Evaluation of First Treatment Timing, Fatal Disease Onset, and Days from First Treatment to Death Associated with Bovine Respiratory Disease in Feedlot Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
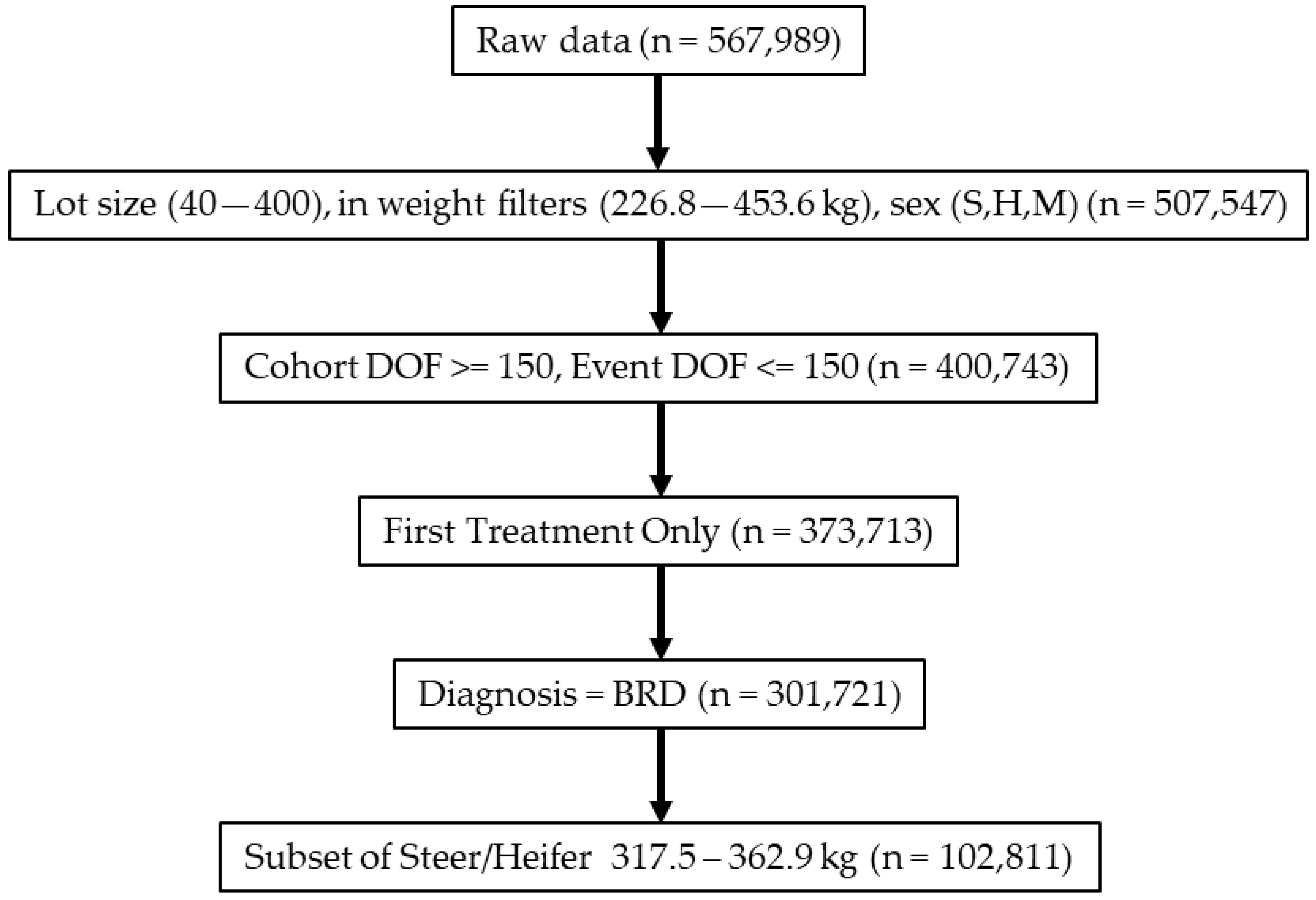
2.2. Data Filtering and Management
2.3. Outcomes of Interest
2.4. Data Analysis
3. Results
3.1. Study Population
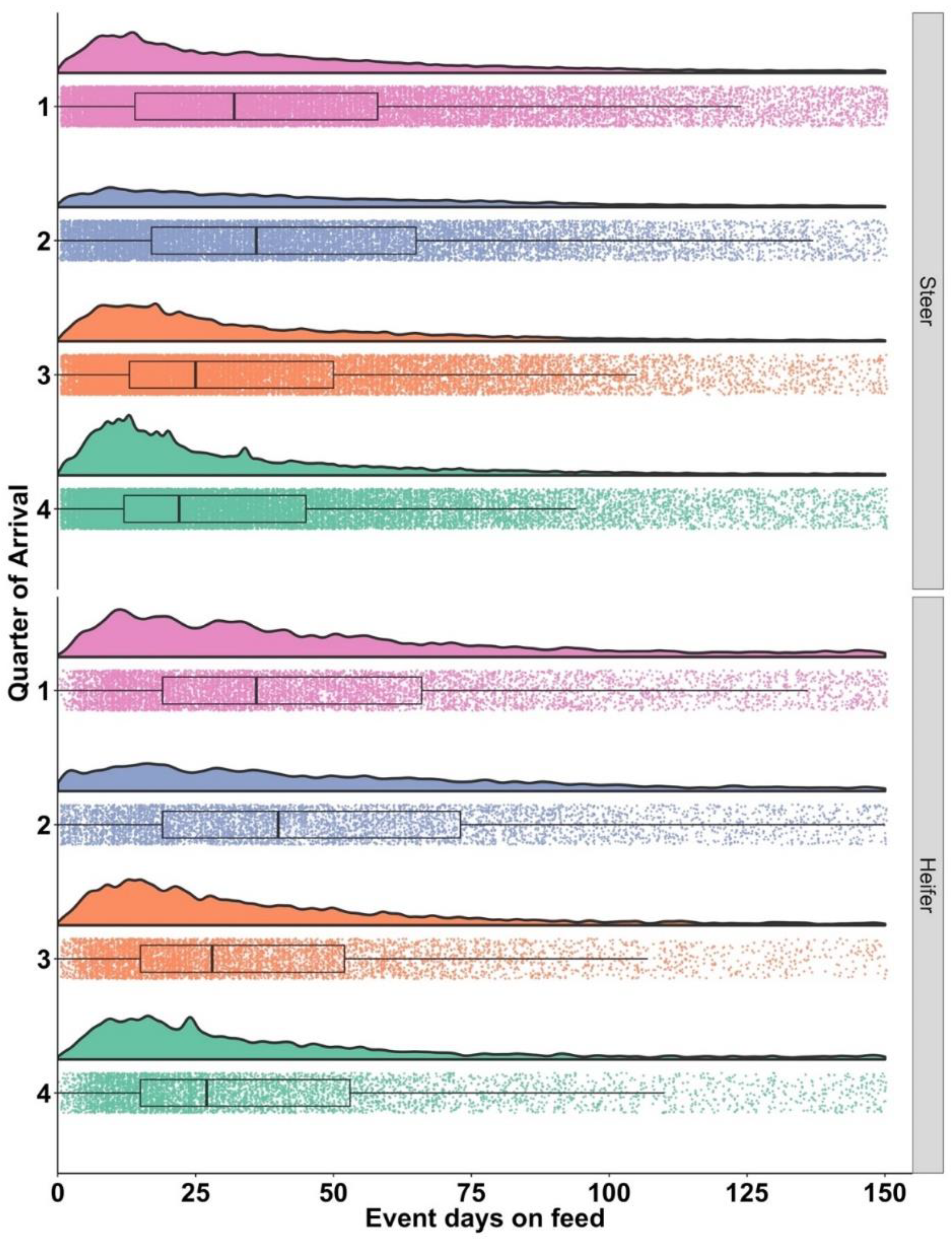
3.2. Days from Arrival to Initial BRD Treatment: Tx1
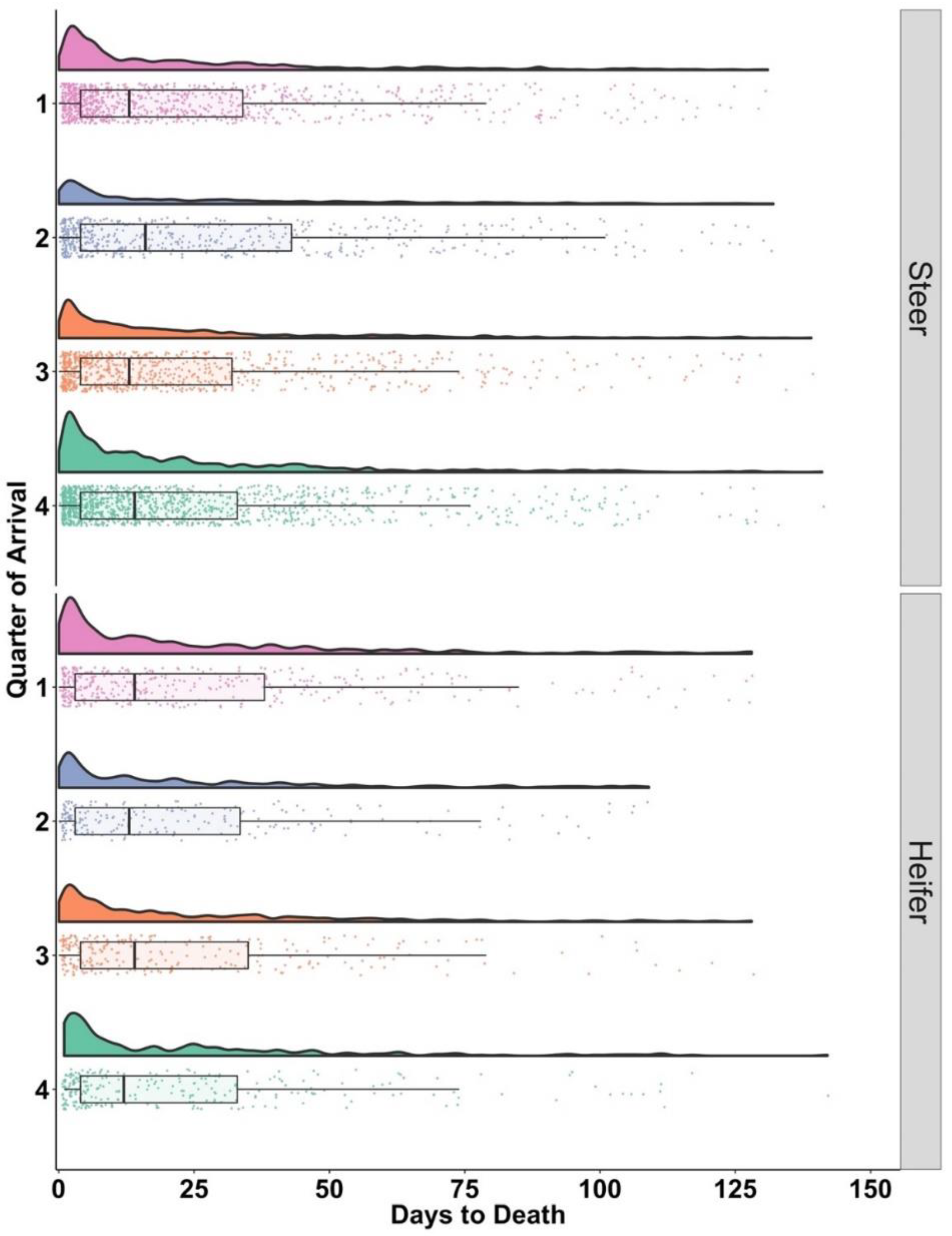
3.3. Days to Death following Initial BRD Treatment: DTD
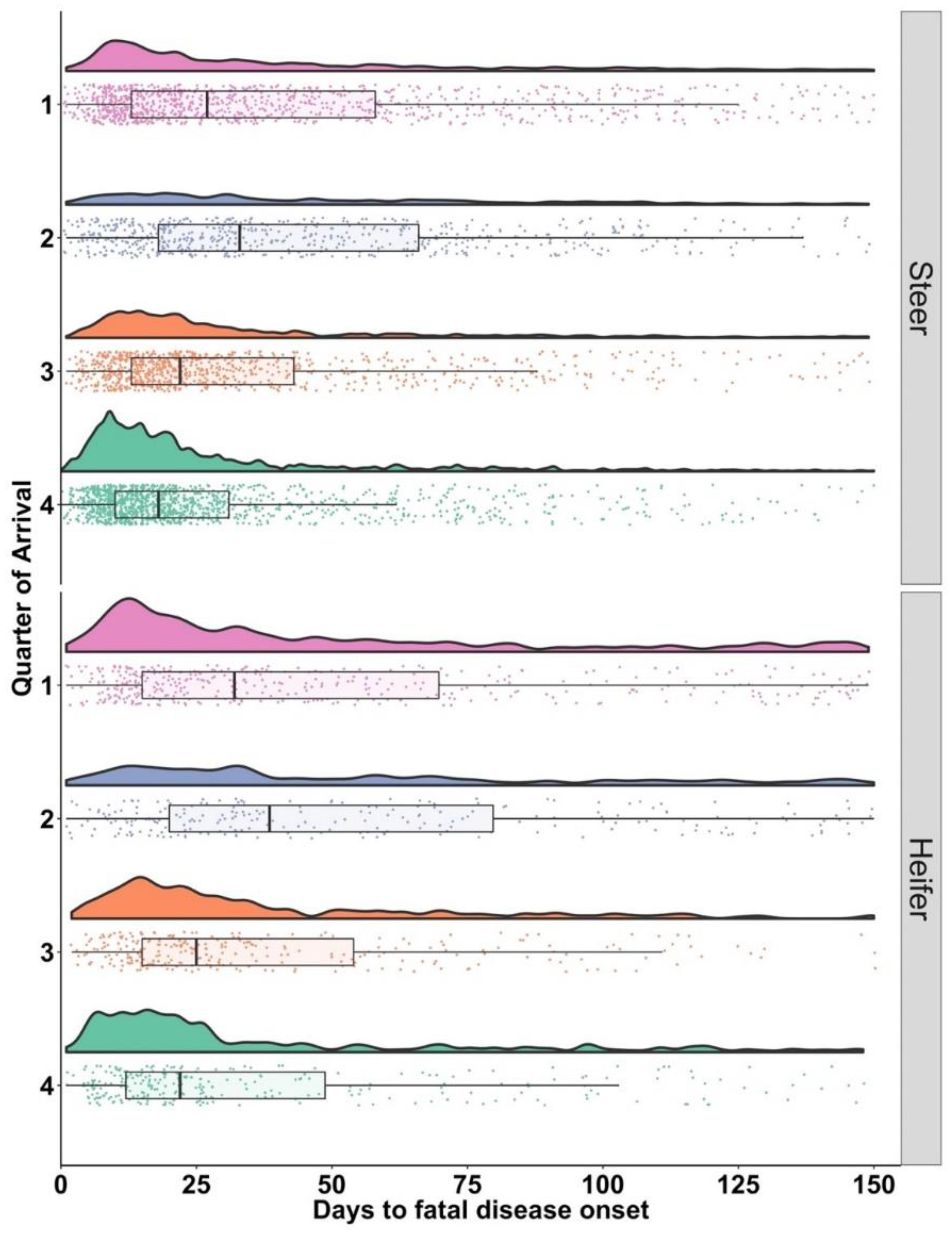
3.4. Onset of BRD in Cases with BRD Associated Fatalities (FDO)
3.5. Wasserstein Distances: Tx1, DTD, FDO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, R.A. North American Cattle Marketing and Bovine Respiratory Disease (BRD). Anim. Health Res. Rev. 2009, 10, 105–108. [Google Scholar] [CrossRef]
