Epidemiologic Factors Supporting Triage of Infected Dog Patients Admitted to a Veterinary Hospital Biological Isolation and Containment Unit
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Isolation and Containment Unit under Study
2.2. Settings
2.3. Participants
2.4. Variables and Measurement
2.5. Bias
2.6. Statistical Methods
3. Results
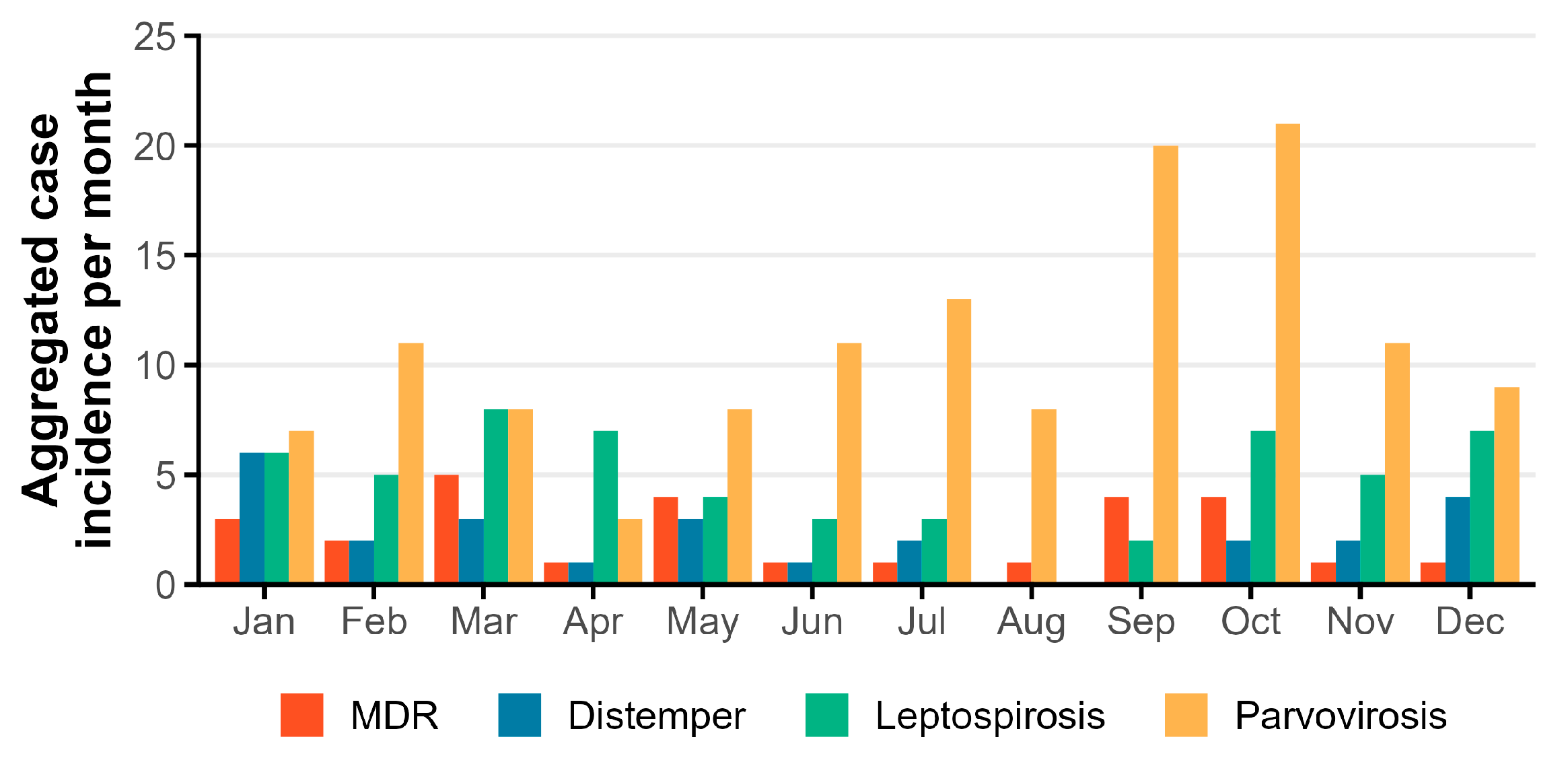
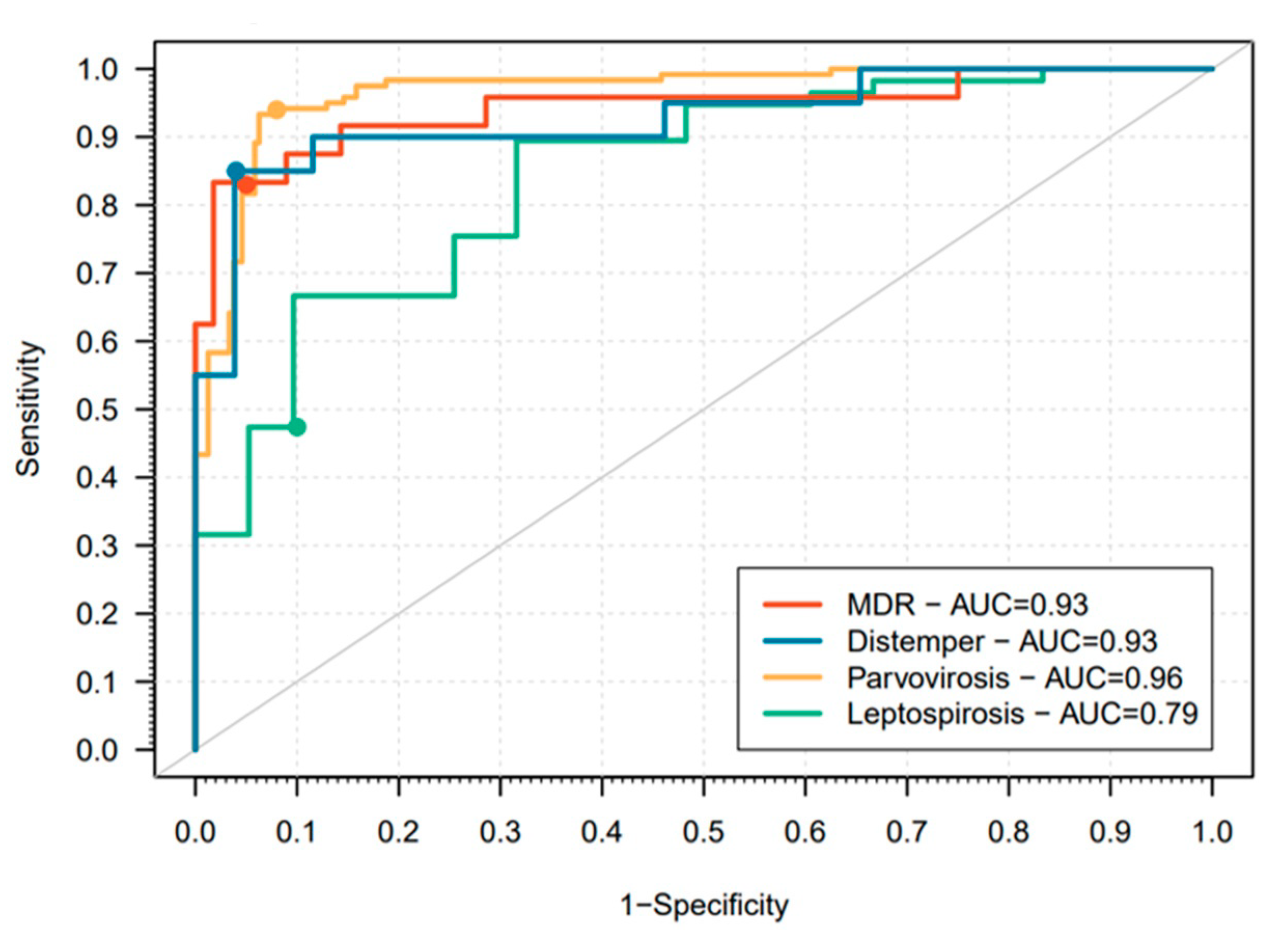
3.1. Analysis of Dogs with a Confirmed Diagnosis of an Infectious Disease
3.2. Analysis of Dogs with a Confirmed Parvovirosis Diagnosis
3.3. Analysis of Dogs with a Confirmed Leptospirosis Diagnosis
3.4. Analysis of Dogs with a Confirmed MDR Infection Diagnosis
3.5. Analysis of Dogs with a Confirmed Distemper Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TH | Teaching hospital |
| BICU | Biological Isolation and Containment Unit |
| ID | Infectious disease |
| SOP | Standard operating procedures |
| PPE | Personal protective equipment |
| IU | Isolation unit (IU) |
| MDR | Multidrug-resistant bacteria |
| CPV-2 | Canine parvovirus type 2 |
| HEPA | High-efficiency particulate air |
| WASAVA | World Small Animal Veterinary Association |
| PCR | Polymerase Chain Reaction |
| CDV | Canine distemper virus |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| UTI | Urinary tract infection |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
Appendix A
| Disease | Parvovirosis | Leptospirosis | MDR | Distemper |
|---|---|---|---|---|
| Variable | p-value Estimate Std error | p-value Estimate Std error | p-value Estimate Std error | p-value Estimate Std error |
| Age Group | ||||
