A Highly Conserved Region in BRCA2 Suppresses the RAD51-Interaction Activity of BRC Repeats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Multiple Sequence Alignment
2.2. Cell Culture, Generate Cell Line, and Antibodies
2.3. Co-Immunoprecipitation
2.4. Mammalian Two-Hybrid Assay
2.5. Phosphorylation Assay by Phos-Tag SDS–PAGE
2.6. Tissue Sampling and DNA Isolation
2.7. PCR and DNA Sequencing
2.8. Statistical Analysis
2.9. Sorting Intolerant from Tolerant (SIFT) Analysis
3. Results
3.1. Location and Alignment of the Highly Conserved Region in BRCA2
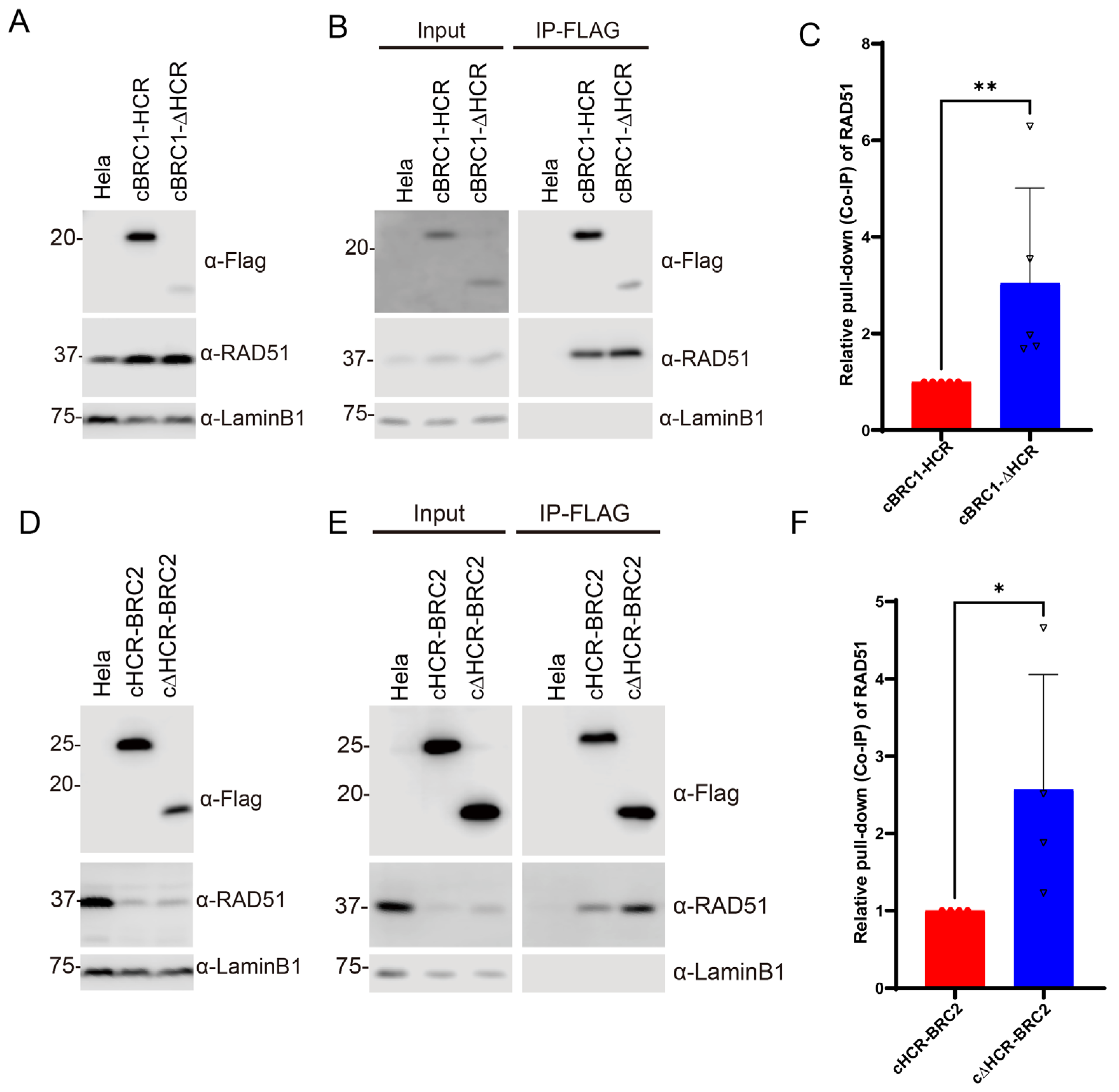
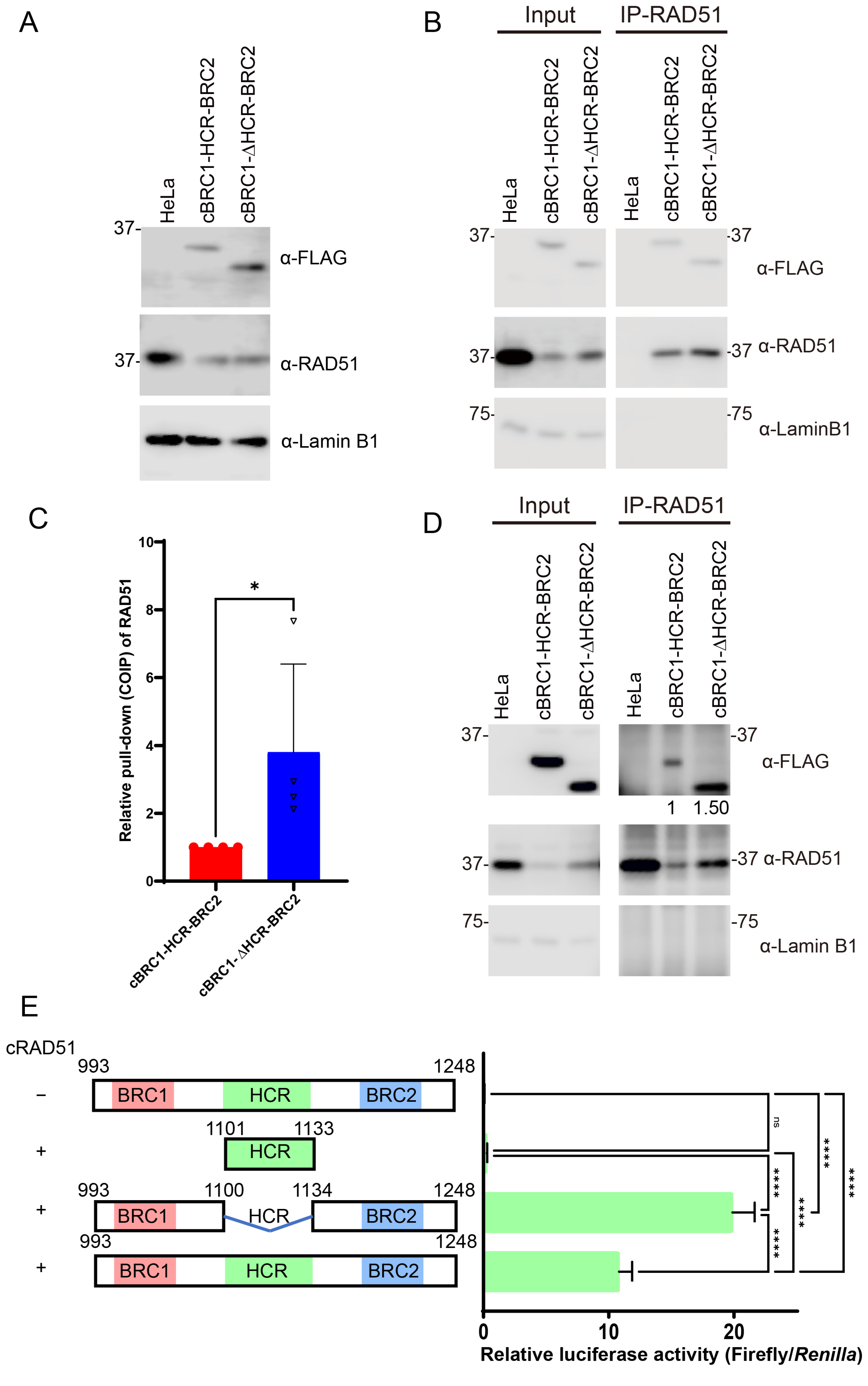
3.2. HCR Attenuates the Interaction Activity of BRC Repeat to RAD51
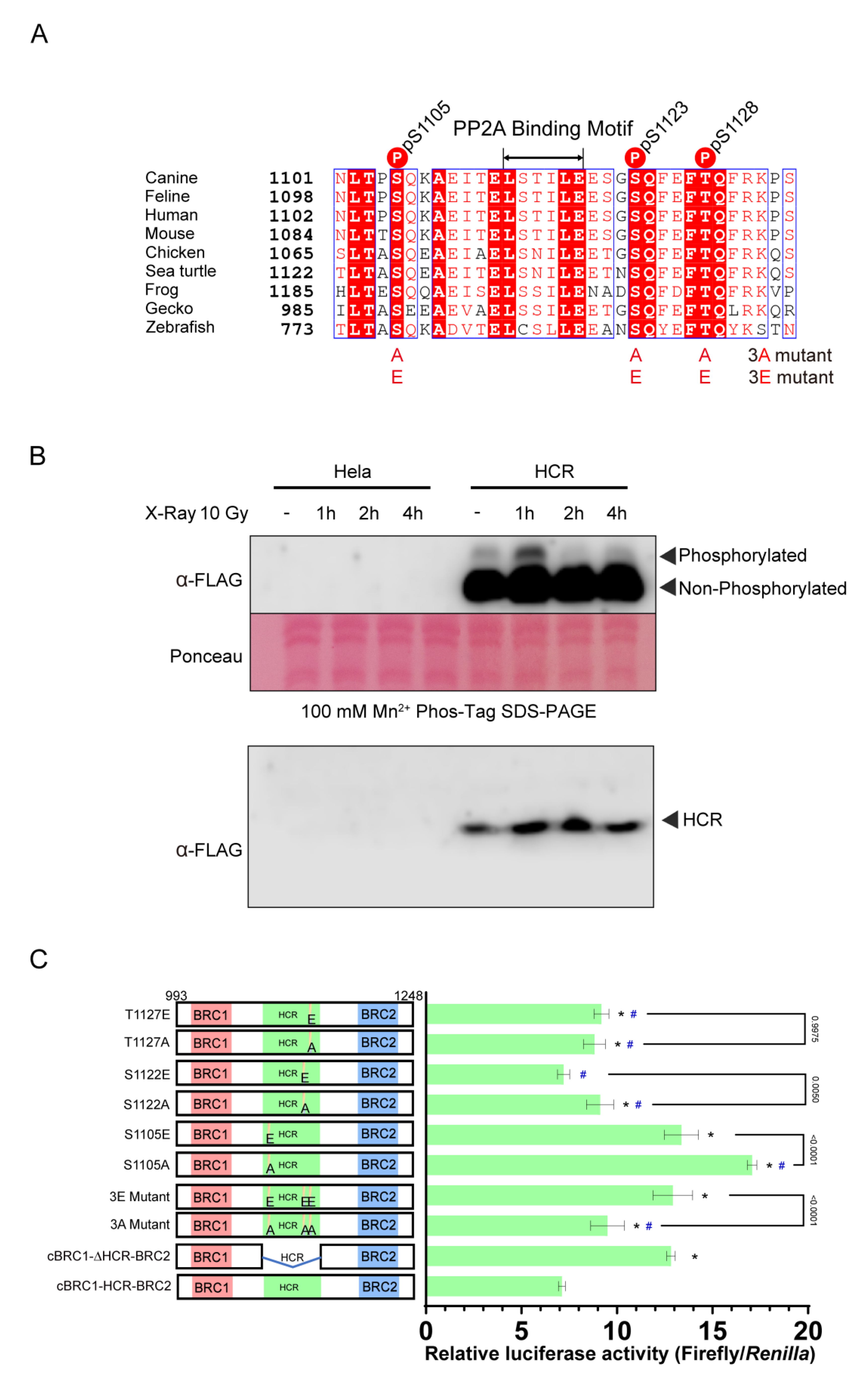
3.3. Mutations of Phosphorylation Sites in HCR Enhance RAD51 Interacting Activity of BRC Repeats
3.4. Canine BRCA2 Mutations in the HCR in Mammary Tumor and Tumor-Free Samples
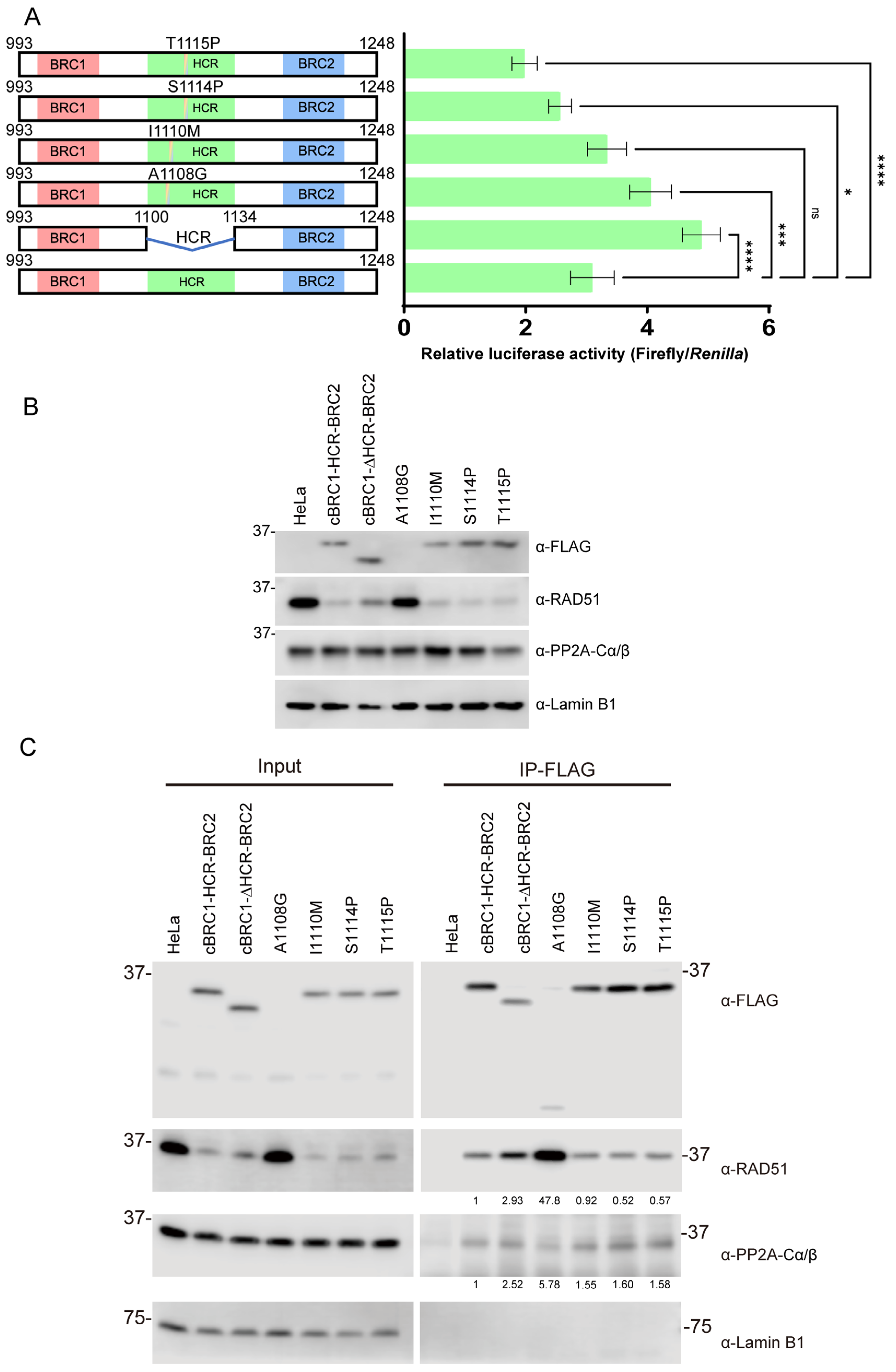
3.5. The RAD51-Interaction Activity of BRC Repeats Was Altered by BRCA2 Mutations in the HCR Found in Mammary Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a Model for Cancer. Annu. Rev. Anim. Biosci. 2014, 4, 199–222. [Google Scholar] [CrossRef]
- Adams, V.J.; Evans, K.M.; Sampson, J.; Wood, J.L. Methods and mortality results of a health survey of purebred dogs in the UK. J. Small Anim. Pract. 