Investigation of the Effects of Monosodium Glutamate on the Embryonic Development of the Eye in Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Preperation of Test Solution
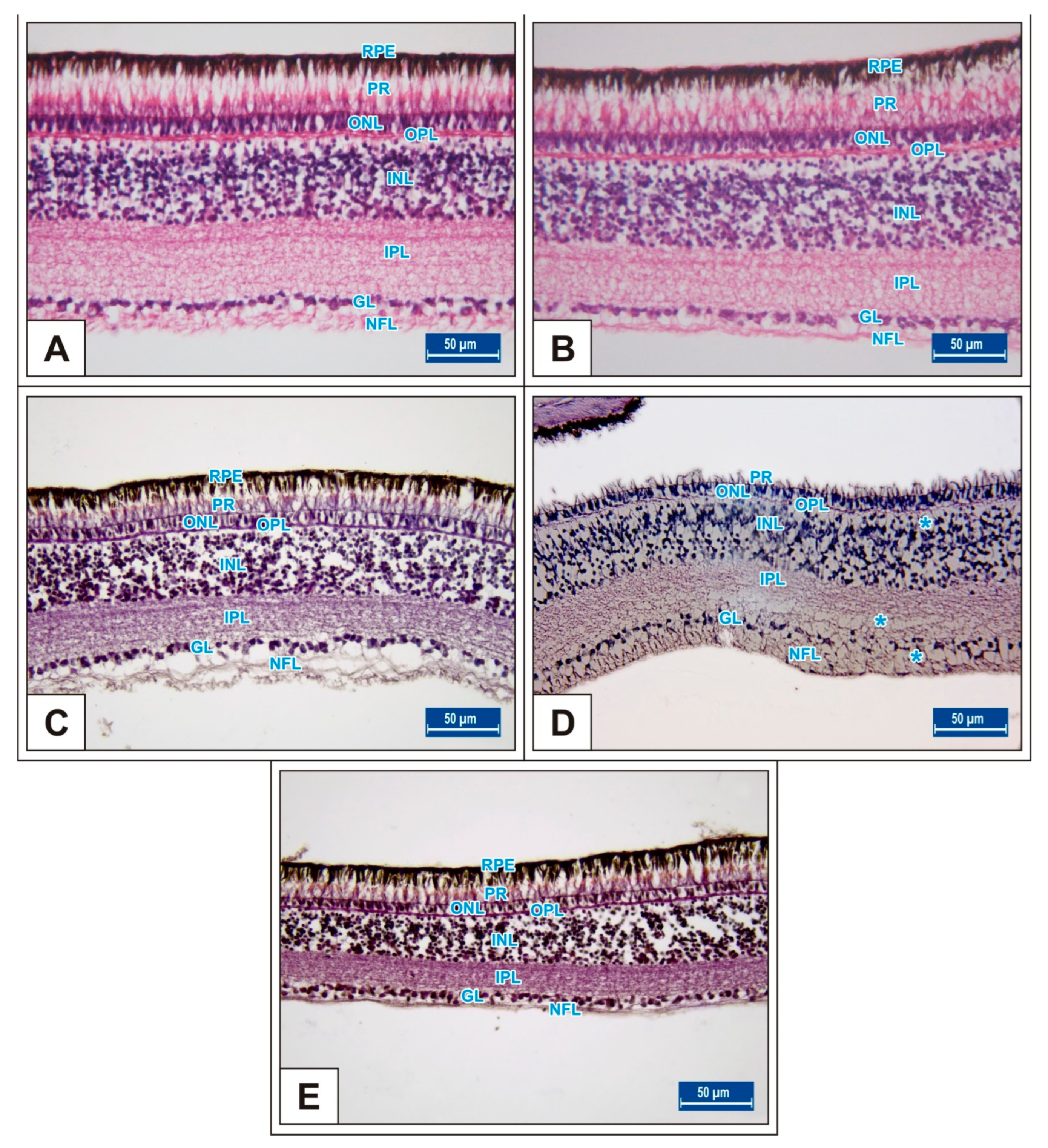
2.2. Tissue Sampling and Histologic Procedures
2.3. Statistical Analysis
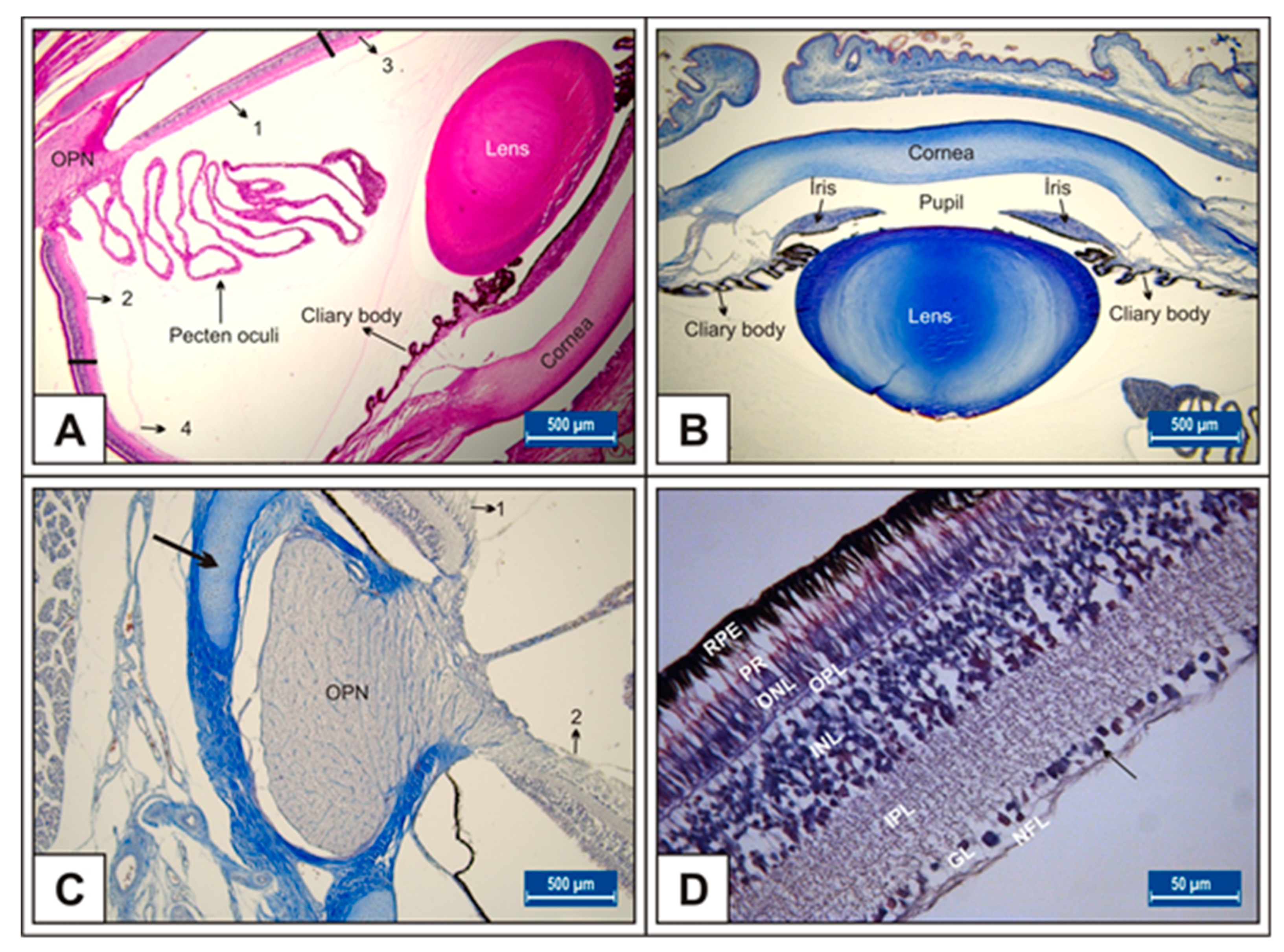
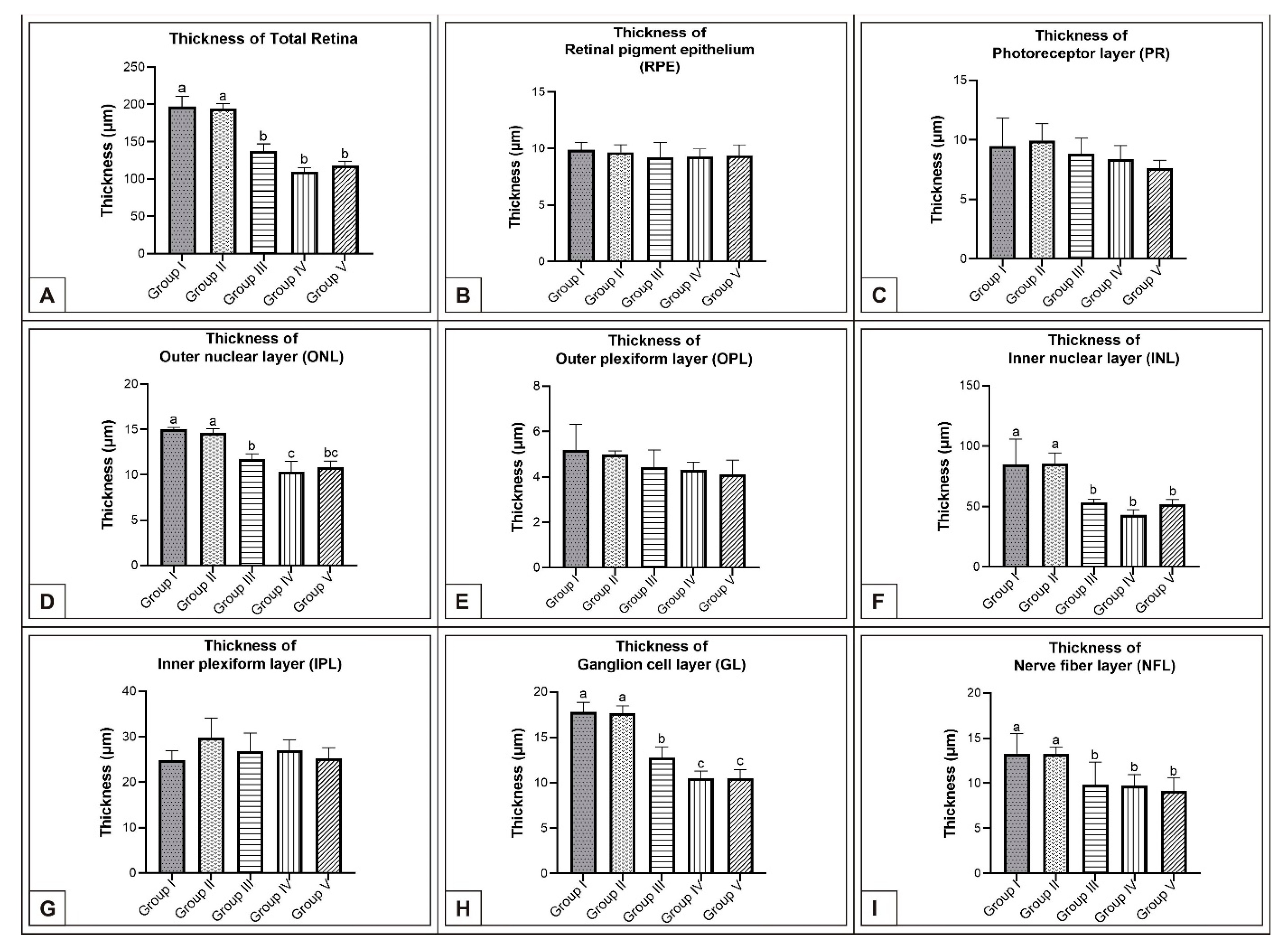
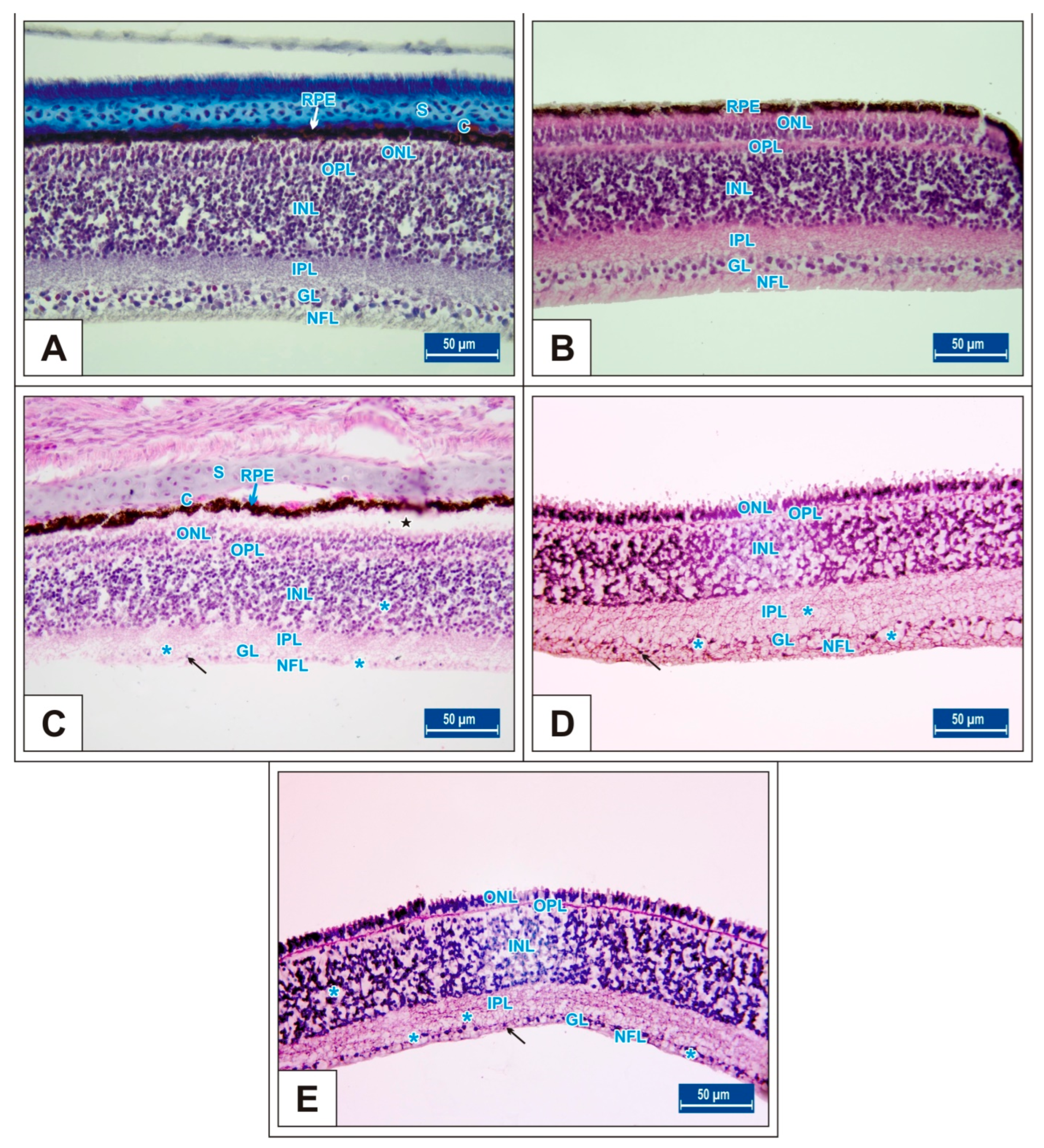
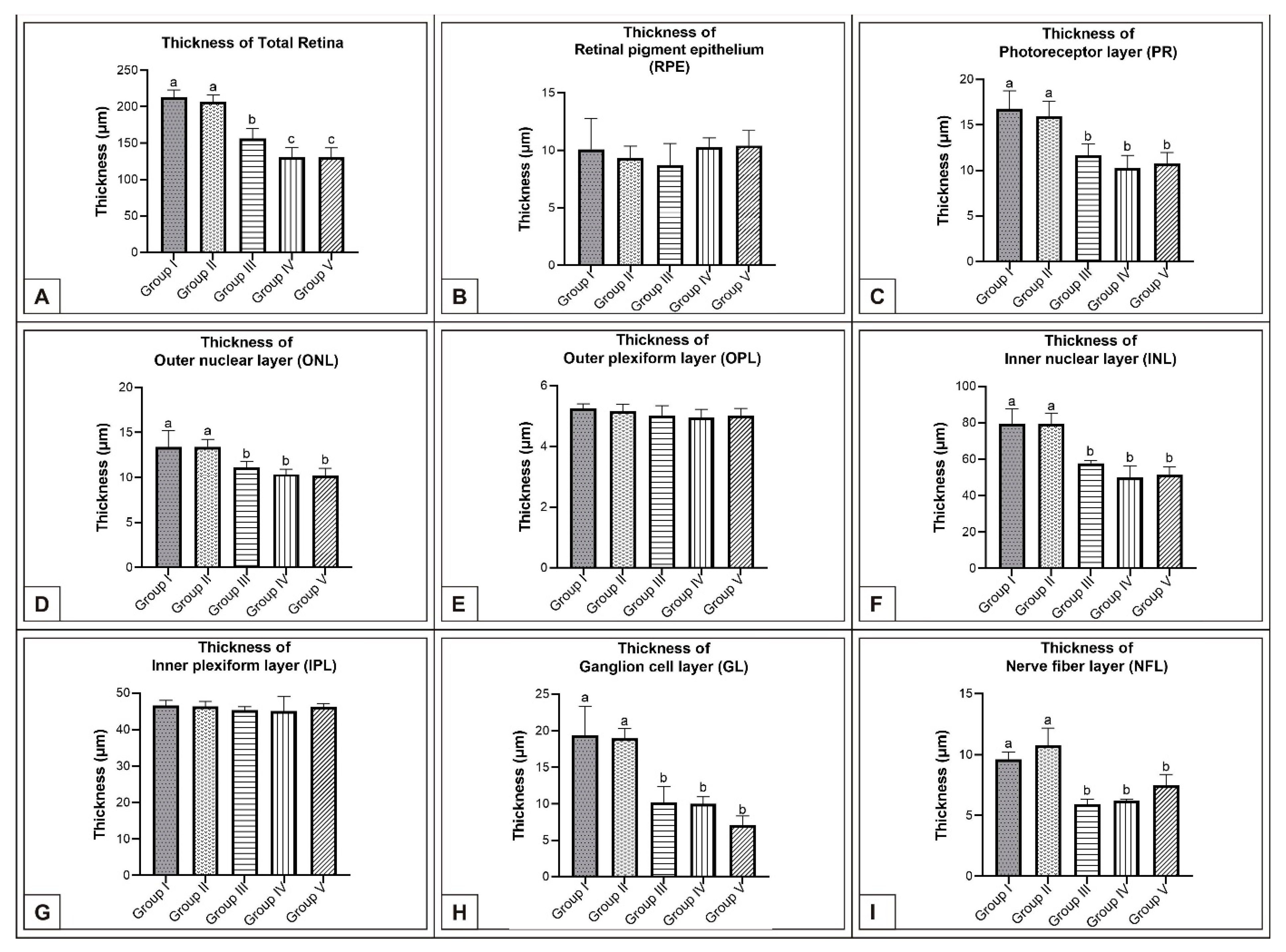
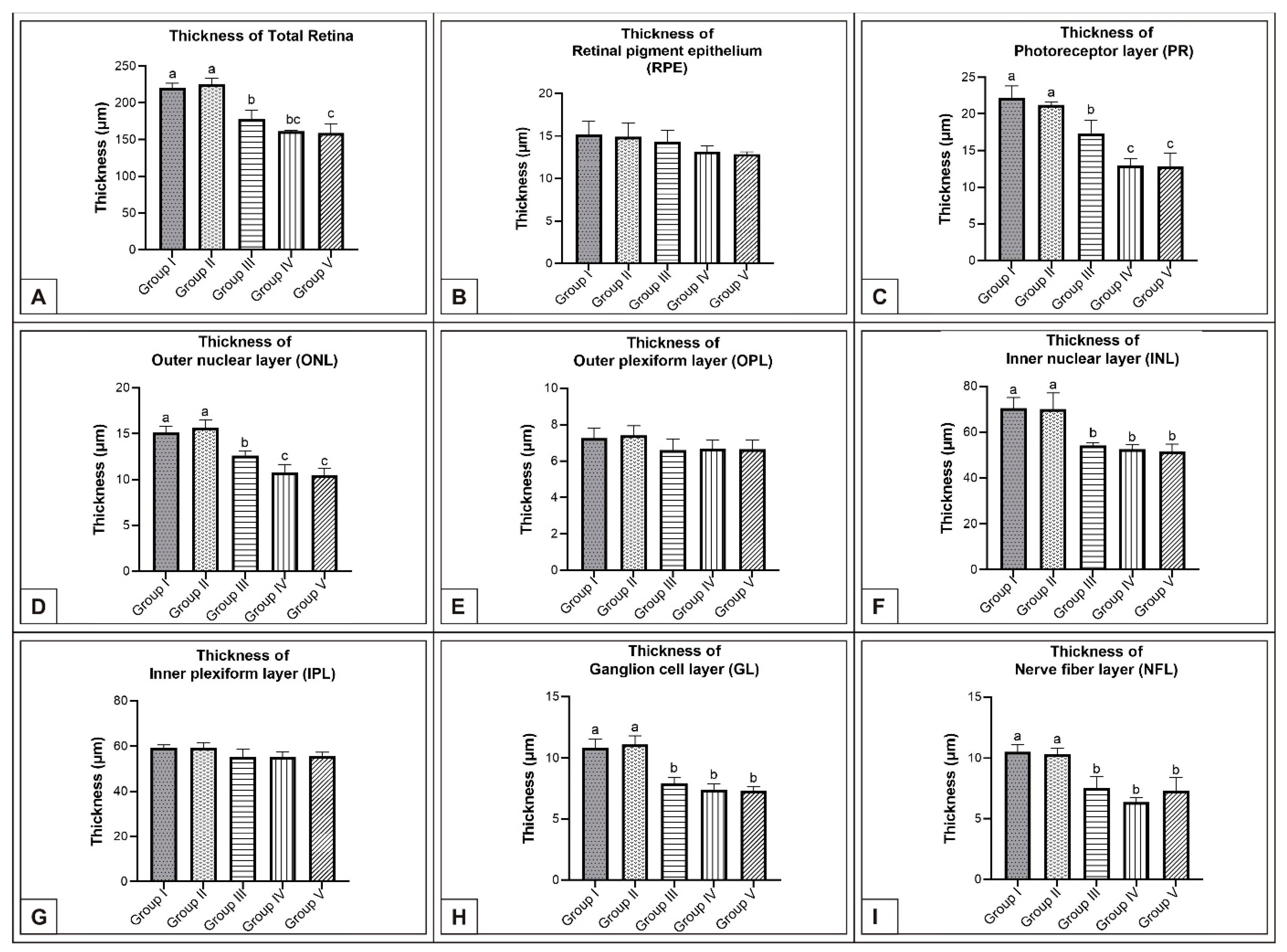
3. Results
3.1. Incubation Day 15
3.2. Incubation Day 18
3.3. Incubation Day 21
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, L.; Salanta, L.-C.; Socaci, S.; Tofana, M.; Fărcaş, A.; Pop, C. A Mini Review About Monosodium Glutamate. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2020, 77, 2020. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A Review of the Alleged Health Hazards of Monosodium Glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Beyreuther, K.; Biesalski, H.K.; Fernstrom, J.D.; Grimm, P.; Hammes, W.P.; Heinemann, U.; Kempski, O.; Stehle, P.; Steinhart, H.; Walker, R. Consensus meeting: Monosodium glutamate—An update. Eur. J. Clin. Nutr. 2007, 61, 304–313. [Google Scholar] [CrossRef]
- Chakraborty, S.P. Patho-physiological and toxicological aspects of monosodium glutamate. Toxicol. Mech. Methods 2019, 29, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Paul, B.D.; Parker, G.M.; Hester, L.D.; Snowman, A.M.; Taniguchi, Y.; Kamiya, A.; Snyder, S.H.; Sawa, A. The glutathione cycle shapes synaptic glutamate activity. Proc. Natl. Acad. Sci. USA 2019, 116, 2701–2706. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kalotra, S.; Bajaj, P.; Singh, H.; Kaur, G. Butanol extract of Tinospora cordifolia ameliorates cognitive deficits associated with glutamate-induced excitotoxicity: A mechanistic study using hippocampal neurons. Neuromolecular Med. 2020, 22, 81–99. [Google Scholar] [CrossRef]
- Derouiche, A.; Rauen, T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: Evidence for coupling of GLAST and GS in transmitter clearance. J. Neurosci. Res. 1995, 42, 131–143. [Google Scholar] [CrossRef]
- Rauen, T.; Rothstein, J.; Wässle, H. Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res. 1996, 286, 325–336. [Google Scholar] [CrossRef]
- Gudiño-Cabrera, G.; Ureña-Guerrero, M.E.; Rivera-Cervantes, M.C.; Feria-Velasco, A.I.; Beas-Zárate, C. Excitotoxicity triggered by neonatal monosodium glutamate treatment and blood–brain barrier function. Arch. Med. Res. 2014, 45, 653–659. [Google Scholar] [CrossRef]
- Banerjee, A.; Mukherjee, S.; Maji, B.K. Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: An overview. Toxicol. Rep. 2021, 8, 938–961. [Google Scholar] [CrossRef]
- Nnadozie, J.O.; Chijioke, U.O.; Okafor, O.C.; Olusina, D.B.; Oli, A.N.; Nwonu, P.C.; Mbagwu, H.O.; Chijioke, C.P. Chronic toxicity of low dose monosodium glutamate in albino Wistar rats. BMC Res. Notes 2019, 12, 593. [Google Scholar] [CrossRef] [PubMed]
- Eweka, A.; Igbigbi, P.; Ucheya, R. Histochemical studies of the effects of monosodium glutamate on the liver of adult Wistar rats. Ann. Med. Health Sci. Res. 2011, 1, 21–30. [Google Scholar] [PubMed]
- Pavlovic, V.; Pavlovic, D.; Kocic, G.; Sokolovic, D.; Sarac, M.; Jovic, Z. Ascorbic acid modulates monosodium glutamate induced cytotoxicity in rat thymus. Bratisl. Lek. Listy 2009, 110, 205–209. [Google Scholar] [PubMed]
