Detection, Quantification and Molecular Characterization of Fowl Adenoviruses Circulating in Ecuadorian Chicken Flocks during 2019–2021
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction
2.3. qPCR to Detect FAdV
2.4. Molecular Characterization of FAdV
2.5. Statistical Analysis
2.6. GenBank Accession Numbers
3. Results
3.1. qPCR Assay—Determination of Standard Curve
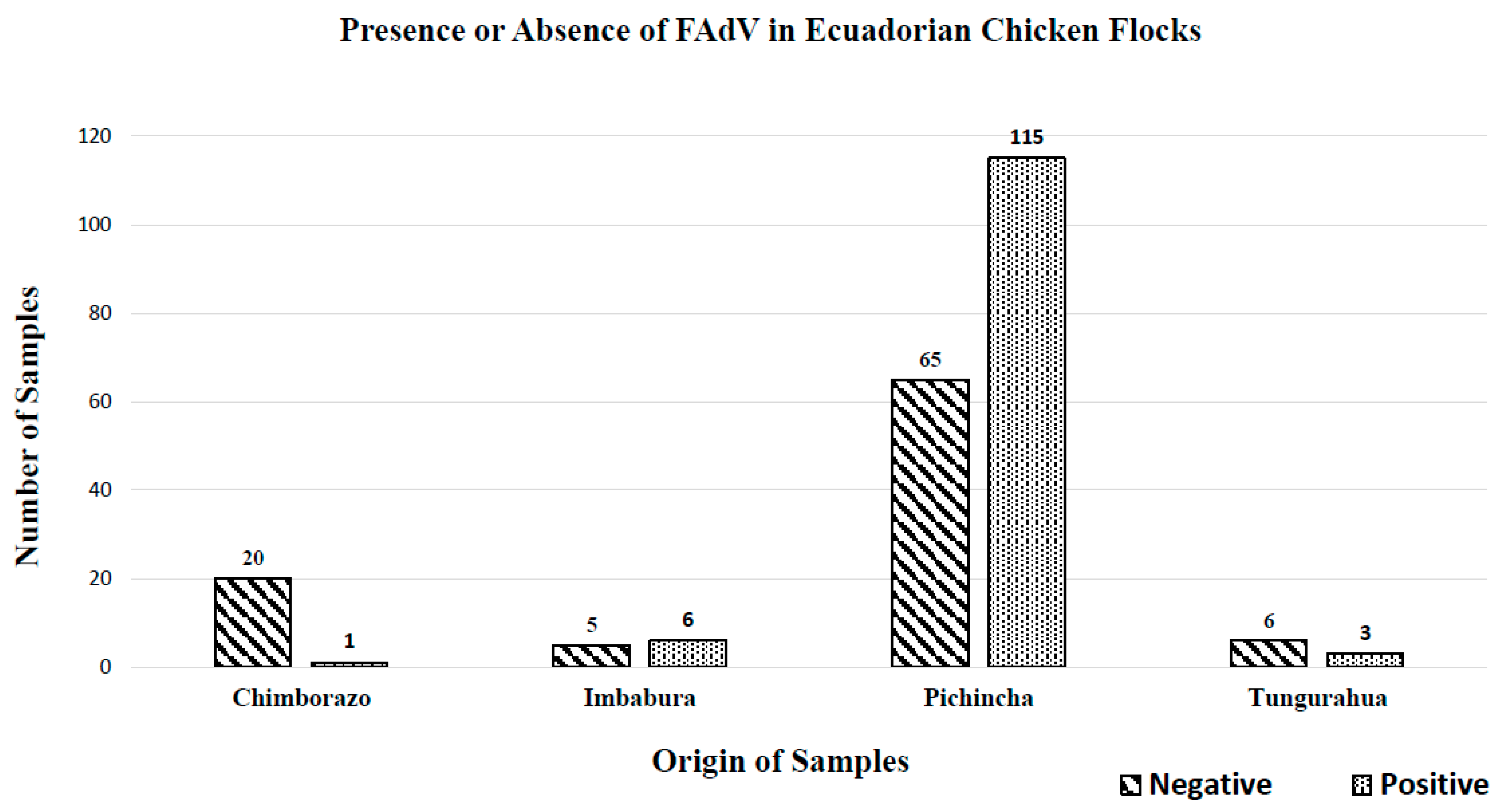
3.2. Detection and Quantification of FAdV
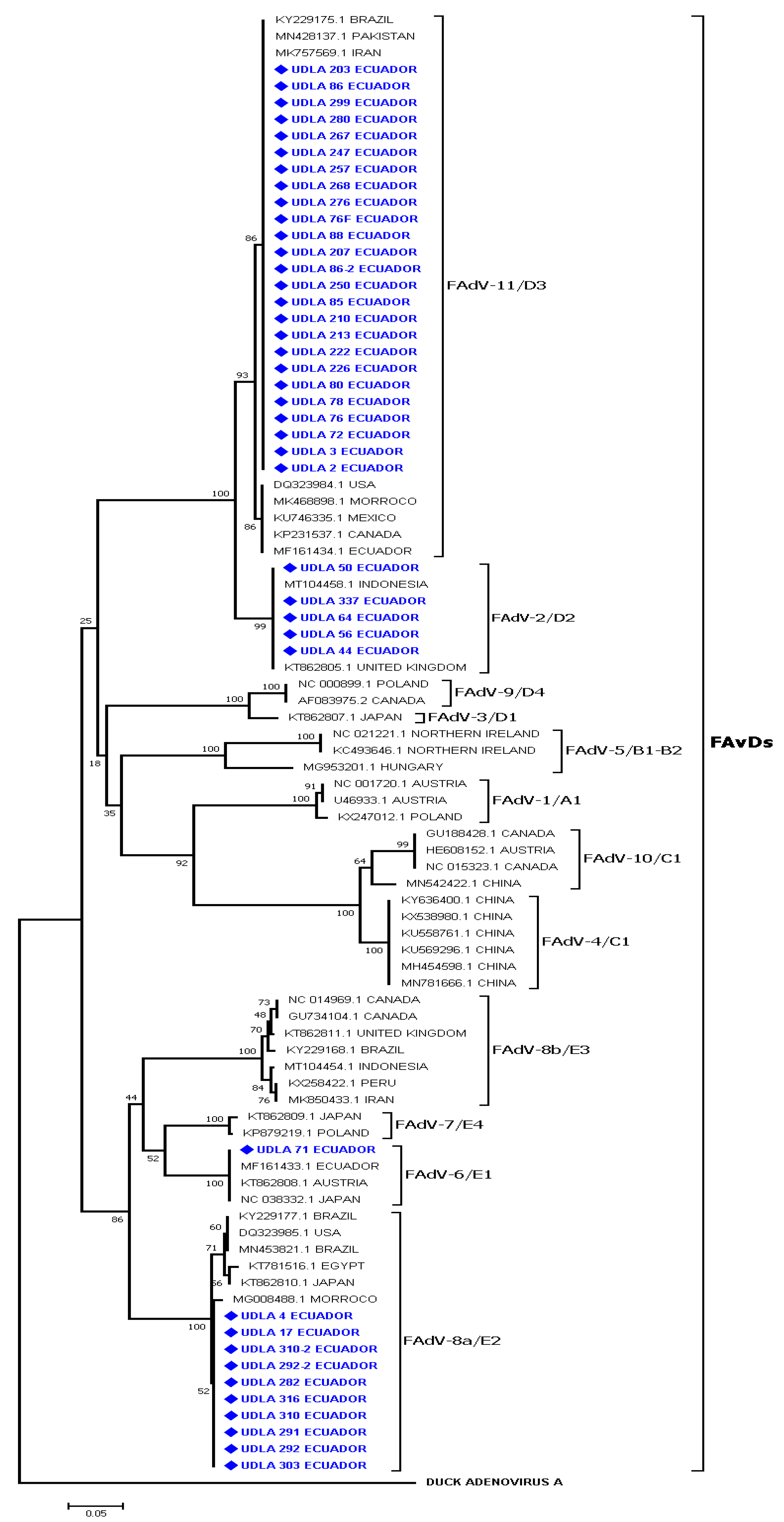
3.3. Sequencing and Phylogenetic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hess, A. Infection, 14th ed.; Swayne, D., Boulaine, E., Logue, C., McDougald, L., Nair, V., Suarez, D.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 322–332. [Google Scholar]
- Benko, K.; Aoki, N.; Arnberg, A.J.; Davison, M.; Echavarria, M.; Hess, M.S.; Jones, G.L.; Kajan, A.E.; Kajon, S.K.; Mittal, I.I.; et al. ICTV Virus Taxonomy Profile: Adenoviridae. J. Gen. Virol. 2022, 103, 1–2. [Google Scholar] [CrossRef]
- Berk, A. The Viruses and Their Replication, 5th ed.; Knipe, S.E., Howley, D.M., Griffin, P.M., Lamb, D.E., Martin, R.A., Roizman, M.A., Straus, B., Eds.; Lippincott Willians & Wilkins: Boston State, MA, USA, 2007; pp. 2356–2394. [Google Scholar]
- Harrach, Z.L.; Tarján, M.; Benkő, M. Adenoviruses across the animal kingdom: A walk in the zoo. FEBS Lett. 2019, 593, 3660–3673. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.D.; Rautenschlein, S.; Mahsoub, H.M.; Pierson, F.W.; Reed, W.M.; Jack, S.W. Adenovirus Infection, 14th ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2020; pp. 321–363. [Google Scholar] [CrossRef]
- Marek, A.; Günes, E.; Schulz, M.; Hess, M.S. Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. J. Virol. Methods 2010, 170, 147–154. [Google Scholar] [CrossRef]
- Nakamura, M.; Mase, Y.; Yamamoto, K.; Takizawa, M.; Kabeya, T.; Wakuda, M.; Matsuda, T.; Chikuba, Y.; Yamamoto, T.; Ohyama, N.; et al. Inclusion Body Hepatitis Caused by Fowl Adenovirus in Broiler Chickens in Case Report—Inclusion Body Hepatitis Caused by Fowl Adenovirus in Broiler Chickens in Japan, 2009–2010. Avian Pathol. 2010, 55, 719–723. [Google Scholar]
- Mettifogo, E.; Nuñez, L.F.; Chacón, J.L.; Santander Parra, S.H.; Astolfi-Ferreira, C.S.; Jerez, J.A.; Jones, R.C.; Piantino Ferreira, A.J. Emergence of Enteric Viruses in Production Chickens is a Concern for Avian Health. Cient. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Niczyporuk, J.S. Phylogenetic and geographic analysis of fowl adenovirus field strains isolated from poultry in Poland. Arch. Virol. 2016, 161, 33–42. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, D.; Torres, B.H.P.; Quezada, E.C.M.; Ferreira, A.J.P. Molecular characterization of fowl adenovirus in commercial chicken flocks in Ecuador. Granja 2018, 28, 84–91. [Google Scholar] [CrossRef]
