L.; Hamper, B.A.; Weeth, L.P. Current Knowledge about the Risks and Benefits of Raw Meat–Based Diets for Dogs and Cats. J. Am. Vet. Med. Assoc. 2013, 243, 1549–1558. [Google Scholar] [CrossRef]
- APPA. The 2017–2018 APPA National Pet Owners Survey Debut; American Pet Products Association: Stamford, CT, USA, 2018. [Google Scholar]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2017. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Gras, L.M.; Smid, J.H.; Wagenaar, J.A.; Koene, M.G.J.; Havelaar, A.H.; Friesema, I.H.M.; French, N.P.; Flemming, C.; Galson, J.D.; Graziani, C.; et al. Increased Risk for Campylobacter jejuni and C. coli Infection of Pet Origin in Dog Owners and Evidence for Genetic Association between Strains Causing Infection in Humans and Their Pets. Epidemiol. Infect. 2013, 141, 2526–2535. [Google Scholar] [CrossRef]
- Davies, R.H.; Lawes, J.R.; Wales, A.D. Raw Diets for Dogs and Cats: A Review, with Particular Reference to Microbiological Hazards. J. Small Anim. Pract. 2019, 60, 329–339. [Google Scholar] [CrossRef]
- Ahmed, F.; Cappai, M.G.; Morrone, S.; Cavallo, L.; Berlinguer, F.; Dessì, G.; Tamponi, C.; Scala, A.; Varcasia, A. Raw meat based diet (RMBD) for household pets as potential door opener to parasitic load of domestic and urban environment. Revival of understated zoonotic hazards? A review. One Health 2021, 13, 100327. [Google Scholar] [CrossRef]
- Waltham. Waltham Faeces Scoring System. Available online: https://waltham.com/media/2020-05/waltham-scoring.pdf?VersionId=27x4BJc1CDcBNfM04N6jLXGJUCEw.vc_ (accessed on 1 January 2023).
- Viganò, F. Capire l’emogasanalisi:Un Metodo Semplice per Interpretare i più Comuni Disturbi Acido-Base. Veterinaria 2002, 6, 45–51. [Google Scholar]
- Candellone, A.; Girolami, F.; Badino, P.; Jarriyawattanachaikul, W.; Odore, R. Changes in the Oxidative Stress Status of Dogs Affected by Acute Enteropathies. Vet. Sci. 2022, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Steingräber, L.; Herold, L. Investigation of Procalcitonin and Beta-defensin2 in the Serum and Feces of Dogs with Acute Diarrhea. Vet. Clin. Pathol. 2022, 50, 55–62. [Google Scholar] [CrossRef]
- Bovens, C.; Tennant, K.; Reeve, J.; Murphy, K.F. Basal Serum Cortisol Concentration as a Screening Test for Hypoadrenocorticism in Dogs. J. Vet. Intern. Med. 2014, 28, 1541–1545. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dogs and Cats; National Academy of Sciences: Washington, DC, USA, 2006. [Google Scholar]
- FEDAF. FEDIAF Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs. Available online: https://europeanpetfood.org/wp-content/uploads/2022/03/Updated-Nutritional-Guidelines.pdf (accessed on 1 March 2022).
- Weese, J.S. Bacterial Enteritis in Dogs and Cats: Diagnosis, Therapy, and Zoonotic Potential. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Nüesch-Inderbinen, M.; Treier, A.; Zurfluh, K.; Stephan, R. Raw Meat-Based Diets for Companion Animals: A Potential Source of Transmission of Pathogenic and Antimicrobial-Resistant Enterobacteriaceae. R. Soc. Open Sci. 2019, 6, 191170. [Google Scholar] [CrossRef]
- ISO 10272; Microbiology of the Food Chain—Horizontal Method for Detection and Enumeration of Campylobacter spp.—Part 1: Detection Method. OIE: Paris, France, 2019.