- Anbarkeh, F.R.; Baradaran, R.; Ghandy, N.; Jalali, M.; Nikravesh, M.R.; Soukhtanloo, M. Effects of monosodium glutamate on apoptosis of germ cells in testicular tissue of adult rat: An experimental study. Int. J. Reprod. Biomed. 2019, 17, 261. [Google Scholar]
- Diab, A.; Hamza, R.Z. Monosodium glutamate induced hepatotoxicity and the possible mitigating effect of vitamin C and propolis. J. Adv. Med. Pharm. Sci. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Eid, R.A.; Al-Shraim, M.; Zaki, M.S.; Kamar, S.S.; Abdel Latif, N.S.; Negm, S.; Al-Ani, B.; Haidara, M.A. Vitamin E protects against monosodium glutamate-induced acute liver injury and hepatocyte ultrastructural alterations in rats. Ultrastruct. Pathol. 2019, 43, 199–208. [Google Scholar] [CrossRef]
- Farombi, E.; Onyema, O. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: Modulatory role of vitamin C, vitamin E and quercetin. Hum. Exp. Toxicol. 2006, 25, 251–259. [Google Scholar] [CrossRef]
- Pavlovic, V.; Pavlovic, D.; Kocic, G.; Sokolovic, D.; Jevtovic-Stoimenov, T.; Cekic, S.; Velickovic, D. Effect of monosodium glutamate on oxidative stress and apoptosis in rat thymus. Mol. Cell Biochem. 2007, 303, 161–166. [Google Scholar] [CrossRef]
- Bölükbaş, F.; Öznurlu, Y. The determination of the effect of in ovo administered monosodium glutamate on the embryonic development of thymus and bursa of Fabricius and percentages of alpha-naphthyl acetate esterase positive lymphocyte in chicken. Environ. Sci. Pollut. Res. 2022, 29, 45338–45348. [Google Scholar] [CrossRef]
- Abd-Elkareem, M.; El-Rahman, A.; Mokhless, A.; Khalil, N.S.A.; Amer, A.S. Antioxidant and cytoprotective effects of Nigella sativa L. seeds on the testis of monosodium glutamate challenged rats. Sci. Rep. 2021, 11, 13519. [Google Scholar] [CrossRef]
- Abdul-Hamid, M.; Galaly, S.R.; Ahmed, R.R.; Hamdalla, H.M. Histopathological and biochemical effect of quercetin on monosodium glutamate supplementation-induced testicular toxicity. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 73. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Harbi, M.S. Monosodium glutamate induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicol. Rep. 2014, 1, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; El-Seify, G.H.; El Haroun, H.M.; Soliman, M.A.E.M.M. Effect of monosodium glutamate on the ovaries of adult female albino rats and the possible protective role of green tea. Menoufia Med. J. 2014, 27, 793. [Google Scholar]
- Bojanić, V.; Bojanić, Z.; Najman, S.; Savić, T.; Jakovljević, V.; Najman, S.; Jančić, S. Diltiazem prevention of toxic effects of monosodium glutamate on ovaries in rats. Gen. Physiol. Biophys. 2009, 28, 149–154. [Google Scholar] [PubMed]
- Eweka, A.O.; Eweka, A.; Om’iniabohs, F.A.E. Histological studies of the effects of monosodium glutamate of the fallopian tubes of adult female Wistar rats. N. Am. J. Med. Sci. 2010, 2, 146–149. [Google Scholar] [CrossRef]
- Rohmawati, W.; Istiananingsih, Y.; Nurdiana, N.; Barlianto, W.; Dwijayasa, P.M. Vitamin CE ve Monosodyum Glutamata Bağlı Ovaryan Toksisite. Cukurova Med. J. 2014, 39, 517–524. [Google Scholar] [CrossRef]