- Blakebrough-Hall, C.; McMeniman, J.P.; González, L.A. An Evaluation of the Economic Effects of Bovine Respiratory Disease on Animal Performance, Carcass Traits, and Economic Outcomes in Feedlot Cattle Defined Using Four BRD Diagnosis Methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.R.; Raper, K.C.; Ward, C.E.; Holland, B.P.; Krehbiel, C.R.; Step, D.L. Economic Effects of Bovine Respiratory Disease on Feedlot Cattle during Backgrounding and Finishing Phases. Prof. Anim. Sci. 2011, 27, 195–203. [Google Scholar] [CrossRef]
- Johnson, K.K.; Pendell, D.L. Market Impacts of Reducing the Prevalence of Bovine Respiratory Disease in United States Beef Cattle Feedlots. Front. Vet. Sci. 2017, 4, 189. [Google Scholar] [CrossRef] [PubMed]
- Booker, C.W. Bovine Respiratory Disease Treatment Failure: Definition and Impact. Anim. Health Res. Rev. 2020, 21, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Buczinski, S.; Ollivett, T.L.; Dendukuri, N. Bayesian Estimation of the Accuracy of the Calf Respiratory Scoring Chart and Ultrasonography for the Diagnosis of Bovine Respiratory Disease in Pre-Weaned Dairy Calves. Prev. Vet. Med. 2015, 119, 227–231. [Google Scholar] [CrossRef]