| <2 years | <0.001 4.44 0.46 | - | - | <0.001 2.05 0.62 |
| ≥2 to and <10 years | - | 0.02 1.03 0.44 | <0.001 3.2 0.84 | - |
| ≥10 years | 0.21 −1.38 1.09 | 0.04 −1.46 0.71 0.2 (0.05–0.9) | <0.001 4.05 0.89 | 0.20 −1.06 0.84 |
| Sex | ||||
| Female | - | - | - | - |
| Male | 0.57 0.12 0.22 | 0.91 −0.04 0.32 | 0.21 0.6 0.49 | 0.14 0.73 0.51 |
| Neuter Status | ||||
| No | - | - | - | - |
| Yes | <0.001 −3.31 0.73 | 0.32 −0.38 0.39 | 0.12 0.89 0.57 | 0.01 −2.58 1.06 |
| Vaccination Status Updated | ||||
| Yes | - | - | - | - |
| No | <0.001 4.05 1.01 | 0.90 0.05 0.37 | <0.001 −2.12 0.59 | 0.14 1.01 0.69 |
| Breed | ||||
| Breed | - | - | - | - |
| Mixed breed | 0.15 0.32 0.22 | 0.82 0.07 0.33 | 0.11 −81 0.51 | 0.05 0.97 0.50 |
| Concomitant Disorders | ||||
| No | - | - | - | - |
| Yes | <0.001 −1.64 0.23 0.2 (0.1–0.3) | <0.001 −1.48 0.34 | <0.001 3.04 0.68 | <0.001 −1.63 0.52 |
| Season | ||||
| Cold (November-April) | - | - | - | - |
| Warm (May-October) | <0.01 0.60 0.22 | 0.04 −0.68 0.33 | 0.88 −0.07 0.46 | 0.19 −0.66 0.51 |
| n | % | |
|---|---|---|
| Ecoli (n = 13; 48.1%) | ||
| Dermatitis | 2 | 7.4% |
| Lymphadenitis | 1 | 3.7% |
| Osteomyelitis | 1 | 3.7% |
| Peritonitis | 1 | 3.7% |
| Rhinitis | 1 | 3.7% |
| UTI | 7 | 25.9% |
| Enterococcus (n = 2; 7.4%) | ||
| Dermatitis | 1 | 3.7% |
| UTI | 1 | 3.7% |
| Klebsiella (n = 3; 11.1%) | ||
| Osteomyelitis | 1 | 3.7% |
| Otitis | 1 | 3.7% |
| Wound | 1 | 3.7% |
| MRSA (n = 1; 3.7%) | ||
| Dermatitis | 1 | 3.7% |
| MRSP (n = 7; 25.9%) | ||
| Dermatitis | 3 | 11.1% |
| Otitis | 3 | 11.1% |
| Wound | 1 | 3.7% |
| Proteus (n = 1; 3.7%) | ||
| Wound | 1 | 3.7% |