2010, 51, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, A.; Bonnett, B.N.; Ohagen, P.; Olson, P.; Hedhammar, A.; von Euler, H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev. Vet. Med. 2005, 69, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Moulton, J.E.; Rosenblatt, L.S.; Goldman, M. Mammary tumors in a colony of beagle dogs. Vet. Pathol. 1986, 23, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Moe, L. Population-based incidence of mammary tumours in some dog breeds. J. Reprod. Fertil. 2001, 57, 439–443. [Google Scholar]
- Evans, D.G.; Shenton, A.; Woodward, E.; Lalloo, F.; Howell, A.; Maher, E.R. Penetrance estimates for BRCA1 and BRCA2based on genetic testing in a Clinical Cancer Genetics service setting: Risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer 2008, 8, 155. [Google Scholar] [CrossRef]
- King, M.C.; Marks, J.H.; Mandell, J.B.; The New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef]
- Rivera, P.; von Euler, H. Molecular biological aspects on canine and human mammary tumors. Vet. Pathol. 2011, 48, 132–146. [Google Scholar] [CrossRef]
- Rivera, P.; Melin, M.; Biagi, T.; Fall, T.; Haggstrom, J.; Lindblad-Toh, K.; von Euler, H. Mammary tumor development in dogs is associated with BRCA1 and BRCA2. Cancer Res. 2009, 69, 8770–8774. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Morimatsu, M.; Ochiai, K.; Nagano, M.; Tomioka, Y.; Sasaki, N.; Hashizume, K.; Iwanaga, T. Novel variations and loss of heterozygosity of BRCA2 identified in a dog with mammary tumors. Am. J. Vet. Res. 2008, 69, 1323–1328. