Analytical Performance Evaluation of the New GEM® Premier™ 5000 in Comparison to the Epoc® Blood Gas Analyzer in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
2.3. Sample Analysis
2.4. Instruments
2.5. Statistical Analysis
3. Results
3.1. Animals and Samples
3.2. Precision (Repeatability)
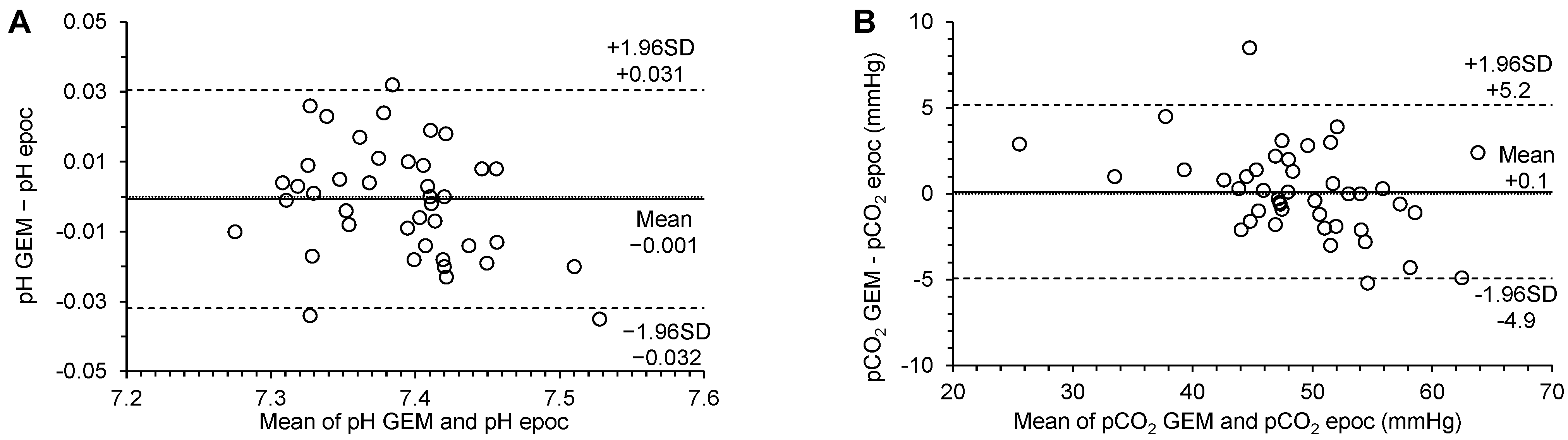
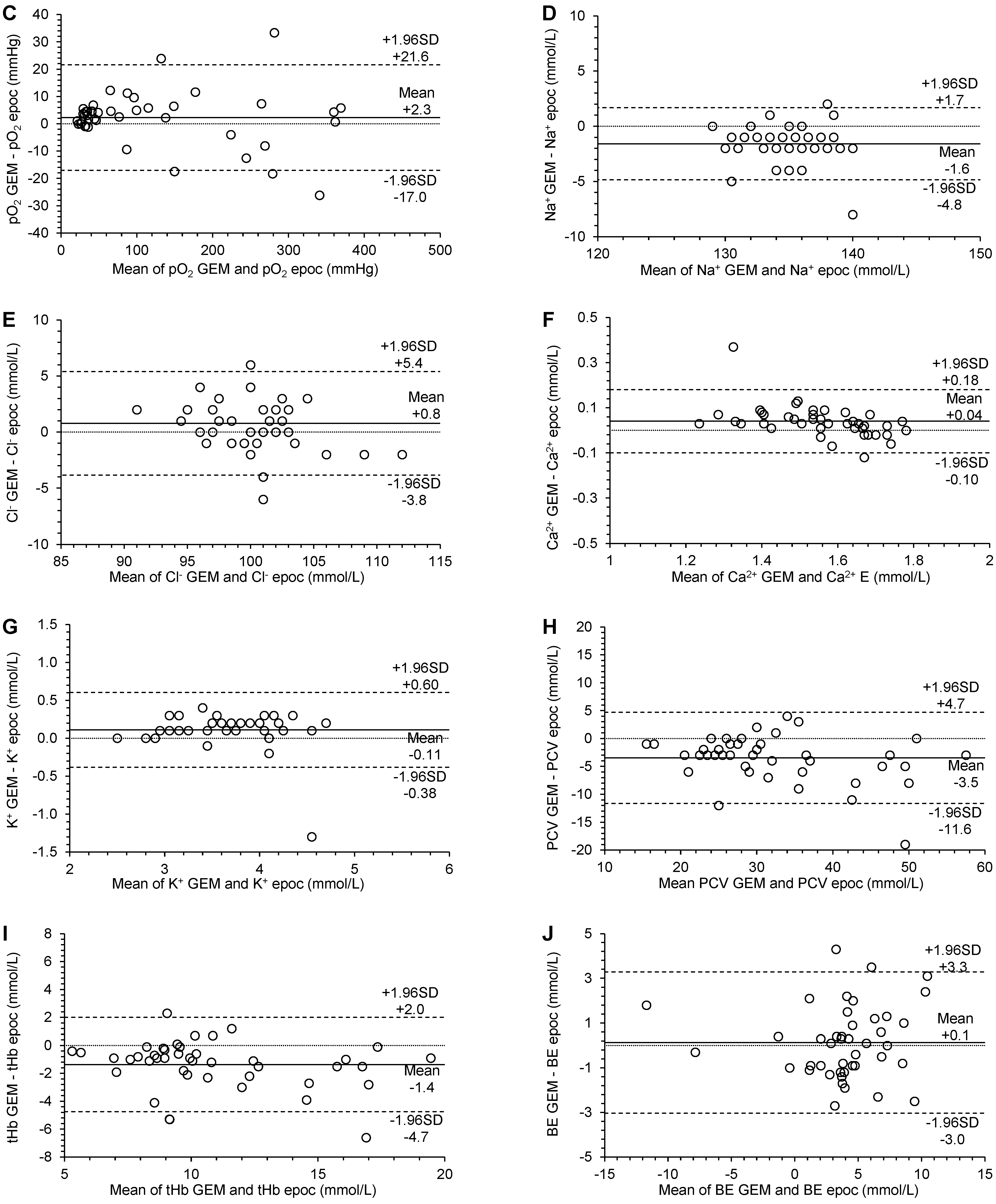
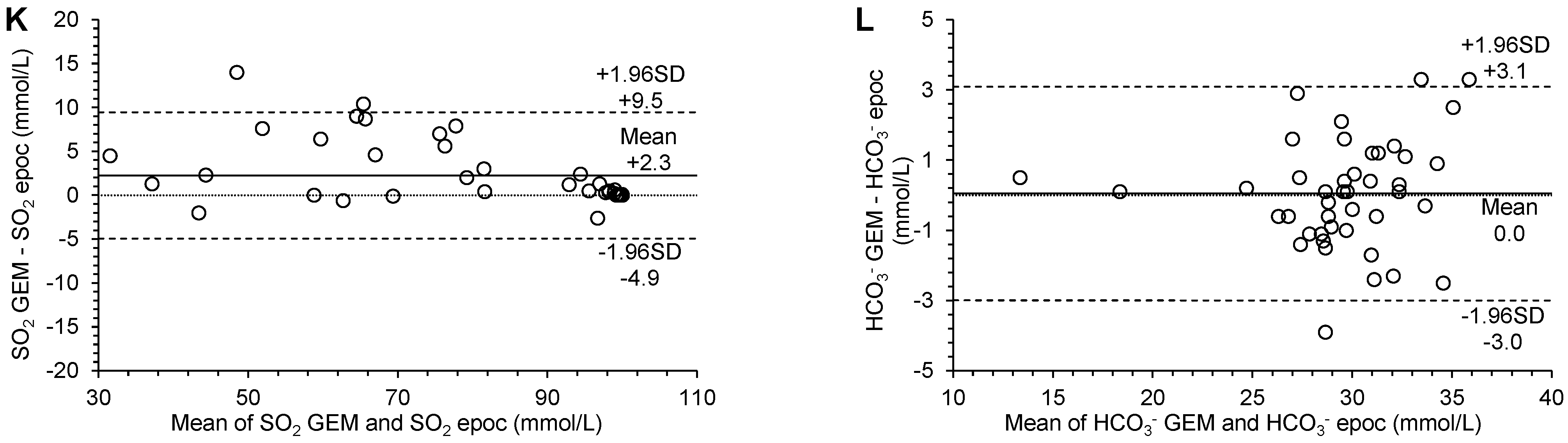
3.3. Agreement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bliskslager, A.T.; Jones, S.L. Obstructive disorders of the gastrointestinal tract. In Equine Internal Medicine, 3rd ed.; Reed, S.M., Bayly, W.M., Sellon, C.D., Eds.; Elsevier Saunders: St. Louis, MO, USA, 1998; pp. 922–936. [Google Scholar]
- Stämpfli, H.; Oliver-Espinosa, O. Clinical Chemistry Tests. In Large Animal Internal Medicine, 5th ed.; Smith, B.P., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2014; p. 362. [Google Scholar]
- Magdesian, K.G.; Southwood, L.L. Monitoring. In Equine Emergency and Critical Care Medicine; Southwood, L.L., Wilkins, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 635–637. [Google Scholar]
- Aguilera-Tejero, J.C.; López, I.; Bas, S.; Bas, S.; Mayer-Valor, R.; Rodríguez, M. Quantitative analysis of acid-base balance in show jumpers before and after exercise. Res. Vet. Sci. 2000, 68, 103–108. [Google Scholar] [CrossRef]
- Andrews, F.M.; Geiser, D.R.; White, S.L.; Williamson, L.H.; Maykuth, P.L.; Green, E.M. Haematological and biochemical changes in horses competing in a 3 Star horse trial and 3-day-event. Equine Vet. J. Suppl. 1995, 20, 57–63. [Google Scholar] [CrossRef]
- Foreman, J.H.; Waldsmith, J.K.; Lalum, R.B. Physical, acid-base and electrolyte changes in horses competing in Training, Preliminary and Intermediate horse trials. Equine Comp. Exerc. Physiol. 2004, 1, 99–105. [Google Scholar] [CrossRef]
- Hinchcliff, K.W.; Kohn, C.W.; Geor, R.; McCutcheon, L.J.; Foreman, J.; Andrews, F.M.; Allen, A.K.; White, S.L.; Williamson, L.H.; Maykuth, P.L. Acid:base and serum biochemistry changes in horses competing at a modified 1Star 3-day event. Equine Vet. J. Suppl. 1995, 20, 105–110. [Google Scholar]