| Total | 27 | 100% |
References
- Benedict, K.M.; Morley, P.S.; van Metre, D.C. Characteristics of Biosecurity and Infection Control Programs at Veterinary Teaching Hospitals. J. Am. Vet. Med. Assoc. 2008, 233, 767–773. [Google Scholar] [CrossRef]
- Stull, J.W.; Bjorvik, E.; Bub, J.; Dvorak, G.; Petersen, C.; Troyer, H.L. 2018 AAHA Infection Control, Prevention, and Biosecurity Guidelines. J. Am. Anim. Hosp. Assoc. 2018, 54, 297–326. [Google Scholar] [CrossRef]
- CCAR Infection Prevention and Control Best Practices for Small Animal Veterinary Clinics; University of Guelph: Guelph, ON, Canada, 2008; Available online: http://scholar.google.com/scholar?q=related:A8CJDQjJQAMJ:scholar.google.com/&hl=en&num=20&as_sdt=0,5%5Cnp (accessed on 20 January 2020).
- Williams, C.J.; Scheftel, J.M.; Elchos, B.L.; Hopkins, S.G.; Levine, J.F. Compendium of Veterinary Standard Precautions for Zoonotic Disease Prevention in Veterinary Personnel: National Association of State Public Health Veterinarians: Veterinary Infection Control Committee 2015. J. Am. Vet. Med. Assoc. 2015, 247, 1252–1277. [Google Scholar] [CrossRef]
- Portner, J.A.; Johnson, J.A. Guidelines for Reducing Pathogens in Veterinary Hospitals: Hospital Design and Special Considerations. Compend. Contin. Educ. Vet. 2010, 32, E1–E8. Available online: https://www.vetfolio.com/learn/article/guideli (accessed on 19 January 2020).
- Siegel, J.; Rhinehart, E.; Jackson, M.; Chiarello, L.; Infection, T.H.; Advisory, C.C.P. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Available online: http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf%0A1 (accessed on 22 January 2020).