- Buczinski, S.; Forté, G.; Francoz, D.; Bélanger, A.-M. Comparison of Thoracic Auscultation, Clinical Score, and Ultrasonography as Indicators of Bovine Respiratory Disease in Preweaned Dairy Calves. J. Vet. Intern. Med. 2014, 28, 234–242. [Google Scholar] [CrossRef]
- Broadway, P.R.; Mauget, S.A.; Burdick Sanchez, N.C.; Carroll, J.A. Correlation of Ambient Temperature with Feedlot Cattle Morbidity and Mortality in the Texas Panhandle. Front. Vet. Sci. 2020, 7, 413. [Google Scholar] [CrossRef]
- Wisnieski, L.; Amrine, D.E.; Renter, D.G. Predictive Models for Weekly Cattle Mortality after Arrival at a Feeding Location Using Records, Weather, and Transport Data at Time of Purchase. Pathogens 2022, 11, 473. [Google Scholar] [CrossRef]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The Epidemiology of Bovine Respiratory Disease: What Is the Evidence for Predisposing Factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar]
- Theurer, M.E.; Johnson, M.D.; Fox, T.; McCarty, T.M.; McCollum, R.M.; Jones, T.M.; Alkire, D.O. Bovine Respiratory Disease during the Mid-Portion of the Feeding Period: Observations of Frequency, Timing, and Population from the Field. Appl. Anim. Sci. 2021, 37, 52–58. [Google Scholar] [CrossRef]
- Smith, K.J.; Amrine, D.E.; Larson, R.L.; Theurer, M.E.; White, B.J. Determining Relevant Risk Factors Associated with Mid- and Late-Feeding-Stage Bovine Respiratory Disease Morbidity in Cohorts of Beef Feedlot Cattle. Appl. Anim. Sci. 2022, 38, 373–379. [Google Scholar] [CrossRef]
- Thompson, P.N.; Stone, A.; Schultheiss, W.A. Use of Treatment Records and Lung Lesion Scoring to Estimate the Effect of Respiratory Disease on Growth during Early and Late Finishing Periods in South African Feedlot Cattle. J. Anim. Sci. 2006, 84, 488–498. [Google Scholar] [CrossRef]