- De la Torre, L.F.N.; Nuñez, S.H.; Santander Parra, C.S.; Astolfi-Ferreira, A.J.; Piantino, F. Molecular characterization of fowl adenovirus group I in commercial broiler chickens in Brazil. Virus Dis. 2018, 29, 83–88. [Google Scholar] [CrossRef]
- Schachner, M.; Matos, B.; Grafl, M.; Hess, M.S. Fowl adenovirus-induced diseases and strategies for their control–A review on the current global situation. Avian Pathol. 2018, 47, 111–126. [Google Scholar] [CrossRef]
- Kaján, I.; Affranio, A.; Tóthné, B.; Kecskeméti, S.; Benkő, M. An emerging new fowl adenovirus genotype. Heliyon 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Mo, J. Historical investigation of fowl adenovirus outbreaks in South Korea from 2007 to 2021: A comprehensive review. Viruses 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Adair, J.M. Adenoviruses, 5th ed.; Zavala, L., Swayne, D., Glisson, J., Pearson, J., Reed, W., Jackwood, M., Woolcock, P., Eds.; The American Association of Avian Pathologists: Kennett Square, PA, USA, 2008; pp. 84–89. [Google Scholar]
- Toro, O.; González, C.; Escobar, L.; Cerda, M.A.; Morales, C. Vertical Induction of the Inclusion Body Hepatitis/Hydropericardium Syndrome with Fowl Adenovirus and Chicken Anemia Virus. Avian Dis. 2001, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, G.; Boschmans, M.; Berg, T.P.V.D.; Decaesstecker, M. Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathol. 2001, 30, 655–660. [Google Scholar] [CrossRef]
- Meulemans, G.; Couvreur, B.; Decaesstecker, M.; Boschmans, M.; Berg, T.P.V.D. Phylogenetic analysis of fowl adenoviruses. Avian Pathol. 2004, 33, 164–170. [Google Scholar] [CrossRef]
- Günes, A.; Marek, M.; Hess, M.S. Species determination of fowl adenoviruses based on the 52K gene region. Avian Dis. 2013, 57, 290–294. [Google Scholar] [CrossRef]
- Green, J.; Sambrook, J. Isolation of high-molecular-weight DNA using organic solvents. Cold Spring Harb. Protoc. 2017, 2017, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Günes, A.; Marek, B.; Grafl, E.; Berger, M.; Hess, M.S. Real-time PCR assay for universal detection and quantitation of all five species of fowl adenoviruses (FAdV-A. to FAdV-E). J. Virol. Methods. 2012, 183, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Li, L.; Luo, L.; Luo, Q.; Zhang, T.; Zhao, K.; Wang, H.; Zhang, R.; Lu, Q.; Pan, Z.; Shao, H.; et al. Genome sequence of a fowl adenovirus serotype 4 strain lethal to chickens, isolated from China. Genome Announc. 2016, 4, 2–3. [Google Scholar] [CrossRef]
