Case–Control Study: Endogenous Procalcitonin and Protein Carbonylated Content as a Potential Biomarker of Subclinical Mastitis in Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Animals and Management
2.3. Inclusion Criteria
2.4. Collection of Samples
2.5. Determination of Plasma PCT and PCC Concentration
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myers, M.J.; Smith, E.R.; Turfle, P.G. Biomarkers in veterinary medicine. Annu. Rev. Anim. Biosci. 2017, 5, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.R.; Skerrett-Byrne, D.A.; Gibb, Z.; Nixon, B.; Swegen, A. The Future of Biomarkers in Veterinary Medicine: Emerging Approaches and Associated Challenges. Animals 2022, 12, 2194. [Google Scholar] [CrossRef]
- Battaglia, F.; Baldoneschi, V.; Meucci, V.; Intorre, L.; Minunni, M.; Scarano, S. Detection of canine and equine procalcitonin for sepsis diagnosis in veterinary clinic by the development of novel MIP-based SPR biosensors. Talanta 2021, 230, 122347. [Google Scholar] [CrossRef] [PubMed]
- Nocera, I.; Bonelli, F.; Vitale, V.; Meucci, V.; Conte, G.; Jose-Cunilleras, E.; Gracia-Calvo, L.A.; Sgorbini, M. Evaluation of plasmatic procalcitonin in healthy, and in systemic inflammatory response syndrome (sirs) negative or positive colic horses. Animals 2021, 11, 2015. [Google Scholar] [CrossRef]
- Matur, E.; Dokuzeylül, B.; Özcan, M.; Çetinkaya, H.; Arslan, M.; Or, E.; Erhan, S.; Çötelioğlu, Ü. Can procalcitonin be used as a clinical biomarker during bacterial, viral and parasitic infections in dogs? Jpn. J. Vet. Res. 2021, 69, 5–17. [Google Scholar]
- Cho, J.G.; Oh, Y.I.; Song, K.H.; Seo, K.W. Evaluation and comparison of serum procalcitonin and heparin-binding protein levels as biomarkers of bacterial infection in cats. J. Feline Med. Surg. 2021, 23, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Maruna, P.; Nedelnikova, K.; Gurlich, R. Physiology and genetics of procalcitonin. Physiol. Res. 2000, 49, S57–S62. [Google Scholar]
- Meisner, M. Pathobiochemistry and clinical use of procalcitonin. Clin. Chim. Acta 2002, 323, 17–29. [Google Scholar] [CrossRef]
- Vijayan, A.L.; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef]
- Müller, B.; White, J.C.; Nylén, E.S.; Snider, R.H.; Becker, K.L.; Habener, J.F. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J. Clin. Endocrinol. Metab. 2001, 86, 396–404. [Google Scholar] [CrossRef]
- Kamat, I.S.; Ramachandran, V.; Eswaran, H.; Guffey, D.; Musher, D.M. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin. Infect. Dis. 2020, 70, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Christ-Crain, M.; Müller, B. Biomarkers in respiratory tract infections: Diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur. Respir. J. 2007, 30, 556–573. [Google Scholar] [CrossRef]
- Brodská, H.; Malíčková, K.; Adámková, V.; Benáková, H.; Šťastná, M.M.; Zima, T. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin. Exper. Med. 2013, 13, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Hochreiter, M.; Koehler, T.; Schweiger, A.M.; Bein, B.; Keck, F.S.; Von Spiegel, T. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: Results of a prospective randomized study. Langenbeck’s Arch. Surg. 2009, 394, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; Gonzalez del Castillo, J.; Jensen, J.U.; Kanizsai, P.L.; Kwa, A.L.H.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. 2019, 57, 1308–1318. [Google Scholar] [CrossRef]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Jose-Cunilleras, E.; Corazza, M.; Tognetti, R.; Guidi, G.; Intorre, L.; Sgorbini, M. Plasma procalcitonin concentration in healthy horses and horses affected by systemic inflammatory response syndrome. J. Vet. Intern. Med. 2015, 29, 1689–1691. [Google Scholar] [CrossRef]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Wagner, B.; Intorre, L.; Sgorbini, M. Kinetics of plasma procalcitonin, soluble CD14, CCL2 and IL-10 after a sublethal infusion of lipopolysaccharide in horses. Vet. Immunol. Immunopathol. 2017, 184, 29–35. [Google Scholar] [CrossRef]
- El-Deeb, W.; Fayez, M.; Elsohaby, I.; Mkrtchyan, H.V.; Alhaider, A. Changes in blood biomarkers in Arabian horses with Clostridium difficile-induced enterocolitis. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101525. [Google Scholar] [CrossRef]