- Aerts, M.; Battisti, A.; Hendriksen, R.; Kempf, I.; Teale, C.; Tenhagen, B.; Veldman, K.; Wasyl, D.; Guerra, B.; Liébana, E.; et al. Technical Specifications on Harmonised Monitoring of Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Food-producing Animals and Food. EFSA J. 2019, 17, e05709. [Google Scholar] [CrossRef]
- WSAVA Gastrointestinal Standardization Group. Summary of Findings and Reports of the WSAVA Gastrointestinal Standardization Group; WSAVA Gastrointestinal Standardization Group: Dundas, ON, Canada, 2008. [Google Scholar]
- Humphrey, T.J.; Martin, K.W.; Slader, J.; Durham, K. Campylobacter spp. in the Kitchen: Spread and Persistence. J. Appl. Microbiol. 2001, 90, 115S–120S. [Google Scholar] [CrossRef]
- Selwet, M.; Cłapa, T.; Galbas, M.; Słomski, R.; Porzucek, F. The Prevalence of Campylobacter spp. and Occurrence of Virulence Genes Isolated from Dogs. Pol. J. Microbiol. 2015, 64, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Murawska, M.; Sypecka, M.; Bartosik, J.; Kwiecień, E.; Rzewuska, M.; Sałamaszyńska-Guz, A. Should We Consider Them as a Threat? Antimicrobial Resistance, Virulence Potential and Genetic Diversity of Campylobacter spp. Isolated from Varsovian Dogs. Antibiotics 2022, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Selwet, M.; Galbas, M.; Słomski, R.; Cłapa, T.; Porzucek, F. Monitoring of Virulence Genes, Drug-Resistance in Campylobacter coli Isolated from Golden Retrievers. Pol. J. Microbiol. 2016, 65, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, M.; Klawe, J.J.; Szczepańska, B.; Spica, D. Occurrence of Virulence Genes among Campylobacter jejuni and Campylobacter coli Isolates from Domestic Animals and Children. Pol. J. Vet. Sci. 2011, 14, 207–211. [Google Scholar] [CrossRef]
- Westgarth, C.; Pinchbeck, G.L.; Bradshaw, J.W.S.; Dawson, S.; Gaskell, R.M.; Christley, R.M. Dog-Human and Dog-Dog Interactions of 260 Dog-Owning Households in a Community in Cheshire. Vet. Rec. 2008, 162, 436–442. [Google Scholar] [CrossRef]
- Solís, D.; Toro, M.; Navarrete, P.; Faúndez, P.; Reyes-Jara, A. Microbiological Quality and Presence of Foodborne Pathogens in Raw and Extruded Canine Diets and Canine Fecal Samples. Front. Vet. Sci. 2022, 9, 799710. [Google Scholar] [CrossRef]
- Candellone, A.; Cerquetella, M.; Girolami, F.; Badino, P.; Odore, R. Acute Diarrhea in Dogs: Current Management and Potential Role of Dietary Polyphenols Supplementation. Antioxidants 2020, 9, 725. [Google Scholar] [CrossRef]