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Morimatsu, M.; Ochiai, K.; Ishiguro-Oonuma, T.; Wada, S.; Orino, K.; Watanabe, K. Reduced canine BRCA2 expression levels in mammary gland tumors. BMC Vet. Res. 2015, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Le, H.P.; Heyer, W.D.; Liu, J. Guardians of the Genome: BRCA2 and Its Partners. Genes 2021, 12, 1229. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Suarez, B.; Gillespie, D.A.; Pawlak, A. DNA damage response proteins in canine cancer as potential research targets in comparative oncology. Vet. Comp. Oncol. 2022, 20, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Thumser-Henner, P.; Nytko, K.J.; Rohrer Bley, C. Mutations of BRCA2 in canine mammary tumors and their targeting potential in clinical therapy. BMC Vet. Res. 2020, 16, 30. [Google Scholar] [CrossRef]
- Bignell, G.; Micklem, G.; Stratton, M.R.; Ashworth, A.; Wooster, R. The BRC repeats are conserved in mammalian BRCA2 proteins. Hum. Mol. Genet. 1997, 6, 53–58. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Ochiai, K.; Morimatsu, M.; Suzuki, Y.; Wada, S.; Taoda, T.; Iwai, S.; Chikazawa, S.; Orino, K.; Watanabe, K. Effects of the missense mutations in canine BRCA2 on BRC repeat 3 functions and comparative analyses between canine and human BRC repeat 3. PLoS ONE 2012, 7, e45833. [Google Scholar] [CrossRef]