- Rose, R.J.; Ilkiw, J.E.; Martin, I.C.A. Blood-gas, Acid-base and Haematological values in Horses during an Endurance ride. Equine Vet. J. 1979, 11, 56–59. [Google Scholar] [CrossRef]
- Rose, R.J.; Ilkiw, J.E.; Arnold, K.S.; Backhouse, J.W.; Sampson, D. Plasma biochemistry in the horse during 3-day event competition. Equine Vet. J. 1980, 12, 132–136. [Google Scholar] [CrossRef]
- Viu, J.; Jose-Cunilleras, E.; Armengou, L.; Cesarini, C.; Tarancón, I.; Rios, J.; Monreal, L. Acid-base imbalances during a 120 km endurance race compared by traditional and simplified strong ion difference methods. Equine Vet. J. Suppl. 2010, 42, 76–82. [Google Scholar] [CrossRef]
- Williamson, L.H.; Andrews, F.M.; Maykuth, P.L.; White, S.L.; Green, E.M. Biochemical changes in Three-day-event horses at the beginning, middle and end of Phase C and after Phase D. Equine Vet. J. Suppl. 1996, 22, 92–98. [Google Scholar] [CrossRef]
- Kirsch, K.; Sandersen, C. Traditional and quantitative analysis of acid-base and electrolyte imbalances in horses competing in cross-country competitions at 2-star to 5-star level. J. Vet. Intern. Med. 2020, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, K.; Detilleux, J.; Serteyn, D.; Sandersen, C. Comparison of two portable clinical analyzers to one stationary analyzer for the determination of blood gas partial pressures and blood electrolyte concentrations in horses. PLoS ONE 2019, 14, e0211104. [Google Scholar] [CrossRef]
- Bardell, D.; West, E.; Senior, M.J. Evaluation of a new handheld point-of-care blood gas analyser using 100 equine blood samples. Vet Anaesth. Analg. 2017, 44, 77–85. [Google Scholar] [CrossRef]
- Elmeshreghi, T.N.; Grubb, T.L.; Greene, S.A.; Ragle, C.A.; Wardrop, J.A. Comparison of Enterprise Point-of-Care and Nova Biomedical Critical Care Xpress analyzers for determination of arterial pH, blood gas, and electrolyte values in canine and equine blood. Vet. Clin. Pathol. 2018, 47, 415–424. [Google Scholar] [CrossRef]