- Rendle, D.I.; Page, S.W. Antimicrobial Resistance in Companion Animals. Equine Vet. J. 2018, 50, 147–152. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance; World Health Organization (WHO): Geneva, Switzerland, 1983. [Google Scholar]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M.; et al. Public Health Risk of Antimicrobial Resistance Transfer from Companion Animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef]
- Ge, B.; Wang, F.; Sjölund-Karlsson, M.; McDermott, P.F. Antimicrobial Resistance in Campylobacter: Susceptibility Testing Methods and Resistance Trends. J. Microbiol. Methods 2013, 95, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Iannino, F.; Salucci, S.; di Donato, G.; Badagliacca, P.; Vincifori, G.; di Giannatale, E. Campylobacter and Antimicrobial Resistance in Dogs and Humans: “One Health” in Practice. Vet. Ital. 2019, 55, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Chalifoux, N.V.; Parker, S.E.; Cosford, K.L. Prognostic Indicators at Presentation for Canine Parvoviral Enteritis: 322 Cases (2001–2018). J. Vet. Emerg. Crit. Care 2021, 31, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.C.; Nunes, T.; Maximino, M.; Malato, J.; Tavares, L.; Almeida, V.; Sepúlveda, N.; Gil, S. Epidemiologic Factors Supporting Triage of Infected Dog Patients Admitted to a Veterinary Hospital Biological Isolation and Containment Unit. Vet. Sci. 2023, 10, 186. [Google Scholar] [CrossRef]
- Carella, E.; Orusa, T.; Viani, A.; Meloni, D.; Borgogno-Mondino, E.; Orusa, R. An Integrated, Tentative Remote-Sensing Approach Based on NDVI Entropy to Model Canine Distemper Virus in Wildlife and to Prompt Science-Based Management Policies. Animals 2022, 12, 1049. [Google Scholar] [CrossRef]
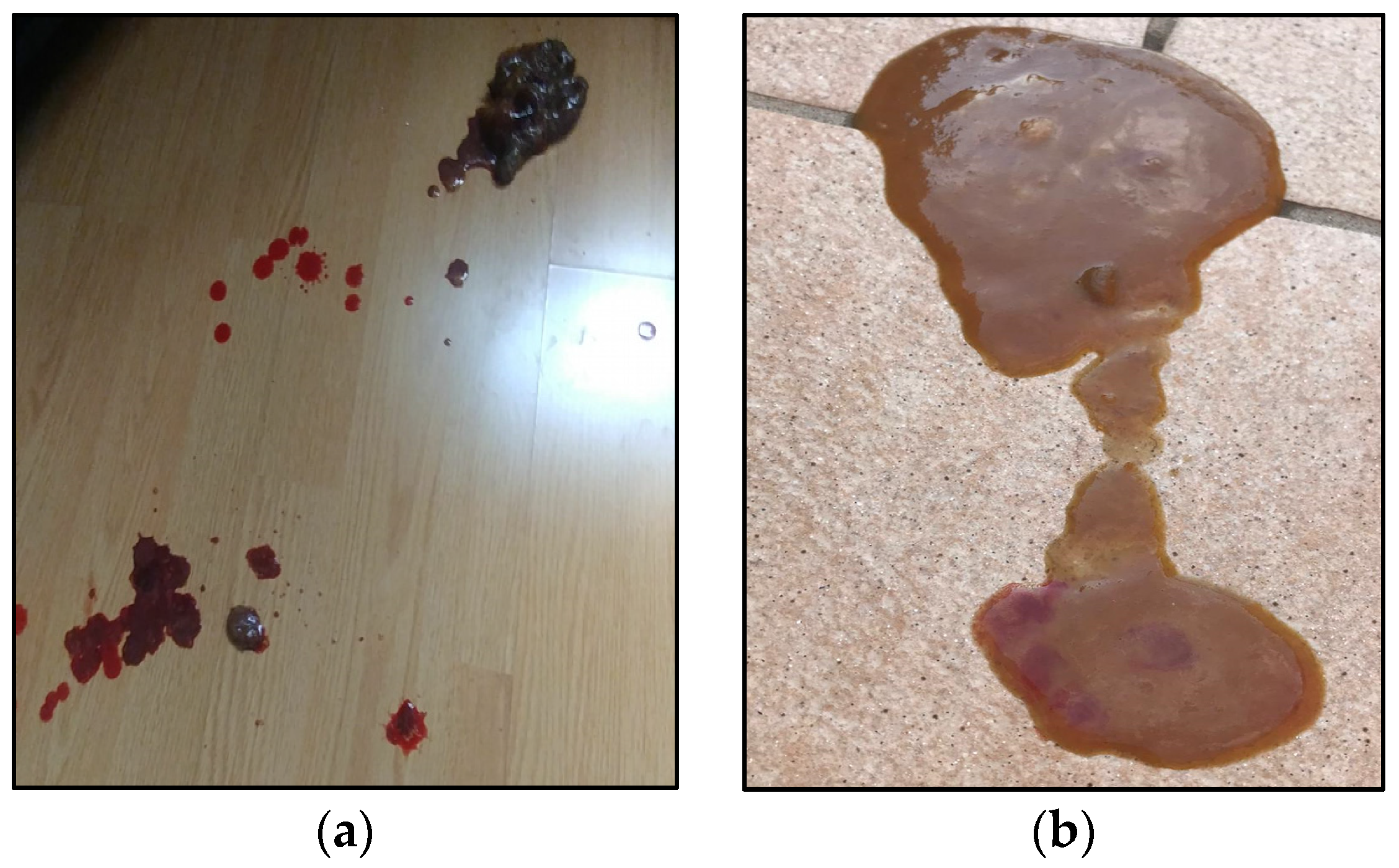
| Clinical/Biochemical Findings | Results | Normality/ Reference Range [17] |
|---|---|---|
| Mental status | Lethargic, dull | Bright and alert |
| % of dehydration | 5–8 | Absent |
| Rectal temperature (°C) | 40.1 | 38–39.2 |
| Heart rate (bpm) | 138 | 100–140 |
| Respiratory rate (brpm) | 22 | 12–20 |
| Capillary refill time (s) | 2 | <2 |
| Body condition score | 3.5/9 | 5/9 |
| pH | 7.4 | 7.32–7.50 * |
| pCO2 (mmHg) | 38 | 33–50 * |
| HCO3− (mmol/L) | 26 | 18–26 * |
| Base excess | 2 | −2 + 2 * |
| Biochemical Findings | Results | Reference Interval |
|---|---|---|
| Hct (%) | 55 | 42–58 |
| WBC (103/µL) | 14.3 | 4.7–11.15 |