- Babcock, A.H.; White, B.J.; Dritz, S.S.; Thomson, D.U.; Renter, D.G. Feedlot Health and Performance Effects Associated with the Timing of Respiratory Disease Treatment. J. Anim. Sci. 2009, 87, 314–327. [Google Scholar] [CrossRef]
- Vogel, G.J.; Bokenkroger, C.D.; Rutten-Ramos, S.C. A Retrospective Evaluation of Animal Mortality in US Feedlots: Rate, Timing, and Cause of Death. Am. Assoc. Bov. Pract. 2015, 49, 11. [Google Scholar]
- Sanderson, M.W.; Dargatz, D.A.; Wagner, B.A. Risk Factors for Initial Respiratory Disease in United States’ Feedlots Based on Producer-Collected Daily Morbidity Counts. Can. Vet. J. 2008, 49, 6. [Google Scholar]
- Booker, C.W.; Lubbers, B.V. Bovine Respiratory Disease Treatment Failure: Impact and Potential Causes. Vet. Clin. Food Anim. Pract. 2020, 36, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Aich, P.; Babiuk, L.A.; Potter, A.A.; Griebel, P. Biomarkers for Prediction of Bovine Respiratory Disease Outcome. Available online: https://www.liebertpub.com/doi/10.1089/omi.2009.0012 (accessed on 16 January 2023).