- Evermann, J.F.; Sellon, R.K.; Sykes, J.E. Laboratory Diagnosis of Viral and Rickettsial Infections and Clinical Epidemiology of Infectious Disease. In Infectious Diseases of the Dog and Cat, 4th ed.; Greene, C.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 1–9. [Google Scholar]
- Goddard, A.; Leisewitz, A.L. Canine Parvovirus Canine Parvovirus. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 1041–1053. [Google Scholar] [CrossRef]
- Carmichael, L.E. An Annotated Historical Account of Canine Parvovirus. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2005, 52, 303–311. [Google Scholar] [CrossRef]
- Reagan, K.L.; Sykes, J.E. Diagnosis of Canine Leptospirosis. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 719–731. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Sykes, J.E. Canine Distemper Virus Infection. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2014; pp. 152–165. [Google Scholar]
- Guptill, L. Patient Management. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 277–298. [Google Scholar] [CrossRef]
- Paulo, C.; Machado, I.; Carvalho, H.; Gomes, J.; Mota, A.D.; Tavares, L.; Almeida, V.; Gil, S. A 5-Year Retrospective Study of Canine and Feline Patients Referred to an Isolation Unit for Infectious Diseases. Vet. Rec. Open 2021, 8, e5. [Google Scholar] [CrossRef]
- Lapsley, J.; Hayes, G.M.; Sumner, J.P. Performance Evaluation and Validation of the Animal Trauma Triage Score and Modified Glasgow Coma Scale in Injured Cats: A Veterinary Committee on Trauma Registry Study. J. Vet. Emerg. Crit. Care 2019, 29, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.E.; Streeter, E.M.; DeCook, R.R. Review of Gunshot Injuries in Cats and Dogs and Utility of a Triage Scoring System to Predict Short-Term Outcome: 37 Cases (2003–2008). J. Am. Vet. Med. Assoc. 2014, 245, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Horzinek, M.C.; Schultz, R.D.; Squires, R.A. WSAVA Guidelines for the Vaccination of Dogs and Cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef]
- Mottola, C.; Alho, A.M.; Gonçalves, T.; Seixas, R. Leptospirosis in Portugal: Current Status and Importance of Control Measures in the Context of Public Health. Revista Electronica de Veterinaria. 2015. Available online: https://www.researchgate.net/publication/281770244_Leptospirosis_in_Portugal_Current_status_and_importance_of_control_measures_in_the_context_of_Public_Health (accessed on 15 September 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 15 November 2022).
- Hosmer, D.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons: Danvers, MA, USA, 2000. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- López-Ratón, M.; Xosé, M.; Alvarez, R.; Cadarso-Suárez, C.; Gude-Sampedro, F. OptimalCutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Miranda, C.; Parrish, C.R.; Thompson, G. Epidemiological Evolution of Canine Parvovirus in the Portuguese Domestic Dog Population. Vet. Microbiol. 2016, 183, 37–42. [Google Scholar] [CrossRef]
- Sykes, J.E. Canine Parvovirus Infections and Other Viral Enteritides. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2014; pp. 141–151. [Google Scholar]
- Hartskeerl, R.A.; Collares-Pereira, M.; Ellis, W.A. Emergence, Control and Re-Emerging Leptospirosis: Dynamics of Infection in the Changing World. Clin. Microbiol. Infect. 2011, 17, 494–501. [Google Scholar] [CrossRef]
- Sykes, J.E.; Rankin, S.C. Isolation and Identification of Aerobic and Anaerobic Bacteria. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2014; pp. 17–28. [Google Scholar]
- São João, T.M.P.L. Caracterização de Quadros Clínicos de Esgana Caninca No Surto Epidémico 2015–2018 Na Área Metropolitana de Lisboa. Dissertação de Mestrado, Universidade de Lisboa, Faculdade de Medicina Veterinária, Lisboa, Portugal, 2019. Available online: http://hdl.handle.net/10400.5/18253 (accessed on 18 September 2021).
- Machado, I. Frequência de Doenças Infeciosas Em Carnívoros Domésticos Hospitalizados Na Unidade Isolamento Do Hospital Escolar Da Faculdade de Medicina Veterinária Da Universidade de Lisboa de Outubro de 2013 a Janeiro de 2016. Dissertação de Mestrado, Universidade de Lisboa, Faculdade de Medicina Veterinária, Lisboa, Portugal, 2016. Available online: http://hdl.handle.net/10400.5/12511 (accessed on 20 September 2021).
- São João, T.; Machado, I.C.; Diogo, R.C.; Gomes, J.F.; Tavares, L.M.; Gil, S.A.; Almeida, V.S. Characterization of Canine Distemper Clinical Presentations during the Epidemic in Lisbon Metropolitan Area, Portugal Caracterização de Quadros Clínicos de Esgana Durante o Surto Registado Entre 2014 e 2018 Na Área Metropolitana de Lisboa, Portugal. Rev. Port. De Ciências Veterinárias 2021, 116, 15–24. [Google Scholar]
- Greene, C.; Decaro, N. Canine Viral Enteritis. In Infectious Diseases of the Dog and Cat, 4th ed.; Greene, C.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 67–80. ISBN 9781455754700. [Google Scholar]
- Greene, C.; Vandevelde, M. Canine Distemper. In Infectious Diseases of the Dog and Cat, 4th ed.; Greene, C.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 25–42. ISBN 9781455754700. [Google Scholar]
- Kim, E.; Choe, C.; Yoo, J.G.; Oh, S.I.; Jung, Y.; Cho, A.; Kim, S.; Do, Y.J. Major Medical Causes by Breed and Life Stage for Dogs Presented at Veterinary Clinics in the Republic of Korea: A Survey of Electronic Medical Records. Peer. J. 2018, 2018, 5161. [Google Scholar] [CrossRef]
- Wensley, S. Survey Suggests Many Pets Do Not Receive Preventive Healthcare. Vet. Rec. 2013, 172, 569. [Google Scholar] [CrossRef]
- IPMA. Portuguese Institute for Sea and Atmosphere. Climate Normals. Available online: https://www.ipma.pt/en/oclima/normais.clima/1971-2000/#535 (accessed on 7 April 2021).