- Raue, M.; Hess, M.S. Hexon based PCRs combined with restriction enzyme analysis for rapid detection and differentiation of fowl adenoviruses and egg drop syndrome virus. J. Virol. Methods 1998, 73, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Mase, M.; Nakamura, K.; Imada, T. Characterization of Fowl adenovirus serotype 4 isolated from chickens with hydropericardium syndrome based on analysis of the short fiber protein gene. J. Vet. Diagn. Investig. 2010, 22, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.R.; Rudis, M.; Vasquez-Lee, R.D.; Montgomery, R.D. A broadly applicable method to characterize large DNA viruses and adenoviruses based on the DNA polymerase gene. Virol. J. 2006, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaján, S.; Sameti, M.; Benko, M. Partial sequence of the DNA-dependent DNA polymerase gene of fowl adenoviruses: A reference panel for a general diagnostic PCR in poultry. Acta Vet. Hung. 2011, 59, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.H. Avian adenoviruses infections with special attention to inclusion body hepatitis/hydropericardium syndrome and egg drop syndrome. Pak. Vet. J. 2011, 31, 85–92. [Google Scholar]
- Şahindokuyucu, F.; Çöven, H.; Kılıç, Ö.; Yılmaz, M.; Kars, Ö.; Yazıcıoğlu, E.; Ertunç, Z.; Yazıcı, Z. First report of fowl aviadenovirus serotypes FAdV-8b and FAdV-11 associated with inclusion body hepatitis in commercial broiler and broiler-breeder flocks in Turkey. Arch. Virol. 2020, 165, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.A.E.; Mohamed, M.; Samir, N.M.; Hagag, A.; Erfan, M.; Said, A.E.S.; Arafa, W.M.M.; Hassan, M.E.; El Zowalaty, M.A.; Shahien, M.A. Epidemiological and molecular analysis of circulating fowl adenoviruses and emerging of serotypes 1, 3, and 8b in Egypt. Heliyon 2021, 7, e08366. [Google Scholar] [CrossRef] [PubMed]
- McFerran, B.M.C.; Adair, B. Avian adenoviruses—A review. Avian Pathol. 1977, 6, 189–217. [Google Scholar] [CrossRef]
- Fadly, B.J.; Riegle, K.; Nazerian, E.A.; Stephens, E.A. Some Observations on an Adenovirus Isolated from Specific Pathogen Free Chickens. Poult. Sci. 1980, 59, 21–27. [Google Scholar] [CrossRef]
- Kang, M.; El-Gazzar, H.S.; Sellers, F.; Dorea, S.M.; Williams, T.; Kim, S.; Collett, E.; Mundt, E. Investigation into the aetiology of runting and stunting syndrome in chickens. Avian Pathol. 2012, 41, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Linnemann, A.H.; Icard, V.; Durairaj, E.; Mundt, H.S.; Sellers, C. Chicken astrovirus as an aetiological agent of runting-stunting syndrome in broiler chickens. J. Gen. Virol. 2018, 1–9. [Google Scholar] [CrossRef]