- Eweka, A.; Om’Iniabohs, F. Histological studies of the effects of monosodium glutamate on the ovaries of adult wistar rats. Ann. Med. Health Sci. Res. 2011, 1, 37–44. [Google Scholar]
- Ajibade, A.; Fakunle, P.; Adetunji, M. Some effects of monosodium glutamate administration on the histo-architecture of the spleen and pancreas of adult Wistar rats. J. Pharm. Biol. Sci. 2015, 3, 39. [Google Scholar]
- Alsalmi, F.; Hamza, R.; El-Shenawy, N. Effect of Green Tea and Zinc oxide Nanoparticles Complex on Histopathology of Spleen of Male Rats Induced by Monosodium Glutamate. Instant J. Hematol. Oncol. 2019, 1, 4–11. [Google Scholar] [CrossRef]
- Hassan, Z.A.; Arafa, M.H.; Soliman, W.I.; Atteia, H.H.; Al-Saeed, H.F. The effects of monosodium glutamate on thymic and splenic immune functions and role of recovery (biochemical and histological study). J. Cytol. Histol. 2014, 5, 1000283. [Google Scholar] [CrossRef]
- Mohamed, D.S.; Abdelhaliem, N.G.; Zakaria, A.M. Histological and immunohistochemical study of the possible protective effect of ascorbic acid on the toxic effect of monosodium glutamate on the spleen of adult male albino rat. Egypt. J. Histol. 2017, 40, 94–104. [Google Scholar] [CrossRef]
- Al-Ghamdi, F.A. Microscopic study of potential toxic effects of monosodium glutamate on liver of chicken embryos aged 16 days. Egypt. Liver J. 2021, 11, 38. [Google Scholar] [CrossRef]
- Solomon, E.N. Changes in Liver Oxidative Stress Biomarkers, Biochemicals and Histological Assess-ment in Lactating Wistar Rats Following Oral Monosodium Glutamate (MSG) Administration. EC Pharmacol. Toxicol. 2020, 8, 14–25. [Google Scholar]
- Araujo, T.R.; Freitas, I.N.; Vettorazzi, J.F.; Batista, T.M.; Santos-Silva, J.C.; Bonfleur, M.L.; Balbo, S.L.; Boschero, A.C.; Carneiro, E.M.; Ribeiro, R.A. Benefits of L-alanine or L-arginine supplementation against adiposity and glucose intolerance in monosodium glutamate-induced obesity. Eur. J. Nutr. 2017, 56, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Du, S.; Xun, P.; Sharma, S.; Wang, H.; Zhai, F.; Popkin, B. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 2011, 93, 1328–1336. [Google Scholar] [CrossRef]
- Madhavadas, S.; Kutty, B.M.; Subramanian, S. Amyloid ß Lowering and Cognition Enhancing Effects of Ghrelin Receptor Analog [D-Lys (3)] GHRP-6 in Rat Model of Obesity. NIScPR Publ. 2014, 51, 257–262. [Google Scholar]
- Špolcová, A.; Mikulášková, B.; Holubová, M.; Nagelová, V.; Pirnik, Z.; Zemenová, J.; Haluzík, M.; Železná, B.; Galas, M.-C.; Maletínská, L. Anorexigenic lipopeptides ameliorate central insulin signaling and attenuate tau phosphorylation in hippocampi of mice with monosodium glutamate-induced obesity. J. Alzheimer’s Dis. 2015, 45, 823–835. [Google Scholar] [CrossRef]
- Chaparro-Huerta, V.; Rivera-Cervantes, M.; Torres-Mendoza, B.; Beas-Zarate, C. Neuronal death and tumor necrosis factor-α response to glutamate-induced excitotoxicity in the cerebral cortex of neonatal rats. Neurosci. Lett. 2002, 333, 95–98. [Google Scholar] [CrossRef]
- Moreno, G.; Perelló, M.; Gaillard, R.C.; Spinedi, E. Orexin a stimulates hypothalamic-pituitary-adrenal (HPA) axis function, but not food intake, in the absence of full hypothalamic NPY-ergic activity. Endocrine 2005, 26, 99–106. [Google Scholar] [CrossRef]
- Abass, M.; Abd El-Haleem, M. Evaluation of monosodium glutamate induced neurotoxicity and nephrotoxicity in adult male albino rats. J. Am. Sci. 2011, 7, 264–276. [Google Scholar]