- Blakebrough-Hall, C.; Hick, P.; Mahony, T.J.; González, L.A. Factors Associated with Bovine Respiratory Disease Case Fatality in Feedlot Cattle. J. Anim. Sci. 2022, 100, skab361. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Schunicht, O.C.; Wildman, B.K.; Hannon, S.J.; Jim, G.K.; Ward, T.I.; Booker, C.W. Comparison of Enrofloxacin and Ceftiofur Sodium for the Treatment of Relapse of Undifferentiated Fever/Bovine Respiratory Disease in Feedlot Cattle. Can. Vet. J. 2012, 53, 57–62. [Google Scholar]
- Bateman, K.G.; Martin, S.W.; Shewen, P.E.; Menzies, P.I. An Evaluation of Antimicrobial Therapy for Undifferentiated Bovine Respiratory Disease. Can. Vet. J. 1990, 31, 689–696. [Google Scholar]
- Fulton, R.W.; Blood, K.S.; Panciera, R.J.; Payton, M.E.; Ridpath, J.F.; Confer, A.W.; Saliki, J.T.; Burge, L.T.; Welsh, R.D.; Johnson, B.J.; et al. Lung Pathology and Infectious Agents in Fatal Feedlot Pneumonias and Relationship with Mortality, Disease Onset, and Treatments. J. Vet. Diagn. Investig. 2009, 21, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Avra, T.D.; Abell, K.M.; Shane, D.D.; Theurer, M.E.; Larson, R.L.; White, B.J. A Retrospective Analysis of Risk Factors Associated with Bovine Respiratory Disease Treatment Failure in Feedlot Cattle. J. Anim. Sci. 2017, 95, 1521. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.; Defoor, P.; King, C.; Gleghorn, J. The Impact of Bovine Respiratory Disease: The Current Feedlot Experience. Anim. Health Res. Rev. 2014, 15, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.L.; Rezende, A.L.; Fonseca, P.D.A.; Massi, R.P.; Nogueira, G.M.; Magalhães, L.Q.; Headley, S.A.; Menezes, G.L.; Alfieri, A.A.; Saut, J.P.E. Bovine Respiratory Disease Complex Associated Mortality and Morbidity Rates in Feedlot Cattle from Southeastern Brazil. J. Infect. Dev. Ctries. 2017, 11, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Ives, S.E.; Richeson, J.T. Use of Antimicrobial Metaphylaxis for the Control of Bovine Respiratory Disease in High-Risk Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 341–350. [Google Scholar] [CrossRef]
- Corbin, M.J. Effects and Economic Implications of Metaphylactic Treatment of Feeder Cattle with Two Different Dosages of Tilmicosin on the Incidence of Bovine Respiratory Disease (BRD)—A Summary of Two Studies. Bov. Pract. 2009, 43, 140–152. [Google Scholar]
- Callan, R.J. Fundamental Considerations in Developing Vaccination Protocols. In American Association of Bovine Practitioners Conference Proceedings, Vancouver, BC, Canada, 13–15 September 2001; Frontier Printers, Inc.: Fort Collins, CO, USA, 2001; pp. 14–22. [Google Scholar]