| RBC (106/µL) | 8.5 | 6.13–8.52 |
| Albumin (g/dL) | 2.9 | 3–3.7 |
| TP (g/dL) | 6.6 | 5.7–7–1 |
| BUN (mg/dL) | 48 | 19–45 |
| Crea (mg/dL) | 0.8 | 0.76–1.24 |
| Ca2+ (mg/dL) | 8 | 9–11–5 |
| Phos (mg/dL) | 4 | 1.9–5.9 |
| Na+ (mEq/L) | 141 | 143–151 |
| K+ (mEq/L) | 4 | 3.9–4.9 |
| Glucose (mg/dL) | 92 | 85–123 |
| ALT (IU/L) | 121 | 10–94 |
| ALP (IU/L) | 220 | 10–250 |
| Basal cortisol (µg/dL) | 2.6 | 1.8–9 |
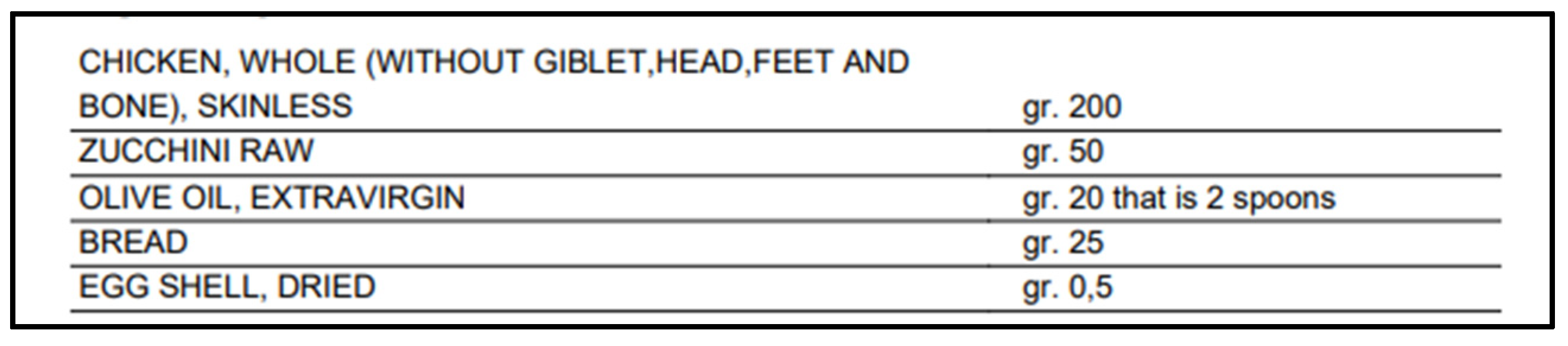
| Macronutrients | ||
| Proteins: 41.61 g | Lipids: 28.15 g | Cholesterol: 150 mg |
| Carbohydrates: 12.98 g | Total Fiber: 1.23 g | Soluble Fiber: 0.31 g |
| Insoluble fiber: 0.37 g | ||
| Minerals | ||
| Potassium: 772.86 mg | Calcium: 22 mg | Sodium: 287.75 mg |
| Phosphorus: 445.50 mg | Zinc: 3.02 mg | Magnesium: 69.10 mg |
| Copper: 0.20 mg | Selenium: 26.90 µg | Chlorine: 220 mg |
| Iodine: 12 µg | Manganese: 0.23 mg | Iron: 2.43 mg |
| Vitamins | ||
| Vitamin B1: 0.34 mg | Vitamin B2: 0.44 mg | Niacin: 12.94 mg |
| Niacin: 12.94 mg | Vitamin C: 8.50 mg | Vitamin B5: 2.35 mg |
| Vitamin B6: 0.68 mg | Total folates: 80.25 µg | Vitamin D: 0.20 µg |
| Biotin: 6 µg | Vitamin B12: 2 µg | Vitamin A eq.: 12.20 µg |
| ß-carotene eq.: 10,320 µg | Vitamin E: 4.76 mg | |
| Fatty acids profile | ||
| Total sat. fatty acids: 5.59 g | Total mono fatty acids: 16.96 g | Total poly fatty acids: 3.52 g |
| Linoleic acid (w6): 2.76 g | Linolenic acid (w3): 0.28 g | Arachidonic acid (w6): 0.26 g |
| EPA (w3): 0.02 g | DHA (w3): 0.06 g | EPA + DHA: 0.08 g |
| Aminoacidic profile | ||
| Tryptophan: 461.25 mg | Threonine: 1981.25 mg | Isoleucine: 2064.25 mg |
| Leucine: 3470 mg | Lysine: 3663.75 mg | Methionine: 1293.50 mg |
| Cystine: 546.25 mg | Phenylalanine: 1774.75 mg | Tyrosine: 1435.50 mg |
| Valina: 2192.50 mg | Arginine: 2786 mg | Histidine: 1437.50 mg |
| Taurine: nc | ||
| Owner Fecal Culture | Puppy Fecal Culture | Puppy Colonic Biopsies Culture | Owner Fecal PCR Assay | Puppy Fecal PCR Assay | Puppy Susceptibility Testing | Puppy FISH on Colonic Biopsies | |
|---|---|---|---|---|---|---|---|
| C. jejuni | x | n.i. | x | x | x | x | x |
| C. upsaliensis | x | x | n.i. | x | x | x | n.i. |
| Bacterial Species | Owner Fecal Culture | Puppy Fecal Culture | Puppy Colonic Biopsies Culture | Puppy FISH Colonic Biopsies | Owner Fecal PCR | Puppy Fecal PCR | Puppy AST |
|---|---|---|---|---|---|---|---|