- Al-Qudsi, F.; Al-Jahdali, A. Effect of monosodium glutamate on chick embryo development. J. Am. Sci 2012, 8, 499–509. [Google Scholar]
- Bölükbaş, F.; Öznurlu, Y. Determining the effects of in ovo administration of monosodium glutamate on the embryonic development of brain in chickens. NeuroToxicology 2023, 94, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Dief, A.E.; Kamha, E.S.; Baraka, A.M.; Elshorbagy, A.K. Monosodium glutamate neurotoxicity increases beta amyloid in the rat hippocampus: A potential role for cyclic AMP protein kinase. Neurotoxicology 2014, 42, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Espinar, A.; García-Oliva, A.; Isorna, E.M.; Quesada, A.; Prada, F.A.; Guerrero, J.M. Neuroprotection by melatonin from glutamate-induced excitotoxicity during development of the cerebellum in the chick embryo. J. Pineal Res. 2000, 28, 81–88. [Google Scholar] [CrossRef]
- Hashem, H.E.; Safwat, E.-D.; Algaidi, S. The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of vitamin C (histological and immunohistochemical study). J. Mol. Histol. 2012, 43, 179–186. [Google Scholar] [CrossRef]
- Miko, A.M.; Shehu, A.M.; Bello, N.; Allyu, I.A.; Tasiu, I.; Abdussalam, A.O.; Isa, A.S. A morphometric study of the teratogenic effect of monosodium glutamate on the developing developing cerebral cortex of Wista Rat(Rattus norvegicus). Niger. J. Sci. Res. 2016, 15, 240–244. [Google Scholar]
- Gurgen, S.G.; Sayın, O.; Çetïn, F.; Sarsmaz, H.Y.; Yazıcı, G.L.N.; Umur, N.; Yucel, A.T. The Effect of Monosodium Glutamate on Neuronal Signaling Molecules in the Hippocampus and the Neuroprotective Effects of Omega-3 Fatty Acids. ACS Chem. Neurosci. 2021, 12, 3028–3037. [Google Scholar] [CrossRef]
- Narayanan, S.N.; Kumar, R.S.; Paval, J.; Nayak, S. Effect of ascorbic acid on the monosodium glutamate-induced neurobehavioral changes in periadolescent rats. Bratisl. Lek Listy 2010, 111, 247–252. [Google Scholar]
- Gim, S.-A.; Park, D.-J.; Kang, J.-B.; Shah, F.-A.; Koh, P.-O. Identification of regulated proteins by resveratrol in glutamate-induced cortical injury of newborn rats. J. Vet. Med. Sci. 2021, 83, 724–733. [Google Scholar] [CrossRef]
- Bellhorn, R.; Lipman, D.; Confino, J.; Burns, M. Effect of monosodium glutamate on retinal vessel development and permeability in rats. Investig. Ophthalmol. Vis. Sci. 1981, 21, 237–247. [Google Scholar]
- Cohen, A.I. An electron microscopic study of the modification by monosodium glutamate of the retinas of normal and “rodless” mice. Am. J. Anat. 1967, 120, 319–355. [Google Scholar] [CrossRef]
- Moustafa, K.A.; Okasha, E.F. The possible protective effect of curcumin on monosodium glutamate-induced retinal changes in adult male albino rats. Egypt. J. Histol. 2016, 39, 87–95. [Google Scholar] [CrossRef]
- Szabadfi, K.; Atlasz, T.; Horváth, G.; Kiss, P.; Hamza, L.; Farkas, J.; Tamás, A.; Lubics, A.; Gábriel, R.; Reglődi, D. Early postnatal enriched environment decreases retinal degeneration induced by monosodium glutamate treatment in rats. Brain Res. 2009, 1259, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zaghlool, S.S.; Hanaf, L.K.; Afifi, N.M.; Ibrahim, E.R. Histological and immunohistochemical study on the protective effect of Ginkgo biloba extract against glutamate-induced neurotoxicity in male albino rat retinal cells. Egypt. J. Histol. 2012, 35, 176–188. [Google Scholar] [CrossRef]