- Munoz, V.I.; Samuelson, K.L.; Tomczak, D.J.; Seiver, H.A.; Smock, T.M.; Richeson, J.T. Comparative Efficacy of Metaphylaxis with Tulathromycin and Pentavalent Modified-Live Virus Vaccination in High-Risk, Newly Received Feedlot Cattle. Appl. Anim. Sci. 2020, 36, 799–807. [Google Scholar] [CrossRef]
- Ambs, A.; Moberly, H.; Capik, S. Delayed versus on Arrival Modified Live Viral Vaccination in Stocker Cattle on Bovine Respiratory Disease. Vet. Evid. 2022, 7. [Google Scholar] [CrossRef]
- Panaretos, V.M.; Zemel, Y. Statistical Aspects of Wasserstein Distances. Annu. Rev. Stat. Its Appl. 2019, 6, 405–431. [Google Scholar] [CrossRef]
- Rustamov, R.; Majumdar, S. Intrinsic Sliced Wasserstein Distances for Comparing Collections of Probability Distributions on Manifolds and Graphs. arXiv 2020, arXiv:2010.15285. [Google Scholar]
- Kolouri, S.; Park, S.; Thorpe, M.; Slepčev, D.; Rohde, G.K. Optimal Mass Transport: Signal Processing and Machine-Learning Applications. IEEE Signal Process. Mag. 2017, 34, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Hallin, M.; Mordant, G.; Segers, J. Multivariate Goodness-of-Fit Tests Based on Wasserstein Distance. Electron. J. Stat. 2021, 15. [Google Scholar] [CrossRef]
- Loneragan, G.H.; Gould, D.H.; Mason, G.L.; Garry, F.B.; Yost, G.S.; Lanza, D.L.; Miles, D.G.; Hoffman, B.W.; Mills, L.J. Association of 3-Methyleneindolenine, a Toxic Metabolite of 3-Methylindole, with Acute Interstitial Pneumonia in Feedlot Cattle. Am. J. Vet. Res. 2001, 62, 1525–1530. [Google Scholar] [CrossRef]
- Woolums, A.R.; Loneragan, G.H.; Hawkins, L.L.; Williams, S.M. A Survey of the Relationship between Management Practices and Risk of Acute Interstitial Pneumonia at US Feedlots. Bov. Pract. 2005, 32, 125–133. [Google Scholar]
- Timsit, E.; Dendukuri, N.; Schiller, I.; Buczinski, S. Diagnostic Accuracy of Clinical Illness for Bovine Respiratory Disease (BRD) Diagnosis in Beef Cattle Placed in Feedlots: A Systematic Literature Review and Hierarchical Bayesian Latent-Class Meta-Analysis. Prev. Vet. Med. 2016, 135, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Babcock, A.H.; Cernicchiaro, N.; White, B.J.; Dubnicka, S.R.; Thomson, D.U.; Ives, S.E.; Scott, H.M.; Milliken, G.A.; Renter, D.G. A Multivariable Assessment Quantifying Effects of Cohort-Level Factors Associated with Combined Mortality and Culling Risk in Cohorts of U.S. Commercial Feedlot Cattle. Prev. Vet. Med. 2013, 108, 38–46. [Google Scholar] [CrossRef]
| Characteristic | Tx1 (DOF) | ||
|---|---|---|---|
| Quarter of Arrival | Quartiles | ||
| Steers | n | 25% | 75% |
| 1 | 22,678 | 14 | 58 |
| 2 | 13,946 | 17 | 65 |
| 3 | 19,342 | 13 | 50 |
| 4 | 23,567 | 12 | 45 |
| Heifers | |||
| 1 | 7223 | 19 | 66 |
| 2 | 5224 | 19 | 73 |
| 3 | 5627 | 15 | 52 |
| 4 | 5204 | 15 | 53 |
| Characteristic | DTD (DOF) | ||
|---|---|---|---|
| Quarter of Arrival | Quartiles | ||