- Westgard, J.O.; Carey, R.N.; Wold, S. Criteria for judging precision and accuracy in method development and evaluation. Clin. Chem. 1974, 20, 825–833. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC); Centers for Medicare and Medical Services (CMS); HHS. Medicare, Medicaid, and CLIA programs: Laboratory requirements relating to quality systems and certain personnel qualifications: Final rule. Fed. Regist. 2003, 68, 3640–3714. [Google Scholar]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- McBride, G.B. A Proposal for Strength-of-Agreement Criteria for Lin’s Concordance Correlation Coefficient; NIWA Client Report; HAM 2005-062; National Institute of Water & Atmospheric Research Ltd.: Hamilton, New Zealand, 2005. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical method for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Passing, H.; Bablok, W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part I. J. Clin. Chem. Clin. Biochem. 1983, 21, 709–720. [Google Scholar] [CrossRef]
- Matburger, A.C.; Henke, J.; Hirschberger, J.; Matburger, S.; Henke, W. Evaluation des tragbaren i-STAT®-Blutgasanalysegerätes im klinischen Einsatz beim Hund. Tierärztl Prax. 2000, 28, 132–137. [Google Scholar]
- Kennedy, S.A.; Constable, P.D.; Sen, I.; Couëtil, L. Effects of syringe type and storage conditions on results of equine blood gas and acid-base analysis. Am. J. Vet. Res. 2012, 73, 979–987. [Google Scholar] [CrossRef]
- Picandet, V.; Jeanneret, S.; Lavoie, J.P. Effects of syringe type and storage temperature on results of blood gas analysis in arterial blood of horses. J. Vet. Intern. Med. 2007, 21, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Mion, M.M.; Bragato, G.; Zaninotto, M.; Alessandroni, J.; Bernardini, S.; Plebani, M. Analytical performance evaluation of the new GEM® Premier™ 5000 analyzer in comparison to the GEM® Premier™ 4000 and the RapidPoint® 405 systems. Clin. Chim. Acta 2018, 486, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Krouwer, J.S.; Tholen, D.; Garber, C.; Goldschmidt, H.M.; Kroll, M.H.; Linnet, K.; Meier, K.; Robinowitz, M.; Kennedy, J.W. Method Comparison and Bias Estimation Using Patient Samples, Approved Guideline, 2nd ed.; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2010. [Google Scholar]