- Dénes, V.; Lakk, M.; Czotter, N.; Gábriel, R. A precise temporal dissection of monosodium glutamate-induced apoptotic events in newborn rat retina in vivo. Neurochem. Res. 2011, 36, 1464–1474. [Google Scholar] [CrossRef]
- Van Rijn, C.; Marani, E.; Rietveld, W. The neurotoxic effect of monosodium glutamate (MSG) on the retinal ganglion cells of the albino rat. Histol. Histopathol. 1986, 1, 3. [Google Scholar]
- Praputpittaya, C.; Wililak, A. Visual Performance in Monosodium Glutamate-Treated Rats. Nutr. Neurosci. 2003, 6, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Swelim, H.H. Monosodium glutamate (MSG) induced retinopath y in adult and neonate CD-1 mice. Egypt J. Med. Lab Sci. 2004, 13, 45–71. [Google Scholar]
- Horvath, G.; Reglodi, D.; Vadasz, G.; Farkas, J.; Kiss, P. Exposure to enriched environment decreases neurobehavioral deficits induced by neonatal glutamate toxicity. Int. J. Mol. Sci. 2013, 14, 19054–19066. [Google Scholar] [CrossRef]
- Kiss, P.; Hauser, D.; Tamas, A.; Lubics, A.; Racz, B.; Horvath, Z.; Farkas, J.; Zimmermann, F.; Stepien, A.; Lengvari, I. Changes in open-field activity and novelty-seeking behavior in periadolescent rats neonatally treated with monosodium glutamate. Neurotox. Res. 2007, 12, 85–93. [Google Scholar] [CrossRef]
- El-Sayyad, H.; Abou-El-Naga, A.; Khalifa, S.; El-Shahari, E.; Jala, H. Abnormal Retinal Structure and Function of Mother Wistar Rats Supplemented Aspartame, Glutamate and Galactose. J. Drug Metab. Toxicol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Alshareef, M.; Alrafiah, A.; Abed, S.; Basingab, F.; Alrofaidi, A. Effect of e-cigarette flavoring agents on the neural retina of chick embryo: Histological and gene expression study. Folia Histochem. Et Cytobiol. 2021, 59, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Wisely, C.E.; Sayed, J.A.; Tamez, H.; Zelinka, C.; Abdel-Rahman, M.H.; Fischer, A.J.; Cebulla, C.M. The chick eye in vision research: An excellent model for the study of ocular disease. Prog. Retin. Eye Res. 2017, 61, 72–97. [Google Scholar] [CrossRef] [PubMed]
- Airaodion, A.I.; Ogbuagu, E.O.; Osemwowa, E.U.; Ogbuagu, U.; Esonu, C.E. Toxicological Effect of Monosodium Glutamate in Seasonings on Human Health. Glob. J. Nutri. Food Sci. 2019, 1, 522. [Google Scholar] [CrossRef]
- Gao, J.; Wu, J.; Zhao, X.; Zhang, W.; Zhang, Y.; Zhang, Z. Transplacental neurotoxic effects of monosodium glutamate on structures and functions of specific brain areas of filial mice. Sheng Li Xue Bao Acta Physiol. Sin. 1994, 46, 44–51. [Google Scholar]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Crossmon, G. A modification of Mallory’s connective tissue stain with a discussion of the principles involved. Anat. Rec. 1937, 69, 33–38. [Google Scholar] [CrossRef]
- Culling, C.F.A.; Allison, R.T.; Barr, W.T. Cellular Pathology Technique, 4th ed.; Culling, C.F.A., Allison, R.T., Barr, W.T., Eds.; Butterworth-Heinemann: Woburn, MA, USA, 1985; pp. 164–180, 214–255, 377–407. [Google Scholar]