- Wong, A.K.; Pero, R.; Ormonde, P.A.; Tavtigian, S.V.; Bartel, P.L. RAD51 interacts with the evolutionarily conserved BRC motifs in the hu man breast cancer susceptibility gene brca2. J. Biol. Chem. 1997, 272, 31941–31944. [Google Scholar] [CrossRef]
- Esashi, F.; Christ, N.; Gannon, J.; Liu, Y.; Hunt, T.; Jasin, M.; West, S.C. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 2005, 434, 598–604. [Google Scholar] [CrossRef]
- Ochiai, K.; Ishiguro-Oonuma, T.; Yoshikawa, Y.; Udagawa, C.; Kato, Y.; Watanabe, M.; Bonkobara, M.; Morimatsu, M.; Omi, T. Polymorphisms of canine BRCA2 BRC repeats affecting interaction with RAD51. Biomed. Res. 2015, 36, 155–158. [Google Scholar] [CrossRef]
- Luo, K.; Li, L.; Li, Y.; Wu, C.; Yin, Y.; Chen, Y.; Deng, M.; Nowsheen, S.; Yuan, J.; Lou, Z. A phosphorylation-deubiquitination cascade regulates the BRCA2-RAD51 axis in homologous recombination. Genes Dev. 2016, 30, 2581–2595. [Google Scholar] [CrossRef]
- Tal, A.; Arbel-Goren, R.; Stavans, J. Cancer-associated mutations in BRC domains of BRCA2 affect homologous recombination induced by Rad51. J. Mol. Biol. 2009, 393, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Ambjørn, S.M.; Duxin, J.P.; Hertz, E.P.T.; Nasa, I.; Duro, J.; Kruse, T.; Lopez-Mendez, B.; Rymarczyk, B.; Cressey, L.E.; Hansen, T.v.O.; et al. A complex of BRCA2 and PP2A-B56 is required for DNA repair by homologous recombination. Nat. Commun. 2021, 12, 5748. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Yoshikawa, Y.; Oonuma, T.; Tomioka, Y.; Hashizume, K.; Morimatsu, M. Interactions between canine RAD51 and full length or truncated BRCA2 BRC repeats. Vet. J. 2011, 190, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef]
- Ozmen, O.; Kul, S.; Risvanli, A.; Ozalp, G.; Sabuncu, A.; Kul, O. Somatic SNPs of the BRCA2 gene at the fragments encoding RAD51 binding sites of canine mammary tumors. Vet. Comp. Oncol. 2017, 15, 1479–1486. [Google Scholar] [CrossRef]
- Maues, T.; El-Jaick, K.B.; Costa, F.B.; Araujo, G.E.F.; Soares, M.V.G.; Moreira, A.S.; Ferreira, M.L.G.; Ferreira, A.M.R. Common germline haplotypes and genotypes identified in BRCA2 exon 11 of dogs with mammary tumours and histopathological analyses. Vet. Comp. Oncol. 2018, 16, 379–384. [Google Scholar] [CrossRef]
- Borge, K.S.; Borresen-Dale, A.L.; Lingaas, F. Identification of genetic variation in 11 candidate genes of canine mammary tumour. Vet. Comp. Oncol. 2011, 9, 241–250. [Google Scholar] [CrossRef]
- Hsu, W.L.; Huang, Y.H.; Chang, T.J.; Wong, M.L.; Chang, S.C. Single nucleotide variation in exon 11 of canine BRCA2 in healthy and cancerous mammary tissue. Vet. J. 2010, 184, 351–356. [Google Scholar] [CrossRef]
- Enginler, S.O.; Akis, I.; Toydemir, T.S.; Oztabak, K.; Haktanir, D.; Gunduz, M.C.; Kirsan, I.; Firat, I. Genetic variations of BRCA1 and BRCA2 genes in dogs with mammary tumours. Vet. Res. Commun. 