- Jindal, D.P.; Patnayak, A.F.; Ziegler, A.; Lago, S.M.; Goyal, S. Experimental reproduction of poult enteritis syndrome: Clinical findings, growth response, and microbiology. Poult. Sci. 2009, 88, 949–958. [Google Scholar] [CrossRef]
- Zsak, R.M.; Cha, J.M.; Day, A.R.M.; Cha, R.M. Chicken parvovirus-induced runting-stunting syndrome in young broilers. Avian Dis. 2013, 57, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, L.F.N.; Santander-Parra, S.H.; Kyriakidis, N.C.; Astolfi-Ferreira, C.S.; Buim, M.R.; De La Torre, D.; Ferreira, A.J.P. Molecular characterization and determination of relative cytokine expression in naturally infected day-old chicks with chicken astrovirus associated to white chick syndrome. Animals 2020, 10, 1195. [Google Scholar] [CrossRef]
- Niczyporuk, H.; Czekaj, H. A comparative pathogenicity analysis of two adenovirus strains, 1/A and 8a/E, isolated from poultry in Poland. Arch. Virol. 2018, 163, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- House, A.; Mazaheri, C.; Prusas, M.; Hess, M.S. Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathol. 1998, 27, 269–276. [Google Scholar] [CrossRef]
- Mase, K.; Nakamura, K. Phylogenetic analysis of fowl adenoviruses isolated from chickens with gizzard erosion in Japan. J. Vet. Med. Sci. 2014, 76, 1535–1538. [Google Scholar] [CrossRef]
- Niczyporuk, W.; Kozdrun, H.; Czekaj, K.; Piekarska, N.; Stys, F. Characterisation of adenovirus strains represented species B and E isolated from broiler chicken flocks in eastern Poland. Heliyon 2021, 7, e06225. [Google Scholar] [CrossRef]
- Grafl, F.; Aigner, D.; Liebhart, A.; Marek, I.; Prokofieva, J.; Bachmeier, M.; Hess, M.S. Vertical transmission and clinical signs in broiler breeders and broilers experiencing adenoviral gizzard erosion. Avian Pathol. 2012, 41, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Steer, J.R.; Sandy, D.; O’Rourke, P.C.; Scott, G.F.; Browning, A.H.; Noormohammadi, A. Chronological analysis of gross and histological lesions induced by field strains of fowl adenovirus serotypes 1, 8b and 11 in one-day-old chickens. Avian Pathol. 2015, 44, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Popowich, D.; Ojkic, S.; Kurukulasuriya, B.; Chow-Lockerbie, T.; Gunawardana, K.; Goonewardene, R.; Karunarathna, L.E.; Ayalew, K.A.; Ahmed, S.K.; et al. Inactivated and live bivalent fowl adenovirus (FAdV8b + FAdV11) breeder vaccines provide broad-spectrum protection in chicks against inclusion body hepatitis (IBH). Vaccine 2018, 36, 744–750. [Google Scholar] [CrossRef]