- Geha, R.S.; Beiser, A.; Ren, C.; Patterson, R.; Greenberger, P.A.; Grammer, L.C.; Ditto, A.M.; Harris, K.E.; Shaughnessy, M.A.; Yarnold, P.R.; et al. Review of alleged reaction to monosodium glutamate and outcome of a multicenter double-blind placebo-controlled study. J. Nut.r 2000, 130, 1058s–1062s. [Google Scholar] [CrossRef]
- Roongruangchai, J.; Viravud, Y.; Plakornkul, V.; Sripaoraya, K.; Boonmark, W.; Roongruangchai, K. The teratogenic effects of monosodium glutamate (MSG) on the development of chick embryos. Siriraj Med. J. 2018, 70, 514–522. [Google Scholar]
- Hajihasani, M.M.; Soheili, V.; Zirak, M.R.; Sahebkar, A.; Shakeri, A. Natural products as safeguards against monosodium glutamate-induced toxicity. Iran. J. Basic Med. Sci. 2020, 23, 416. [Google Scholar] [CrossRef]
- Kazmi, Z.; Fatima, I.; Perveen, S.; Malik, S.S. Monosodium glutamate: Review on clinical reports. Int. J. Food Prop. 2017, 20, 1807–1815. [Google Scholar] [CrossRef]
- Baad-Hansen, L.; Cairns, B.; Ernberg, M.; Svensson, P. Effect of systemic monosodium glutamate (MSG) on headache and pericranial muscle sensitivity. Cephalalgia Int. J. Headache 2010, 30, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, Y.; Nagamura, Y. Does monosodium glutamate really cause headache? A systematic review of human studies. J. Headache Pain. 2016, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Abu Elnaga, N.A.; Sarhan, M.; Mansour, H. Teratogenicity of Monosodium Glutamate on The Pregnant Rats and Their Fetuses. Egypt. J. Hosp. Med. 2019, 74, 1737–1747. [Google Scholar] [CrossRef]
- Umukoro, S.; Oluwole, G.O.; Olamijowon, H.E.; Omogbiya, A.I.; Eduviere, A.T. Effect of monosodium glutamate on behavioral phenotypes, biomarkers of oxidative stress in brain tissues and liver enzymes in mice. World J. Neurosci. 2015, 5, 339. [Google Scholar] [CrossRef]
- Boutry, C.; Matsumoto, H.; Airinei, G.; Benamouzig, R.; Tomé, D.; Blachier, F.; Bos, C. Monosodium glutamate raises antral distension and plasma amino acid after a standard meal in humans. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G137–G145. [Google Scholar] [CrossRef]
- Adebayo, O. Lipid peroxidation and antioxidant status of the cerebrum, cerebellum and brain stem following dietary monosodium glutamate administration in mice. Asian J. Clin. Nutr. 2011, 3, 71–77. [Google Scholar] [CrossRef]
- Ohguro, H.; Katsushima, H.; Maruyama, I.; Maeda, T.; Yanagihashi, S.; Metoki, T.; Nakazawa, M. A high dietary intake of sodium glutamate as flavoring (ajinomoto) causes gross changes in retinal morphology and function. Exp. Eye Res. 2002, 75, 307–315. [Google Scholar] [CrossRef]
- Gu, L.; Liang, X.; Wang, L.; Yan, Y.; Ni, Z.; Dai, H.; Gao, J.; Mou, S.; Wang, Q.; Chen, X. Functional metabotropic glutamate receptors 1 and 5 are expressed in murine podocytes. Kidney Int. 2012, 81, 458–468. [Google Scholar] [CrossRef]
- El-Gohari, K.M.; Bahei-Eldin, I.A.; Habib, E.M.; Saad, S.A.; Rady, H.Y.; Said, A.M. Neuroprotection of the rat’s retinal ganglion cells against glutamate-induced toxicity. J. Egypt. Ophthalmol. Soc. 2016, 109, 135. [Google Scholar]
- Ali, H.S.; El-Gohary, A.A.; Metwally, F.G.; Sabra, N.M.; El Sayed, A.A. Mono sodium glutamate induced damage in rabbit retina: Electroretinographic and histologic studies. Glob. J Pharm. 2012, 6, 148–159. [Google Scholar]
- AlThanoon, S.A.; Abd, A.A. Histopathological Changes Induced by Monosodium Glutamate and Sodium Nitrite in the Development of Eye in Albino Mice Mus musculus. J. Educ. Sci. 2021, 30, 3. [Google Scholar] [CrossRef]