| Steers | n | 25% | 75% |
| 1 | 927 | 4 | 34 |
| 2 | 557 | 4 | 43 |
| 3 | 806 | 4 | 32 |
| 4 | 1257 | 4 | 33 |
| Heifers | |||
| 1 | 307 | 3 | 38 |
| 2 | 215 | 3 | 34 |
| 3 | 261 | 4 | 35 |
| 4 | 270 | 4 | 33 |
| Characteristic | FDO (DOF) | ||
|---|---|---|---|
| Quarter of Arrival | Quartiles | ||
| Steers | n | 25% | 75% |
| 1 | 1009 | 13 | 58 |
| 2 | 605 | 18 | 66 |
| 3 | 847 | 13 | 43 |
| 4 | 1309 | 10 | 31 |
| Heifers | |||
| 1 | 398 | 15 | 70 |
| 2 | 232 | 20 | 80 |
| 3 | 275 | 15 | 54 |
| 4 | 284 | 12 | 49 |
| Quarter of Comparison | Wasserstein Distance | ||
|---|---|---|---|
| First Treatment (Tx1) | Days to Death (DTD) | Days to Fatal Disease Onset (FDO) | |
| Steers | |||
| 1–2 | 3.63 | 6.96 | 4.99 |
| 1–3 | 6.06 | 6.41 | 6.46 |
| 1–4 | 7.78 | 12.00 | 12.88 |
| 2–3 | 9.56 | 13.21 | 10.73 |
| 2–4 | 11.29 | 18.90 | 17.20 |
| 3–4 | 9.56 | 5.70 | 6.56 |
| Heifers | |||
| 1–2 | 3.10 | 6.38 | 6.90 |
| 1–3 | 8.72 | 9.41 | 9.30 |
| 1–4 | 7.39 | 12.05 | 10.40 |
| 2–3 | 11.42 | 14.93 | 15.94 |
| 2–4 | 10.13 | 17.74 | 17.18 |
| 3–4 | 1.78 | 4.53 | 4.10 |
| Combined | |||
| 1–2 | 3.57 | 6.67 | 5.29 |
| 1–3 | 6.75 | 7.52 | 7.40 |
| 1–4 | 8.05 | 13.00 | 13.19 |
| 2–3 | 10.21 | 13.88 | 12.30 |
| 2–4 | 11.51 | 19.45 | 18.15 |
| 3–4 | 2.15 | 5.58 | 5.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, K.J.; White, B.J.; Amrine, D.E.; Larson, R.L.; Theurer, M.E.; Szasz, J.I.; Bryant, T.C.; Waggoner, J.W. Evaluation of First Treatment Timing, Fatal Disease Onset, and Days from First Treatment to Death Associated with Bovine Respiratory Disease in Feedlot Cattle. Vet. Sci. 2023, 10, 204. https://doi.org/10.3390/vetsci10030204
Smith KJ, White BJ, Amrine DE, Larson RL, Theurer ME, Szasz JI, Bryant TC, Waggoner JW. Evaluation of First Treatment Timing, Fatal Disease Onset, and Days from First Treatment to Death Associated with Bovine Respiratory Disease in Feedlot Cattle. Veterinary Sciences. 2023; 10(3):204. https://doi.org/10.3390/vetsci10030204
Chicago/Turabian StyleSmith, Kristen J., Brad J. White, David E. Amrine, Robert L. Larson, Miles E. Theurer, Josh I. Szasz, Tony C. Bryant, and Justin W. Waggoner. 2023. "Evaluation of First Treatment Timing, Fatal Disease Onset, and Days from First Treatment to Death Associated with Bovine Respiratory Disease in Feedlot Cattle" Veterinary Sciences 10, no. 3: 204. https://doi.org/10.3390/vetsci10030204
APA StyleSmith, K. J., White, B. J., Amrine, D. E., Larson, R. L., Theurer, M. E., Szasz, J. I., Bryant, T. C., & Waggoner, J. W. (2023). Evaluation of First Treatment Timing, Fatal Disease Onset, and Days from First Treatment to Death Associated with Bovine Respiratory Disease in Feedlot Cattle. Veterinary Sciences, 10(3), 204. https://doi.org/10.3390/vetsci10030204