2014, 38, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.; Radhika, G.; Aravindakshan, T.V.; Thomas, N.; Devi, S.S. Association of A4304G in exon eleven of Brca2 gene with canine mammary tumour. J. Vet. Anim. Sci. 2022, 53, 401–406. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Colombo, J.; Goncalves, F.M.; de Carvalho, L.A.L.; Costa, D.S.; Henrique, T.; Novais, A.A.; Moschetta-Pinheiro, M.G.; Chuffa, L.G.A.; Coutinho, L.L.; et al. Liquid biopsy can detect BRCA2 gene variants in female dogs with mammary neoplasia. Vet. Comp. Oncol. 2022, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Moschetta-Pinheiro, M.G.; Novais, A.A.; Stoppe, B.R.; Bonini, E.D.; Goncalves, F.M.; Fukumasu, H.; Coutinho, L.L.; Chuffa, L.G.A.; Zuccari, D. Liquid Biopsy as a Diagnostic and Prognostic Tool for Women and Female Dogs with Breast Cancer. Cancers 2021, 13, 5233. [Google Scholar] [CrossRef] [PubMed]

| Variant | SIFT Score | Mutant Homozygosity | Heterozygosity | Remained Non-Mutant Sample | ||||
|---|---|---|---|---|---|---|---|---|
| Nucleotide Change | Amino Acid Change | Tumor Sample | Tumor-Free Sample | Tumor Sample | Tumor-Free Sample | Tumor Sample | Tumor-Free Sample | |
| 2135G>A | E643K | 0.29 | 1 | 0 | 2 | 2 | 69 | 76 |
| 2213A>G | N669D | 0.36 | 1 | 1 | 3 | 2 | 68 | 75 |
| 2329T>C | Synonymous | 2 | 0 | 3 | 1 | 67 | 77 | |
| 2609A>C | K801Q | 0 | 13 | 24 | 23 | 20 | 36 | 36 |
| 2696A>G | I830V | 0.59 | 1 | 1 | 3 | 2 | 67 | 78 |
| 3538T>G | I1110M | 0 | 1 | 0 | 0 | 2 | 71 | 76 |