- Öznurlu, Y.; Özaydın, T.; Sur, E.; Özparlak, H. The effects of in ovo administered bisphenol A on tibial growth plate histology in chicken. Birth Defects Res. 2021; in press. [Google Scholar] [CrossRef]
- Bölükbaş, F.; Öznurlu, Y. Yumurtaya verilen monosodyum glutamat’ın tavuk embriyolarında medulla spinalisin servikal bölgesinin embriyonik gelişimi üzerindeki etkilerinin belirlenmesi. J. Adv. VetBio Sci. Tech. 2021, 6, 298–311. [Google Scholar] [CrossRef]
- Atallah, M.N.; Badawy, G.M.; El–Garawani, I.M.; Abdallah, F.S.; El–Borm, H.T. Neurotoxic effect of nalufin on the histology, ultrastructure, cell cycle and apoptosis of the developing chick embryo and its amelioration by selenium. Food Chem. Toxicol. 2021, 158, 112693. [Google Scholar] [CrossRef]
- Kmecick, M.; Vieira da Costa, M.C.; Oliveira Ribeiro, C.A.; Ortolani-Machado, C.F. Morphological evidence of neurotoxic effects in chicken embryos after exposure to perfluorooctanoic acid (PFOA) and inorganic cadmium. Toxicology 2019, 427, 152286. [Google Scholar] [CrossRef] [PubMed]
- Hocking, P.M.; Guggenheim, J.A. The chick as an animal model of eye disease. Drug Discov. Today Dis. Model. 2013, 10, e225–e230. [Google Scholar] [CrossRef]
- Vergara, M.N.; Canto-Soler, M.V. Rediscovering the chick embryo as a model to study retinal development. Neural. Dev. 2012, 7, 22. [Google Scholar] [CrossRef]
- Trejo-Reveles, V.; McTeir, L.; Summers, K.; Rainger, J. An analysis of anterior segment development in the chicken eye. Mech. Dev. 2018, 150, 42–49. [Google Scholar] [CrossRef]
- Berg, C.; Halldin, K.; Brunström, B. Effects of bisphenol A and tetrabromobisphenol A on sex organ development in quail and chicken embryos. Environ. Toxicol. Chem. Int. J. 2001, 20, 2836–2840. [Google Scholar] [CrossRef]
- Jessl, L.; Lenz, R.; Massing, F.G.; Scheider, J.; Oehlmann, J. Effects of estrogens and antiestrogens on gonadal sex differentiation and embryonic development in the domestic fowl (Gallus gallus domesticus). PeerJ 2018, 6, e5094. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Ang, H.-P.; Poh, R.; Chaurasia, S.S.; Peh, G.; Liu, J.; Tan, D.T.; Vithana, E.N.; Mehta, J.S. Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6179–6189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, G.; Huang, W.; He, M. Distribution of central and peripheral corneal thickness in Chinese children and adults: The Guangzhou twin eye study. Cornea 2008, 27, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Nemesure, B.; Wu, S.-Y.; Hennis, A.; Leske, M.C.; Group, B.E.S. Corneal thickness and intraocular pressure in the Barbados eye studies. Arch. Ophthalmol. 2003, 121, 240–244. [Google Scholar] [CrossRef]
- Ueno, Y.; Hiraoka, T.; Miyazaki, M.; Ito, M.; Oshika, T. Corneal thickness profile and posterior corneal astigmatism in normal corneas. Ophthalmology 2015, 122, 1072–1078. [Google Scholar] [CrossRef]
- Inomata, T.; Mashaghi, A.; Hong, J.; Nakao, T.; Dana, R. Scaling and maintenance of corneal thickness during aging. PloS ONE 2017, 12, e0185694. [Google Scholar] [CrossRef]
- Al-Qudsi, F.; Azzouz, S. Effect of electromagnetic mobile radiation on chick embryo development. Life Sci. J. 2012, 9, 983–991. [Google Scholar]