- Jiang, F. Bioclimatic and Altitudinal Variables Influence the Potential Distribution of Canine Parvovirus Type 2 Worldwide. Ecol. Evol. 2018, 8, 4534–4543. [Google Scholar] [CrossRef]
- Kalli, I.; Leontides, S.; Adamama-Moraitou, K.; Rallis, T.; Koutinas, A.F. Factors Affecting the Occurrence, Duration of Hospitalization and Final Outcome in Canine Parvovirus Infection. Res. Vet. Sci. 2010, 89, 174–178. [Google Scholar] [CrossRef]
- Horecka, K.; Porter, S.; Susan Amirian, E.; Jefferson, E. A Decade of Treatment of Canine Parvovirus in an Animal Shelter: A Retrospective Study. Animals 2020, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.; Kalli, I.; Rallis, T. Canine Parvoviral Enteritis: An Update on the Clinical Diagnosis, Treatment, and Prevention. Vet. Med. Res. Rep. 2016, 7, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Savigny, M.R.; Macintire, D.K. Use of Oseltamivir in the Treatment of Canine Parvoviral Enteritis. J. Vet. Emerg. Crit. Care 2010, 20, 132–142. [Google Scholar] [CrossRef]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.E.; Sykes, J. European Consensus Statement on Leptospirosis in Dogs and Cats. J. Small Anim. Pract. 2015, 56, 159–179. [Google Scholar] [CrossRef]
- Major, A.; Schweighauser, A.; Francey, T. Increasing Incidence of Canine Leptospirosis in Switzerland. Int. J. Environ. Res. Public Health 2014, 11, 7242–7260. [Google Scholar] [CrossRef]
- Sykes, J.E. Leptospirosis. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2014; pp. 474–486. [Google Scholar]
- Goldstein, R.E.; Greene, C.E.; Moore, G.E.; Schultz, R.D.; Sykes, J.E. Leptospirosis. In Infectious Diseases of the Dog and Cat, 4th ed.; Greene, C.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 431–447. [Google Scholar]
- Duarte, R.B.V.da.S. Contributo Para o Estudo Da Leptospirose Canina Na Grande Área Metropolitana de Lisboa. Dissertação de Mestrado, Universidade de Lisboa, Faculdade de Medicina Veterinária, Lisboa, Portugal, 2015. Available online: http://hdl.handle.net/10400.5/10098 (accessed on 16 September 2021).
- Ward, M.P.; Guptill, L.F.; Prahl, A.; Ching Wu, C. Serovar-Specific Prevalence and Risk Factors for Leptospirosis among Dogs: 90 Cases (1997–2002). J. Am. Vet. Med. Assoc. 2004, 224, 1958–1963. [Google Scholar] [CrossRef]
- Knöpfler, S.; Mayer-Scholl, A.; Luge, E.; Klopfleisch, R.; Gruber, A.D.; Nöckler, K.; Kohn, B. Evaluation of Clinical, Laboratory, Imaging Findings and Outcome in 99 Dogs with Leptospirosis. J. Small Anim. Pract. 2017, 58, 582–588. [Google Scholar] [CrossRef]
- Birnbaum, N.; Barr, S.C.; Center, S.A.; Schermerhorn, T.; Randolph, J.F.; Simpson, K.W. Naturally Acquired Leptospirosis in 36 Dogs: Serological and Clinicopathological Features. J. Small Anim. Pract. 1998, 39, 231–236. [Google Scholar] [CrossRef]
- Boey, K.; Shiokawa, K.; Rajeev, S. Leptospira Infection in Rats: A Literature Review of Global Prevalence and Distribution. PLoS Negl. Trop Dis. 2019, 13, e0007499. [Google Scholar] [CrossRef]
- Auffray, J.C.; Renaud, S.; Claude, J. Rodent Biodiversity Human Health and Pest Control in a Changing Environments rodent Biodiversity in Changing Environments. Kasetsart J. Nat. Sci. 2009, 43, 83–93. [Google Scholar]
- Adin, C.A.; Cowgill, L.D. Treatment and Outcome of Dogs with Leptospirosis: 36 Cases (1990–1998). J. Am. Vet. Med. Assoc. 2000, 216, 371–375. [Google Scholar] [CrossRef]
- Qekwana, D.N.; Phophi, L.; Naidoo, V.; Oguttu, J.W.; Odoi, A. Antimicrobial Resistance among Escherichia Coli Isolates from Dogs Presented with Urinary Tract Infections at a Veterinary Teaching Hospital in South Africa. BMC Vet. Res. 2018, 14, 228. [Google Scholar] [CrossRef]