- Hughes, J.; Bardell, D. Determination of reference intervals for equine arterial blood-gas, acid-base and electrolyte analysis. Vet. Anaesth. Analg. 2019, 46, 765–771. [Google Scholar] [CrossRef]
- Taylor, L.E.; Kronfeld, D.S.; Ferrante, P.L.; Wilson, J.A.; Tiegs, W. Blood-gas measurements adjusted for temperature at three sites during incremental exercise in the horse. J. Appl. Physiol. 1998, 85, 1030–1036. [Google Scholar] [CrossRef]
- Bisson, J.; Younker, J. Correcting arterial blood gases for temperature: (When) is it clinically significant? Nurs. Crit. Care 2006, 11, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Linnet, K. Necessary sample size for method comparison studies based on regression analysis. Clin. Chem. 1999, 45, 882–894. [Google Scholar] [CrossRef]
| Measured Variables | GEM5000 | |||
|---|---|---|---|---|
| Mean ± SD of the 1st Sample | CV (%) of the 1st Sample | Mean CV (%) of All Duplicates | Precision Target (CV%) | |
| pH | 7.53 ± 0.025 | 0.33 | 0.07 | <0.1 [13,16] |
| pCO2 (mmHg) | 38 ± 2 | 5.26 | 0.87 | <2.4 [12,16] |
| pO2 (mmHg) | 79.5 ± 8.5 | 10.69 | 3.18 | <4.8 [12,16] |
| Na+ (mmol/L) | 132 ± 0 | 0 | 0.3 | <0.3 [12,16] |
| Cl− (mmol/L) | 95.5 ± 0.5 | 0.52 | 0.42 | <0.6 [14,16] |
| iCa2+ (mmol/L) | 1.67 ± 0.01 | 0.59 | 1.1 | <0.9 [14,16] |
| K+ (mmol/L) | 4.65 ± 0.15 | 3.22 | 0.8 | <2.3 [14,16] |
| PCV (%) | 31.5 ± 1.5 | 4.76 | 3.84 | <1.35 [14,16] |
| tHb (g/dL) | 10.7 ± 0.5 | 4.47 | 4.84 | <1.43 [14,16] |
| BE (mmol/L) | 8.8 ± 0.6 | 6.82 | 34.8 | <38.2 [16] |
| SO2 (%) | 96.75 ± 1.15 | 1.19 | 1.04 | <5 [23] |
| HCO3− (mmol/L) | 32.05 ± 1.15 | 0.47 | 1.74 | <5 [23] |
| Measured Variables | epoc | GEM5000 | ||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| pH | 7.390 ± 0.056 | 7.280–7.545 | 7.390 ± 0.052 | 7.270–7.510 |
| pCO2 (mmHg) | 48.7 ± 7.7 | 24.1–64.9 | 48.9 ± 6.6 | 27.0–65.0 |
| pO2 (mmHg) | 117.9 ± 111.3 | 21.0–366.2 | 120.2 ± 109.7 | 22.0–372.0 |