- Kilcoyne, I.; Nieto, J.E.; Dechant, J.E. Diagnostic value of plasma and peritoneal fluid procalcitonin concentrations in horses with strangulating intestinal lesions. J. Am. Vet. Med. Assoc. 2020, 256, 927–933. [Google Scholar] [CrossRef]
- Bonelli, F.; Meucci, V.; Divers, T.; Radcliffe, R.; Jose-Cunilleras, E.; Corazza, M.; Guidi, G.; Tognetti, R.; Castagnetti, C.; Sgorbini, M. Evaluation of plasma procalcitonin concentrations in healthy foals and foals affected by septic systemic inflammatory response syndrome. J. Equine Vet. Sci. 2015, 35, 645–649. [Google Scholar] [CrossRef]
- Barton, A.K.; Rieger, M.; Teschner, D.; Gehlen, H. Procalcitonin—A Useful Biomarker for Pneumonia Associated with Rhodococcus equi? Mod. Res. Inflamm. 2016, 5, 13–19. [Google Scholar] [CrossRef]
- El-Deeb, W.; Fayez, M.; Alhumam, N.; Elsohaby, I.; Quadri, S.A.; Mkrtchyan, H. The effect of staphylococcal mastitis including resistant strains on serum procalcitonin, neopterin, acute phase response and stress biomarkers in Holstein dairy cows. PeerJ 2021, 9, e11511. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Madrigali, A.; Sgorbini, M.; Meucci, V.; Battaglia, F.; Guélat-Brechbuehl, M.; Sala, G.; Meylan, M. Case–Control study: Evaluation of plasma procalcitonin concentration as an indicator of inflammation in healthy and sick cows. Res. Vet. Sci. 2023, 153, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Siegert, S.; Fischer, A. Procalcitonin as an Endogenous Biomarker for Mastitis in Cows. Animals 2023, 13, 2204. [Google Scholar] [CrossRef]
- Ercan, N.; Tuzcu, N.; Başbug, O.; Tuzcu, M.; Alim, A. Diagnostic value of serum procalcitonin, neopterin, and gamma interferon in neonatal calves with septicemic colibacillosis. J. Vet. Diagn. Investig. 2016, 28, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Boccardo, A.; Pravettoni, D.; Meylan, M.; Belloli, A.; Sgorbini, M. Plasma procalcitonin concentration in healthy calves and those with septic systemic inflammatory response syndrome. Vet. J. 2018, 234, 61–65. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, W.; Elsohaby, I.; Fayez, M.; Mkrtchyan, H.V.; El-Etriby, D.; ElGioushy, M. Use of procalcitonin, neopterin, haptoglobin, serum amyloid A and proinflammatory cytokines in diagnosis and prognosis of bovine respiratory disease in feedlot calves under field conditions. Acta Trop. 2020, 204, 105336. [Google Scholar] [CrossRef]
- Akyüz, E.; Gökce, G. Neopterin, procalcitonin, clinical biochemistry, and hematology in calves with neonatal sepsis. Trop. Anim. Health Prod. 2021, 53, 354. [Google Scholar] [CrossRef]
- Akyüz, E.; Merhan, O.; Aydın, U.; Sezer, M.; Kuru, M.; Karakurt, E.; Yıldız, U.; Bozukluhan, K.; Batı, Y.U.; Yıldız, A.; et al. Neopterin, procalcitonin, total sialic acid, paraoxonase-1 and selected haematological indices in calves with aspiration pneumonia. Acta Vet. BRNO 2022, 91, 115–124. [Google Scholar] [CrossRef]
- El-Deeb, W.; Iacob, O.; Fayez, M.; Elsohaby, I.; Alhaider, A.; Mkrtchyan, H.V.; Ibrahim, A.; Alhumam, N. Assessment of the immune response of clinically infected calves to Cryptosporidium parvum infection. Agriculture 2022, 12, 1151. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidat. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Jha, J.C.; Banal, C.; Chow, B.S.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and kidney disease: Role of oxidative stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar] [PubMed]
- Hasselgren, P.O. Pathways of muscle protein breakdown in injury and sepsis. Curr. Opin. Clin. Nutr. Metab. Care 1999, 2, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kehrer, J.P. Oxidative changes in hypoxic-reoxygenated rabbit heart: A consequence of hypoxia rather than reoxygenation. Free Radic. Res. Comm. 1991, 14, 179–185. [Google Scholar] [CrossRef]
- Abu-Zidan, F.M.; Plank, L.D.; Windsor, J.A. Proteolysis in severe sepsis is related to oxidation of plasma protein. Eur. J. Surg. 2002, 168, 119–123. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Nocera, I.; Bonelli, F.; Meucci, V.; Rinnovati, R.; Spadari, A.; Intorre, L.; Pretti, C.; Sgorbini, M. Evaluation of Protein Carbonyl Content in Healthy and Sick Hospitalized Horses. Front. Vet. Sci. 2020, 7, 582886. [Google Scholar] [CrossRef]
- Molayi-Jabdaragi, N.; Esmaeilnejad, B.; Mohammadi, V. Evaluation of oxidative/nitrosative stress biomarkers and DNA damage in buffaloes naturally infected with Theileria annulata. Microb. Pathog. 2020, 138, 103821. [Google Scholar] [CrossRef]