| C. jejuni | + | n.i. | + | + | + | + | S: NAL (8 mg/mL), CIP (<0.12 mg/mL), GEN (1 mg/mL), ERY (≤1 mg/mL), STR (4 mg/mL) R: TET (64 mg/mL) |
| C. upsaliensis | + | + | n.i. | n.i. | + | + | NA: CIP (<2 mg/mL), ERY (<0.016 mg/mL), STR (<0.064 mg/mL), TET (<0.016 mg/mL) |
| Morphological Features | ||||
|---|---|---|---|---|
| Normal | Mild | Moderate | Marked | |
| Surface epithelial injury/Villous stunting | LG | S | ||
| Crypt hyperplasia/Crypt distension * | S | L | ||
| Crypt dilation/distortion * | LS | |||
| Fibrosis/atrophy | LG | S | ||
| Inflammation | ||||
| Normal | Mild | Moderate | Marked | |
| Intra-epitelial lymphocytes § | G | S | ||
| Lamina propria Lymphocytes and plasma cells | G | L | S | |
| Lamina propria eosinophils | LSG | |||
| Lamina propria neutrophils | LSG | |||
| Lamina propria macrophages | LSG | |||
| Other notes | ||||
| Edema | LSG | |||
| Superficial erosions | L | |||
| Final diagnosis | ||||
| Mild chronic gastritis; Moderate-to-severe lymphoplasmacellular enteritis with signs of fibrosis; Moderate lymphoplasmacellular colitis with signs of erosions. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candellone, A.; Badino, P.; Girolami, F.; Cerquetella, M.; Nebbia, P.; Aresu, L.; Zoppi, S.; Bergero, D.; Odore, R. Concomitant Campylobacteriosis in a Puppy and in Its Caregiver: A One Health Perspective Paradigm in Human-Pet Relationship. Vet. Sci. 2023, 10, 244. https://doi.org/10.3390/vetsci10040244
Candellone A, Badino P, Girolami F, Cerquetella M, Nebbia P, Aresu L, Zoppi S, Bergero D, Odore R. Concomitant Campylobacteriosis in a Puppy and in Its Caregiver: A One Health Perspective Paradigm in Human-Pet Relationship. Veterinary Sciences. 2023; 10(4):244. https://doi.org/10.3390/vetsci10040244
Chicago/Turabian StyleCandellone, Alessia, Paola Badino, Flavia Girolami, Matteo Cerquetella, Patrizia Nebbia, Luca Aresu, Simona Zoppi, Domenico Bergero, and Rosangela Odore. 2023. "Concomitant Campylobacteriosis in a Puppy and in Its Caregiver: A One Health Perspective Paradigm in Human-Pet Relationship" Veterinary Sciences 10, no. 4: 244. https://doi.org/10.3390/vetsci10040244
APA StyleCandellone, A., Badino, P., Girolami, F., Cerquetella, M., Nebbia, P., Aresu, L., Zoppi, S., Bergero, D., & Odore, R. (2023). Concomitant Campylobacteriosis in a Puppy and in Its Caregiver: A One Health Perspective Paradigm in Human-Pet Relationship. Veterinary Sciences, 10(4), 244. https://doi.org/10.3390/vetsci10040244