- Steer-Cope, J.R.; Sandy, D.; O’Rourke, P.C.; Scott, G.F.; Browning, A.H.; Noormohammadi, A. Vaccination with FAdV-8a induces protection against inclusion body hepatitis caused by homologous and heterologous strains. Avian Pathol. 2019, 48, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Chitradevi, K.; Sukumar, P.; Suresh, G.A.; Balasubramaniam, D.; Kannan, D. Molecular typing and pathogenicity assessment of fowl adenovirus associated with inclusion body hepatitis in chicken from India. Trop. Anim. Health Prod. 2021, 53, 412. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Lv, K.; Wang, Z.; Yang, Y.; Li, H.; Chen, H. Characterization of Co-infection With Fowl Adenovirus Serotype 4 and 8a. Front. Microbiol. 2021, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, M.; Sun, Q.; Zeng, Y.; Huang, J.; Dong, L.; Li, S.; Huang, M.; Liao, M. The first complete genome sequence and pathogenicity characterization of fowl adenovirus serotype 2 with inclusion body hepatitis and hydropericardium in China. Front. Vet. Sci. 2022, 9, 951554. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Chen, P.; Zhang, S.; Sun, J.; Yuan, W. Pathogenicity and molecular characterization of a fowl adenovirus 4 isolated from chicken associated with IBH and HPS in China. BMC Vet. Res. 2018, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ishag, H.Z.A.; Terab, A.M.A.; El Tigani-Asil, E.T.A.; Bensalah, O.K.; Khalil, N.A.H.; Khalafalla, A.I.; Al Hammadi, Z.M.A.H.; Shah, A.A.M.; Al Muhairi, S.S.M.; Al Hammadi, A.A.M.; et al. Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE. Vet. Sci. 2022, 9, 154. [Google Scholar] [CrossRef]
- Changjing, L.; Haiying, W.; Dongdong, W.; Jingjing, W.; Youming, W.; Shouchun, L.; Jida, L.; Ping, W.; Jianlin, X.; Shouzhen, C.; et al. Characterization of fowl adenoviruses isolated between 2007 and 2014 in China. Vet. Microbiol. 2016, 197, 62–67. [Google Scholar] [CrossRef]
- Guan, Y.; Tian, X.; Han, X.; Yang, H.; Wang, H. Complete genome sequence and pathogenicity of fowl adenovirus serotype 4 involved in hydropericardium syndrome in Southwest China. Microb. Pathog. 2018, 117, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yuan, J.; Yu, Y.; Zhang, W.; Ai, Y.; Wang, Y. Identification, pathogenicity of novel fowl adenovirus serotype 4 SDJN0105 in shandong, china and immunoprotective evaluation of the newly developed inactivated oil-emulsion FAdV-4 vaccine. Viruses 2019, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.C.; Corredor, B.D.; Griffin, P.J.; Krell, É.; Nagy, E. Fowl adenovirus 4 (FAdV-4)-based infectious clone for vaccine vector development and viral gene function studies. Viruses 2018, 10, 97. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Q.; Kan, Y.; Mu, W.; Zhang, J.; Chen, L.; Li, H.; Fu, T.; Li, Z.; Wan, Z.; et al. FAdV-4 without Fiber-2 Is a Highly Attenuated and Protective Vaccine Candidate. Microbiol. Spectr. 2022, 10, e01436-21. [Google Scholar] [CrossRef]