| Na+ (mmol/L) | 136 ± 3 | 129–144 | 134 ± 3 | 128–139 |
| Cl− (mmol/L) | 100 ± 4 | 90–113 | 101 ± 4 | 92–111 |
| iCa2+ (mmol/L) | 1.53 ± 0.16 | 1.14–1.78 | 1.57 ± 0.12 | 1.25–1.79 |
| K+ (mmol/L) | 3.6 ± 0.6 | 2.5–5.2 | 3.7 ± 0.5 | 2.5–4.8 |
| Hct (%) | 34 ± 11 | 16–59 | 30 ± 9 | 15–56 |
| tHb (g/dL) | 11.5 ± 3.7 | 5.5–20.2 | 10.2 ± 3.3 | 5.1–19.0 |
| BE (mmol/L) | 3.8 ± 4.1 | −12.6–10.7 | 4.0 ± 4.1 | −10.8–12.0 |
| SO2 (%) | 80.4 ± 22.0 | 29.3–100.0 | 82.7 ± 20.1 | 33.8–100.0 |
| HCO3− (mmol/L) | 29.5 ± 3.9 | 13.1–35.8 | 29.5 ± 4.1 | 13.6–37.5 |
| Measured Variables | Concordance Correlation Analysis | |||
|---|---|---|---|---|
| Lin’s rccc (95% CI) | Precision ρ | Accuracy Cb | ||
| pH | 0.956 (0.923 to 0.976) | 0.960 | 0.997 | substantial agreement |
| pCO2 (mmHg) | 0.935 (0.889 to 0.962) | 0.946 | 0.989 | moderate agreement |
| pO2 (mmHg) | 0.996 (0.992 to 0.998) | 0.996 | 1.000 | almost perfect agreement |
| Na+ (mmol/L) | 0.720 (0.569 to 0.824) | 0.833 | 0.865 | poor agreement |
| Cl− (mmol/L) | 0.811 (0.685 to 0.890) | 0.839 | 0.967 | poor agreement |
| iCa2+ (mmol/L) | 0.837 (0.739 to 0.901) | 0.897 | 0.933 | poor agreement |
| K+ (mmol/L) | 0.875 (0.785 to 0.929) | 0.895 | 0.977 | poor agreement |
| Hct (%) | 0.865 (0.779 to 0.920) | 0.925 | 0.936 | poor agreement |
| tHb (g/dL) | 0.814 (0.699 to 0.888) | 0.885 | 0.921 | poor agreement |
| BE (mmol/L) | 0.922 (0.862 to 0.957) | 0.923 | 0.999 | moderate agreement |
| SO2 (%) | 0.979 (0.965 to 0.988) | 0.989 | 0.990 | substantial agreement |
| HCO3− (mmol/L) | 0.926 (0.869 to 0.959) | 0.927 | 0.999 | moderate agreement |
| Measured Variables | Passing–Bablok Regression | Bland–Altman Plot | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept (95% CI) | Slope (95% CI) | Residual Standard Deviation (95% CI) | Bias (95% CI) | Lower Limit of Agreement (95% CI) | Upper Limit of Agreement (95% CI) | Passing Bablok | Bland–Altman | |||
| pH | 0.67 (−0.07 to 1.31) | 0.91 (0.82 to 1.01) | 0.011 (−0.022 to 0.022) | −0.001 (−0.006 to 0.004) | −0.032 (−0.040 to −0.024) | 0.031 (0.022 to 0.039) | no significant constant bias | no significant proportional bias | no significant systematic bias | p = 0.7682 |