- Gibson, J.S.; Morton, J.M.; Cobbold, R.N.; Sidjabat, H.E.; Filippich, L.J.; Trott, D.J. Multidrug-Resistant E. Coli and Enterobacter Extraintestinal Infection in 37 Dogs. J. Vet. Intern. Med. 2008, 22, 844–850. [Google Scholar] [CrossRef]
- Tenney, J.; Hudson, N.; Alnifaidy, H.; Li, J.T.C.; Fung, K.H. Risk Factors for Aquiring Multidrug-Resistant Organisms in Urinary Tract Infections: A Systematic Literature Review. Saudi. Pharm. J. 2018, 26, 678–684. [Google Scholar] [CrossRef]
- La Fauci, V.; Alessi, V. Antibiotic Resistance: Where Are We Going? Ann. Di. Ig. 2018, 30, 52–57. [Google Scholar] [CrossRef]
- Tuerena, I.; Williams, N.J.; Nuttall, T.; Pinchbeck, G. Antimicrobial-Resistant Escherichia Coli in Hospitalised Companion Animals and Their Hospital Environment. J. Small Anim. Pract. 2016, 57, 339–347. [Google Scholar] [CrossRef]
- Worthing, K.A.; Brown, J.; Gerber, L.; Trott, D.J.; Abraham, S.; Norris, J.M. Methicillin-Resistant Staphylococci amongst Veterinary Personnel, Personnel-Owned Pets, Patients and the Hospital Environment of Two Small Animal Veterinary Hospitals. Vet. Microbiol. 2018, 223, 79–85. [Google Scholar] [CrossRef]
- Sehulster, L.M.; Chinn, R.Y.W.; Arduino, M.J.; Carpenter, J.; Donlan, R.; Ashford, D.; Besser, R.; Fields, B.; McNeil, M.M.; Whitney, C.; et al. Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Am. Soc. Healthc. Eng. Am. Hosp. Assoc. 2004, 1, 15–17. Available online: https://www.cdc.gov/infectioncontrol/guidelines/en (accessed on 18 January 2022).
- Mahajan, S.; Dey, S.; Kumar, A.; Panigrahi, P.; Karunanithy, M. Association and Risk of Canine Distemper with Respect to Age, Sex and Breed of Dogs Suffering from Demyelinating Neuropathies. Int. J. Livest. Res. 2018, 8, 164–171. [Google Scholar] [CrossRef]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and Immunopathology of Systemic and Nervous Canine Distemper. Vet. Immunol. Immunopathol. 2009, 127, 23. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and Molecular Pathogenesis of Canine Distemper Virus. Virol. J. 2019, 16, 1–12. [Google Scholar] [CrossRef]
- Arjo, W.M.; Gese, E.M.; Bromley, C.; Kozlowski, A.; Williams, E.S. Serologic Survey for Diseases in Free-Ranging Coyotes (Canis Latrans) from Two Ecologically Distinct Areas of Utah. J. Wildl. Dis. 2003, 39, 449–455. [Google Scholar] [CrossRef]
- Headley, S.A.; Graça, D.L. Canine Distemper: Epidemiological Findings of 250 Cases. Braz. J. Vet. Res. Anim. Sci. 2000, 37, 136–140. [Google Scholar] [CrossRef]
- Carella, E.; Orusa, T.; Viani, A.; Meloni, D.; Borgogno-mondino, E.; Orusa, R. An Integrated, Tentative Remote-Sensing Approach Based on NDVI Entropy to Model Canine Distemper Virus in Wildlife and to Prompt Science-Based Management Policies. Animals 2022, 12, 1049. [Google Scholar] [CrossRef]
- Cleaveland, S.; Appel, M.G.J.; Chalmers, W.S.K.; Chillingworth, C.; Kaare, M.; Dye, C. Serological and Demographic Evidence for Domestic Dogs as a Source of Canine Distemper Virus Infection for Serengeti Wildlife. Vet. Microbiol. 2000, 72, 217–227. [Google Scholar] [CrossRef]
- Hiebl, A.; Auer, A.; Bagrinovschi, G.; Stejskal, M.; Hirt, R.; Rümenapf, H.T.; Tichy, A.; Künzel, F. Detection of Selected Viral Pathogens in Dogs with Canine Infectious Respiratory Disease in Austria. J. Small Anim. Pract. 2019, 60, 594–600. [Google Scholar] [CrossRef]