- De Luca, A.; Schachner, T.; Mitra, S.; Heidl, D.; Liebhart, M.; Hess, M.S. Fowl adenovirus (FAdV) fiber-based vaccine against inclusion body hepatitis (IBH) provides type-specific protection guided by humoral immunity and regulation of B and T cell response. Vet. Res. 2020, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Schachner, A.; Heidl, S.; Hess, M.S. Vaccination with a fowl adenovirus chimeric fiber protein (crecFib-4/11) simultaneously protects chickens against hepatitis-hydropericardium syndrome (HHS) and inclusion body hepatitis (IBH). Vaccine 2022, 15, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xie, W.; Zhang, J.; Zhang, W.; Wang, M.; Lian, Z.; Zhao, D.; Ren, S.; Xie, Y.; Lin, T.; et al. A Novel Recombinant FAdV-4 Virus with Fiber of FAdV-8b Provides Efficient Protection against Both FAdV-4 and FAdV-8b. Viruses 2022, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Min, H.T.L.; Lai, J.; Mo, J. Epidemiology of fowl adenovirus (FAdV) infections in South Korean chickens during 2013–2019 following introduction of FAdV-4 vaccines. Avian Pathol. 2021, 50, 182–189. [Google Scholar] [CrossRef]
| Virus | Gene Target | Primer Name | Primer Sequence | Amplicon Size (bp) | Assay | Reference |
|---|---|---|---|---|---|---|
| FAdV | 52K and pIIIa | 52K-fw | 5′-ATG GCK CAG ATG GCY AAG G-3′ | 176 | qPCR | [21] |
| 52K-rv | 5′-AGC GCC TGG GTC AAA CCG A-3′ | |||||
| 52K-F | 5′-TGT ACG AYT TCG TSC ARA C-3′ | 773 | PCR | |||
| 52K-R | 5′-TAR ATG GCG CCY TGC TC-3′ | |||||
| Hexon | Hexon A | 5′-CAARTTCAGRCAGACGGT-3′ | 897 | [17] | ||
| Hexon B | 5′-TAGTGATGMCGSGACATCAT-3′ |
| Lineage | Age | Number of Analyzed Samples | Clinical Signs | Negative Samples to FAdV | Sample Origin | Positive Samples to FAdV | Average of Viral Gene Copies/mg of tissue | Sample Origin | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dwarfism | Stunting | Diarrhea | ||||||||||
| Broiler | Days | 7 | 16 | 16/16 | 16/16 | 14/16 | 13 | (10) EC-H, (2) EC-I, (1) EC-P | 3 | 327.51 | a | (1) EC-H, (1) EC-P, (1) EC-T |
| 12 | 2 | 2/2 | 2/2 | 2/2 | 2 | 866,152.96 | (2) EC-P | |||||
| 14 | 9 | 9/9 | 6/9 | 9/9 | 5 | (3) EC-H, (1) EC-I, (1) EC-P | 4 | 54,394.66 | (2) EC-I, (2) EC-P | |||
| 21 | 5 | 5/5 | 4/5 | 5/5 | 3 | (3) EC-P | 2 | 3842.90 | (2) EC-P | |||