| 4481A>C | T1425P | 0 | 5 | 1 | 2 | 8 | 64 | 72 |
| 4512A>G | K1435R | 0.35 | 18 | 8 | 12 | 8 | 41 | 65 |
| 5788G>A | Synonymous | 1 | 0 | 3 | 1 | 67 | 80 | |
| 6886A>G | I2226M | 0.05 | 1 | 1 | 4 | 2 | 66 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Kitano, T.; Morimatsu, M.; Ochiai, K.; Ishiguro-Oonuma, T.; Oosumi, K.; Lin, X.; Orino, K.; Yoshikawa, Y. A Highly Conserved Region in BRCA2 Suppresses the RAD51-Interaction Activity of BRC Repeats. Vet. Sci. 2023, 10, 145. https://doi.org/10.3390/vetsci10020145
Zhu Z, Kitano T, Morimatsu M, Ochiai K, Ishiguro-Oonuma T, Oosumi K, Lin X, Orino K, Yoshikawa Y. A Highly Conserved Region in BRCA2 Suppresses the RAD51-Interaction Activity of BRC Repeats. Veterinary Sciences. 2023; 10(2):145. https://doi.org/10.3390/vetsci10020145
Chicago/Turabian StyleZhu, Zida, Taisuke Kitano, Masami Morimatsu, Kazuhiko Ochiai, Toshina Ishiguro-Oonuma, Kosuke Oosumi, Xianghui Lin, Koichi Orino, and Yasunaga Yoshikawa. 2023. "A Highly Conserved Region in BRCA2 Suppresses the RAD51-Interaction Activity of BRC Repeats" Veterinary Sciences 10, no. 2: 145. https://doi.org/10.3390/vetsci10020145
APA StyleZhu, Z., Kitano, T., Morimatsu, M., Ochiai, K., Ishiguro-Oonuma, T., Oosumi, K., Lin, X., Orino, K., & Yoshikawa, Y. (2023). A Highly Conserved Region in BRCA2 Suppresses the RAD51-Interaction Activity of BRC Repeats. Veterinary Sciences, 10(2), 145. https://doi.org/10.3390/vetsci10020145