- Blixenkrone-Møller, M.; Svansson, V.; Have, P.; Örvell, C.; Appel, M.; Rode Pedersen, I.; Henrik Dietz, H.; Henriksen, P. Studies on Manifestations of Canine Distemper Virus Infection in an Urban Dog Population. Vet. Microbiol. 1993, 37, 163–173. [Google Scholar] [CrossRef]
- Carvalho, O.V.; Botelho, C.V.; Ferreira, C.G.T.; Scherer, P.O.; Soares-Martins, J.A.P.; Almeida, M.R.; Silva Júnior, A. Immunopathogenic and Neurological Mechanisms of Canine Distemper Virus. Adv. Virol. 2012, 2012, 163860. [Google Scholar] [CrossRef]
- Kapil, S.; Yeary, T.J. Canine Distemper Spillover in Domestic Dogs from Urban Wildlife. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, V.J. Canine Distemper. In Drug Therapy for Infectious Diseases of the Dog and Cat; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 64–65. ISBN 9781118557341. [Google Scholar]
| Disease | Tests |
|---|---|
| Parvovirosis (n = 130) | Rapid immunomigration test (n = 62) 1 Real-time PCR (RT-qPCR) (n = 68) |
| Leptospirosis (n = 57) | Single dosage IgM measure by semi-quantitative indirect immunofluorescence for IgM detection (n = 54) 2 PCR on Blood/Urine (n = 3) |
| MDR (n = 28) | Bacterial culture and antibiotic susceptibility testing from the adequate tissue (urine, skin, effusion liquid, infected wounds, and others, n = 28) |
| Distemper (n = 26) | Serology for antibody IgM/IgG detection (n = 19) 3 Real time PCR (RT-qPCR) from oronasal, rectal, cerebrospinal fluid or blood sample (n = 6) Rapid immunomigration test (n = 1) 4 |
| Parameter | Category | All Infectious (n = 263) | Parvovirosis (n = 130) | Controls (n = 260) | Leptospirosis (n = 57) | Controls (n = 114) | MDR (n = 28) | Controls (n = 56) | Distemper (n = 26) | Controls (n = 52) |
|---|---|---|---|---|---|---|---|---|---|---|
| Median Age [range] Q1; Q3 | 0.8 [0.1–18] 0.3; 6.0 | 0.3 [0.1–14] 0.2; 0.48 | 8.0 [0.1–17] 4.0; 11.0 | 6 [0.2–15] 3.0; 8.0 | 6 [0.1–15] 2.0; 10.75 | 9.5 [0.4–16] 7.0; 12.0 | 0.6 [0.1–14] 0.2; 2.5 | 1 [0.2–15] 0.4; 4.75 | 8 [0.6–15] 5.0; 11.0 | |
| Age group (years) n(%) | <2 | 156 (59.3) | 123 (94.6) | 33 (12.7) | 9 (15.8) | 27 (23.7) | 2 (7.1) | 41 (73.2) | 15 (57.7) | 6 (11.5) |
| ≥2 and <10 | 81 (30.8) | 6 (4.6) | 137 (52.7) | 45 (78.9) | 48 (42.1) | 12 (42.9) | 10 (17.9) | 9 (34.6) | 28 (53.8) | |
| ≥10 | 26 (9.9) | 1 (0.8) | 90 (34.6) | 3 (5.3) | 39 (43.2) | 14 (50.0) | 5 (8.9) | 2 (7.7) | 18 (34.6) | |
| Sex n(%) | Female | 112 (42.6) | 56 (43.1) | 120 (46.2) | 28 (49.1) | 55 (48.2) | 9 (32.1) | 26 (46.4) | 8 (30.8) | 25 (48.1) |
| Male | 151 (57.4) | 74 (56.9) | 140 (53.8) | 29 (50.9) | 59 (51.8) | 19 (67.9) | 30 (53.6) | 18 (69.2) | 27 (51.9) | |
| Neuter Status n(%) | No | 236 (89.7) | 128 (98.5) | 182 (70) | 45 (78.9) | 82 (71.9) | 20 (71.4) | 48 (85.7) | 25 (96.2) | 34 (65.4) |
| Yes | 27 (10.3) | 2 (1.5) | 78 (30) | 12 (21.1) | 32 (28.1) | 8 (28.6) | 8 (14.3) | 1 (3.8) | 18 (34.6) | |
| Vaccination Status n(%) | Updated | 39 (14.8) | 1 (0.8) | 78 (30.0) | 15 (26.3) | 29 (25.4) | 12 (42.9) | 6 (10.7) | 3 (11.5) | 17 (32.7) |
| Not updated | 195 (74.1) | 119 (91.5) | 162 (62.3) | 39 (68.4) | 79 (69.3) | 12 (42.9) | 50 (89.3) | 17 (65.4) | 35 (67.3) | |
| Unknown | 29 (11.0) | 10(7.7) | 20 (7.7) | 3 (5.3) | 6 (5.3) | 4 (14.3) | - | 6 (23.1) | - | |
| Breed n(%) | Breed | 151 (57.4) | 71(54.6) | 162 (62.3) | 35 (61.4) | 72 (63.2) | 21 (75.0) | 32 (57.1) | 12 (46.2) | 36 (69.2) |
| Mixed breed | 112 (42.6) | 59(45.4) | 98 (37.7) | 22 (38.6) | 42 (36.8) | 7 (25.0) | 24 (42.9) | 14 (53.8) | 16 (30.8) | |