| 25 | 3 | 3/3 | 3/3 | 1/3 | 1 | (1) EC-P | 2 | 142.89 | (2) EC-P | |||
| 26 | 1 | 1/1 | 1/1 | 1/1 | 1 | 33.10 | (1) EC-P | |||||
| 27 | 1 | 1/1 | 1/1 | 1/1 | 1 | DNQ | (1) EC-P | |||||
| 28 | 4 | 4/4 | 3/4 | 4/4 | 2 | (2) EC-P | 2 | 1,199,504.49 | (2) EC-P | |||
| 29 | 1 | 1/1 | 1/1 | 1/1 | 1 | (1) EC-P | ||||||
| 30 | 1 | 1/1 | 1/1 | 1/1 | 1 | 556.63 | (1) EC-P | |||||
| 31 | 1 | 1/1 | 1/1 | 1/1 | 1 | DNQ | (1) EC-P | |||||
| 32 | 1 | 1/1 | 1/1 | 1/1 | 1 | 14,050.52 | (1) EC-P | |||||
| 33 | 1 | 1/1 | 1/1 | 1/1 | 1 | 188.93 | (1) EC-P | |||||
| 34 | 1 | 1/1 | 1/1 | 1/1 | 1 | (1) EC-P | ||||||
| 35 | 14 | 14/14 | 10/14 | 14/14 | 10 | (1) EC-H, (9) EC-P | 4 | 2,999,651.62 | (4) EC-P | |||
| 36 | 1 | 1/1 | 0/1 | 1/1 | 1 | 668,606.68 | (1) EC-P | |||||
| 42 | 131 | 120/131 | 120/131 | 131/131 | 43 | (6) EC-H, (2) EC-I, (35) EC-P | 88 | 270,140.35 | (4) EC-I, (84) EC-P | |||
| Breeder Hens | Days | 4 | 4 | 4/4 | 2/4 | 4/4 | 2 | (2) EC-P | 2 | 4,660,482.12 | a | (2) EC-P |
| Layer Hens | Weeks | 14 | 1 | 1/1 | 1/1 | 1/1 | 1 | 393.39 | b | (1) EC-P | ||
| 30 | 3 | 3/3 | 2/2 | 3/3 | 1 | (1) EC-P | 2 | 883.37 | (2) EC-P | |||
| 35 | 6 | 6/6 | 5/6 | 6/6 | 4 | (4) EC-P | 2 | 7976.76 | (2) EC-P | |||
| 40 | 5 | 5/5 | 5/5 | 5/5 | 3 | (3) EC-P | 2 | 3,601,094.58 | (2) EC-P | |||
| 45 | 3 | 3/3 | 2/2 | 3/3 | 1 | (1) EC-P | 2 | 37,149.28 | (2) EC-T | |||
| 48 | 4 | 4/4 | 2/4 | 4/4 | 4 | (4) EC-T | ||||||
| 50 | 2 | 2/2 | 2/2 | 2/2 | 2 | (2) EC-T | ||||||
| Total | 96 | 125 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santander-Parra, S.H.; Caza, M.; Nuñez, L. Detection, Quantification and Molecular Characterization of Fowl Adenoviruses Circulating in Ecuadorian Chicken Flocks during 2019–2021. Vet. Sci. 2023, 10, 115. https://doi.org/10.3390/vetsci10020115
Santander-Parra SH, Caza M, Nuñez L. Detection, Quantification and Molecular Characterization of Fowl Adenoviruses Circulating in Ecuadorian Chicken Flocks during 2019–2021. Veterinary Sciences. 2023; 10(2):115. https://doi.org/10.3390/vetsci10020115
Chicago/Turabian StyleSantander-Parra, Silvana H., Manuel Caza, and Luis Nuñez. 2023. "Detection, Quantification and Molecular Characterization of Fowl Adenoviruses Circulating in Ecuadorian Chicken Flocks during 2019–2021" Veterinary Sciences 10, no. 2: 115. https://doi.org/10.3390/vetsci10020115
APA StyleSantander-Parra, S. H., Caza, M., & Nuñez, L. (2023). Detection, Quantification and Molecular Characterization of Fowl Adenoviruses Circulating in Ecuadorian Chicken Flocks during 2019–2021. Veterinary Sciences, 10(2), 115. https://doi.org/10.3390/vetsci10020115