- Blanks, J.C.; Reif-Lehrer, L.; Casper, D. Effects of monosodium glutamate on the isolated retina of the chick embryo as a function of age: A morphological study. Exp. Eye Res. 1981, 32, 105–124. [Google Scholar] [CrossRef]
- Reif-Lehrer, L.; Bergenthal, J.; Hanninen, L. Effects of monosodium glutamate on chick embryo retina in culture. Investig. Ophthalmol. Vis. Sci. 1975, 14, 114–124. [Google Scholar]
- Kujawa-Hadryś, M.; Tosik, D.; Bartel, H. Changes in thickness of each layer of developing chicken cornea after administration of caffeine. Folia Histochem. Cytobiol. 2010, 48, 273–277. [Google Scholar] [CrossRef]
- Moayed, A.A.; Hariri, S.; Song, E.S.; Choh, V.; Bizheva, K. In vivo volumetric imaging of chicken retina with ultrahigh-resolution spectral domain optical coherence tomography. Biomed. Opt. Express 2011, 2, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Zareen, N.; Khan, M.; Minhas, L. Histological stages of retinal morphogenesis in chicken–a descriptive laboratory research. Ital. J. Zool. 2011, 78, 45–52. [Google Scholar] [CrossRef]
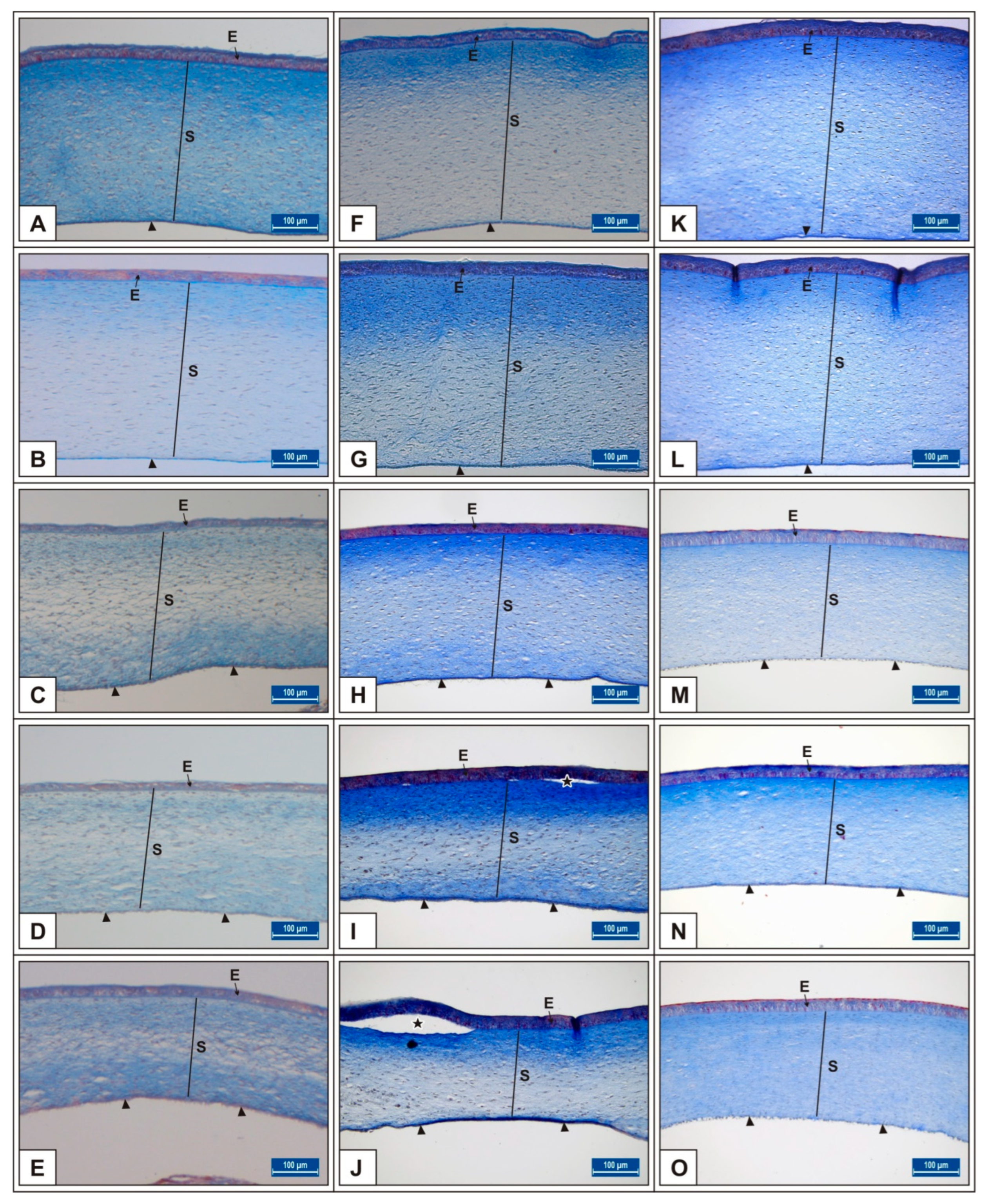

| Groups n = 6 | Day 15 of Incubation | Day 18 of Incubation | Day 21 of Incubation | |||
|---|---|---|---|---|---|---|
| Thickness of epithelium | Total corneal thickness | Thickness of epithelium | Total corneal thickness | Thickness of epithelium | Total corneal thickness | |
| Group I | 25.22 ± 2.02 a | 328.42 ± 30.41 a | 29.68 ± 2.54 a | 416,70 ± 5.62 a | 33.12 ± 3.35 a | 417.85 ± 12.98 a |
| Group II | 24.99 ± 2.18 a | 318.59 ± 5.59 a | 29.24 ± 1.19 a | 413.51 ± 10.18 a | 33.33 ± 6.11 a | 413.05 ± 13.44 a |
| Group III | 15.96 ± 1.67 b | 249.22 ± 14.60 b | 24.72 ± 1.80 b | 308.73 ± 14.68 b | 27.16 ± 1.77 b | 377.58 ± 21.98 b |
| Group IV | 13.15 ± 1.71 c | 222.85 ± 21.31 c | 22.82 ± 1.15 c | 295.67 ± 7.16 c | 26.30 ± 1.45 b | 365.91 ± 13.43 bc |
| Group V | 13.71 ± 1.61 c | 210.04 ± 17.15 c | 22.22 ± 1.86 c | 291.36 ± 6.39 c | 24.92 ± 2.34 b | 359.04 ± 11.60 c |
| Ganglion Cell Numbers | |||
|---|---|---|---|
| Groups n = 6 | Day 15 of incubation | Day 18 of incubation | Day 21 of incubation |
| Group I | 22.58 ± 2.91 a | 21.47 ± 3.21 a | 18.94 ± 4.86 a |
| Group II | 21.01 ± 3.28 a | 22,52 ± 4.32 a | 17.58 ± 3.07 a |
| Group III | 9.71 ± 3.56 b | 12.74 ± 2.93 b | 10.84 ± 4.36 b |
| Group IV | 7.66 ± 3.04 b | 9.11 ± 2.76 c | 10.33 ± 1.93 b |
| Group V | 7.44 ± 1.56 b | 8.86 ± 2.94 c | 8.27 ± 2.78 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bölükbaş, F.; Öznurlu, Y. Investigation of the Effects of Monosodium Glutamate on the Embryonic Development of the Eye in Chickens. Vet. Sci. 2023, 10, 99. https://doi.org/10.3390/vetsci10020099
Bölükbaş F, Öznurlu Y. Investigation of the Effects of Monosodium Glutamate on the Embryonic Development of the Eye in Chickens. Veterinary Sciences. 2023; 10(2):99. https://doi.org/10.3390/vetsci10020099
Chicago/Turabian StyleBölükbaş, Ferhan, and Yasemin Öznurlu. 2023. "Investigation of the Effects of Monosodium Glutamate on the Embryonic Development of the Eye in Chickens" Veterinary Sciences 10, no. 2: 99. https://doi.org/10.3390/vetsci10020099
APA StyleBölükbaş, F., & Öznurlu, Y. (2023). Investigation of the Effects of Monosodium Glutamate on the Embryonic Development of the Eye in Chickens. Veterinary Sciences, 10(2), 99. https://doi.org/10.3390/vetsci10020099