| Concomitant Diseases n(%) | No | 148 (56.3) | 148(56.3) | 77 (29.6) | 36 (63.2) | 32 (28.1) | 3 (10.7) | 32 (28.1) | 15 (57.7) | 11 (21.2) |
| Yes | 115 (43.7) | 115(43.7) | 183 (70.4) | 21 (36.8) | 82 (71.9) | 25 (89.3) | 82 (71.9) | 11 (42.3) | 41 (78.8) | |
| Hospital stay length (days) Median [Range] | All | 4.0 [1.0–20.0] | 4.5 [1.0–18.0] | - | 5.0 [1.0–16.0] | - | 3.0 [1.0–13.0] | - | 3.5 [1.0–20.0] | - |
| Only Discharge | 5.0 [1.0–20.0] | 5.0 [1.0–18.0] | - | 7.0 [1.0–16.0] | - | 3.0 [1.0–13.0] | - | 4.0 [1.0–20.0] | - | |
| Hospital stay Outcome n(%) | Discharge | 189 (71.9) | 109 (83.8) | - | 31 (54.4) | 20 (71.4) | - | 13 (50.0) | - | |
| Euthanasia | 40 (15.2) | 1 (0.8) | - | 18 (31.6) | - | 7 (25.0) | - | 11 (42.3) | - | |
| Dead | 34 (12.9) | 20 (15.4) | 8 (14.0) | 1 (3.6) | 2 (7.7) | |||||
| Final Outcome n(%) | Discharge | 181 (68.8) | 107 (82.3) | - | 29 (50.9) | - | 15 (53.6) | - | 9 (34.6) | - |
| Euthanasia | 46 (17.5) | 1 (0.8) | - | 18 (31.6) | - | 12 (42.9) | - | 15 (57.7) | - | |
| Dead | 36 (13.7) | 22 (16.9) | - | 10 (31.6) | 1 (3.6) | 2 (7.7) | - |
| Disease | Parvovirosis | Leptospirosis | MDR | Distemper |
|---|---|---|---|---|
| Variable | p-value Estimate Std error | p-value Estimate Std error | p-value Estimate Std error | p-value Estimate Std error |
| Age group | ||||
| <2 years | <0.001 4.26 0.53 | - | - | <0.001 3.78 1.17 |
| ≥2 to and <10 years | - | <0.001 1.65 0.50 | 0.16 1.59 1.14 | - |
| ≥10 years | 0.43 −0.89 1.13 | 0.6 −0.40 0.77 | <0.01 3.51 1.19 | 0.62 −0.62 1.26 |
| Sex | ||||
| Female | - | - | - | - |
| Male | - | - | - | 0.6 −0.43 0.86 |
| Neuter Status | ||||
| No | - | - | - | - |
| Yes | 0.09 −1.65 0.98 | - | 0.56 −0.53 0.92 | 0.04 −3.25 1.55 |
| Vaccination Status Updated | ||||
| Yes | - | - | - | - |
| No | <0.001 4.25 1.09 | - | 0.02 −2.07 0.85 | 0.03 2.70 1.26 |
| Breed | ||||
| Breed | - | - | - | - |
| Mixed breed | 0.98 −0.01 0.43 | - | - | 0.11 1.27 0.8 |
| Concomitant Disorders | ||||
| No | - | - | - | - |
| Yes | 0.06 −0.82 0.43 | <0.001 −1.62 0.41 | 0.03 2.39 1.10 | 0.3 −0.89 0.84 |
| Season | ||||
| Cold (November-April) | - | - | - | - |
| Warm (May-October) | 0.5 0.25 0.43 | 0.07 −0.68 0.38 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, I.C.; Nunes, T.; Maximino, M.; Malato, J.; Tavares, L.; Almeida, V.; Sepúlveda, N.; Gil, S. Epidemiologic Factors Supporting Triage of Infected Dog Patients Admitted to a Veterinary Hospital Biological Isolation and Containment Unit. Vet. Sci. 2023, 10, 186. https://doi.org/10.3390/vetsci10030186
Machado IC, Nunes T, Maximino M, Malato J, Tavares L, Almeida V, Sepúlveda N, Gil S. Epidemiologic Factors Supporting Triage of Infected Dog Patients Admitted to a Veterinary Hospital Biological Isolation and Containment Unit. Veterinary Sciences. 2023; 10(3):186. https://doi.org/10.3390/vetsci10030186
Chicago/Turabian StyleMachado, Inês Cunha, Telmo Nunes, Miguel Maximino, João Malato, Luís Tavares, Virgilio Almeida, Nuno Sepúlveda, and Solange Gil. 2023. "Epidemiologic Factors Supporting Triage of Infected Dog Patients Admitted to a Veterinary Hospital Biological Isolation and Containment Unit" Veterinary Sciences 10, no. 3: 186. https://doi.org/10.3390/vetsci10030186
APA StyleMachado, I. C., Nunes, T., Maximino, M., Malato, J., Tavares, L., Almeida, V., Sepúlveda, N., & Gil, S. (2023). Epidemiologic Factors Supporting Triage of Infected Dog Patients Admitted to a Veterinary Hospital Biological Isolation and Containment Unit. Veterinary Sciences, 10(3), 186. https://doi.org/10.3390/vetsci10030186