| pCO2 (mmHg) | 7.13 (2.38 to 11.30) | 0.85 (0.76 to 0.95) | 1.690 (−3.312 to 3.312) | −0.1 (−0.7 to 0.9) | −4.9 (−6.3 to −3.6) | 5.2 (3.8 to 6.6) | significant constant bias | significant proportional bias | no significant systematic bias | p = 0.7512 |
| pO2 (mmHg) | 2.84 (0.24 to 5.00) | 1.01 (0.98 to 1.04) | 7.253 (−14.215 to 14.215) | 2.3 (−0.7 to 5.3) | −17.0 (−22.2 to −11.8) | 21.6 (16.4 to 26.8) | significant constant bias | no significant proportional bias | no significant systematic bias | p = 0.1356 |
| Na+ (mmol/L) | −2.0 (−2.00 to 15.50) | 1.0 (0.88 to 1.00) | 1.230 (−2.410 to 2.410) | −1.6 (−2.1 to −1.1) | −4.8 (−5.7 to −4.0) | 1.7 (0.8 to 2.6) | no significant constant bias | no significant proportional bias | significant systematic bias | p < 0.0001 |
| Cl− (mmol/L) | 14.86 (1.00 to 27.82) | 0.86 (0.73 to 1.00) | 1.614 (−3.163 to 3.163) | 0.8 (0.1 to 1.5) | −3.8 (−5.1 to −2.6) | 5.4 (4.1 to 6.7) | significant constant bias | no significant proportional bias | significant systematic bias | p = 0.0364 |
| Ca2+ (mmol/L) | 0.34 (0.18 to 0.51) | 0.81 (0.70 to 0.90) | 0.045 (−0.088 to 0.088) | 0.04 (0.02 to 0.06) | −0.10 (−0.14 to −0.06) | 0.18 (0.14 to 0.22) | significant constant bias | significant proportional bias | significant systematic bias | p = 0.0006 |
| K+ (mmol/L) | 0.10 (−0.26 to 0.10) | 1.00 (1.00 to 1.11) | 0.180 (−0.352 to 0.352) | 0.11 (0.03 to 0.19) | −0.38 (−0.51 to −0.25) | 0.60 (0.47 to 0.74) | no significant constant bias | no significant proportional bias | significant systematic bias | p = 0.0056 |
| HTC (%) | −0.47 (−3.00 to 2.90) | 0.92 (0.81 to 1.00) | 2.840 (−5.566 to 5.566) | −3.5 (−4.7 to −2.2) | −11.6 (−13.8 to −9.4) | 4.7 (2.5 to 6.9) | no significant constant bias | no significant proportional bias | significant systematic bias | p < 0.0001 |
| tHb (g/dL) | −0.04 (−1.23 to 1.12) | 0.91 (0.79 to 1.02) | 1.212 (−2.376 to 2.376) | −1.4 (−1.9 to −0.8) | −4.7 (−5.7 to −3.8) | 2.0 (1.1 to 2.9) | no significant constant bias | no significant proportional bias | significant systematic bias | p < 0.0001 |
| BE (mmol/L) | −0.10 (−1.45 to 0.34) | 1.07 (0.91 to 1.33) | 1.163 (−2.279 to 2.279) | 0.1 (−0.4 to 0.6) | −3.0 (−3.9 to −2.2) | 3.3 (2.4 to 4.1) | no significant constant bias | no significant proportional bias | no significant systematic bias | p = 0.6059 |
| SO2 (%) | 12.97 (6.10 to 15.73) | 0.87 (0.84 to 0.94) | 2.372 (−4.649 to 4.649) | 2.3 (1.1 to 3.4) | −4.9 (−6.9 to −3.0) | 9.4 (7.5 to 11.4) | no significant constant bias | significant proportional bias | significant systematic bias | p = 0.0002 |
| HCO3− (mmol/L) | −3.46 (−11.04 to 1.27) | 1.12 (0.96 to 1.38) | 1.116 (−2.188 to 2.188) | 0.0 (−0.4 to 0.5) | −3.0 (−3.8 to −2.2) | 3.1 (2.3 to 3.9) | no significant constant bias | no significant proportional bias | no significant systematic bias | p = 0.8377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandersen, C.; Dmitrovic, P.; Dupont, J.; Cesarini, C.; Guyot, H.; Serteyn, D.; Kirsch, K. Analytical Performance Evaluation of the New GEM® Premier™ 5000 in Comparison to the Epoc® Blood Gas Analyzer in Horses. Vet. Sci. 2023, 10, 114. https://doi.org/10.3390/vetsci10020114
Sandersen C, Dmitrovic P, Dupont J, Cesarini C, Guyot H, Serteyn D, Kirsch K. Analytical Performance Evaluation of the New GEM® Premier™ 5000 in Comparison to the Epoc® Blood Gas Analyzer in Horses. Veterinary Sciences. 2023; 10(2):114. https://doi.org/10.3390/vetsci10020114
Chicago/Turabian StyleSandersen, Charlotte, Petra Dmitrovic, Julien Dupont, Carla Cesarini, Hugues Guyot, Didier Serteyn, and Katharina Kirsch. 2023. "Analytical Performance Evaluation of the New GEM® Premier™ 5000 in Comparison to the Epoc® Blood Gas Analyzer in Horses" Veterinary Sciences 10, no. 2: 114. https://doi.org/10.3390/vetsci10020114
APA StyleSandersen, C., Dmitrovic, P., Dupont, J., Cesarini, C., Guyot, H., Serteyn, D., & Kirsch, K. (2023). Analytical Performance Evaluation of the New GEM® Premier™ 5000 in Comparison to the Epoc® Blood Gas Analyzer in Horses. Veterinary Sciences, 10(2), 114. https://doi.org/10.3390/vetsci10020114