- Ruggerone, B.; Troia, R.; Murgia, E.; Giunti, M.; Dondi, F.; Paltrinieri, S. Diagnosis of sepsis in dogs by measuring carbonylated proteins (PCos) and paraoxonase (PON-1). Int. J. Health Anim. Sci. Food Saf. 2018, 5. [Google Scholar] [CrossRef]
- Ruggerone, B.; Scavone, D.; Troìa, R.; Giunti, M.; Dondi, F.; Paltrinieri, S. Comparison of protein carbonyl (PCO), paraoxonase-1 (PON1) and C-reactive protein (CRP) as diagnostic and prognostic markers of septic inflammation in dogs. Vet. Sci. 2021, 8, 93. [Google Scholar] [CrossRef]
- Possenti, C.G.R.; Horn, R.C.; Diaz, J.D.S.; Seifert, Q.C.S.; Golle, D.P.; Koefender, J.; Mori, N.C.; Júnior, V.S.R. Mint Extract Present Antioxidant Action on the Erythrocytes of Dairy Cows Suffering from Mastitis? J. Agric. Sci. 2016, 8, 105–111. [Google Scholar] [CrossRef]
- Andrei, S.M.S.R.D.; Matei, S.; Rugină, D.; Bogdan, L.; Ştefănuţ, C. Interrelationships between the content of oxidative markers, antioxidative status, and somatic cell count in cow’s milk. Czech J. Anim. Sci. 2016, 61, 407–413. [Google Scholar] [CrossRef]
- Bae, H.; Jeong, C.H.; Cheng, W.N.; Hong, K.; Seo, H.G.; Han, S.G. Oxidative stress-induced inflammatory responses and effects of N-acetylcysteine in bovine mammary alveolar cells. J. Dairy Res. 2017, 84, 418–425. [Google Scholar] [CrossRef]
- Hogeveen, H.; Huijps, K.; Lam, T.J.G.M. Economic aspects of mastitis: New developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and classification of mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Harmon, R.J. Physiology of mastitis and factors affecting somatic cell counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Lakshmi, K.; Jayavardhanan, K. Screening of milk samples for sub-clinical and clinical mastitis by using CMT and SCC. JMSCR 2016, 4, 10853–10855. [Google Scholar] [CrossRef]
- Schukken, Y.; Wilson, D.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R. Monitoring udder health and milk quality using somatic cell counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef]
- Adkins, P.R.; Middleton, J.R. Methods for diagnosing mastitis. Vet. Clin. Food Anim. Pract. 2018, 34, 479–491. [Google Scholar] [CrossRef]
- Adkins, P.R.; Middleton, J.R.; Fox, L.K.; Pighetti, K.; Petersson-Wolfe, C. Laboratory Handbook on Bovine Mastitis; National Mastitis Council Inc.: New Prague, MN, USA, 2017. [Google Scholar]
- Wattenburger, K.; Schmidt, R.; Placheta, L.; Middleton, J.R.; Adkins, P.R.F. Evaluation of 4 different teat disinfection methods prior to collection of milk samples for bacterial culture in dairy cattle. J. Dairy Sci. 2020, 103, 4579–4587. [Google Scholar] [CrossRef]
- Meucci, V.; Orsetti, C.; Sgorbini, M.; Battaglia, F.; Cresci, M.; Bonelli, F. Can Procalcitonin Be Dosed in Bovine Milk Using a Commercial ELISA Kit? Animals 2020, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I. Determination of carbonyl content in oxidatively modified proteins. Meth Enzymol. 1990, 186, 464–478. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagents. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.K.; Pelli, A.; Rieger, M.; Gehlen, H. Procalcitonin as a biomarker in equine chronic pneumopathies. BMC Vet. Res. 2016, 12, 281. [Google Scholar] [CrossRef]
- Shaheen, T.; Ahmad, S.B.; Rehman, M.U.; Muzamil, S.; Bhat, R.R.; Hussain, I.; Bashir, N.; Mir, M.U.R.; Paray, B.A.; Dawood, M.A. Investigations on cytokines and proteins in lactating cows with and without naturally occurring mastitis. J. King Saud Univ. Sci. 2020, 32, 2863–2867. [Google Scholar] [CrossRef]
- Turk, R.; Rošić, N.; Kuleš, J.; Horvatić, A.; Gelemanovic, A.; Galen, A.; Ljubić, B.B.; Benić, M.; Stevanović, V.; Mrljak, V.; et al. Milk and serum proteomes in subclinical and clinical mastitis in Simmental cows. J. Proteom. 2021, 244, 104277. [Google Scholar] [CrossRef]
- Kumari, T.; Bhakat, C.; Choudhary, R. A review on subclinical mastitis in dairy cattle. Int. J. Pure Appl. Biosci. 2018, 6, 1291–1299. [Google Scholar]
- Feezor, R.J.; Oberholzer, C.; Baker, H.V.; Novick, D.; Rubinstein, M.; Moldawer, L.L.; Pribble, J.; Souza, S.; Dinarello, C.A.; Ertel, W.; et al. Molecular characterization of the acute inflammatory response to infections with gram-negative versus gram-positive bacteria. Infect. Immun. 2003, 71, 5803–5813. [Google Scholar] [CrossRef]
- Gruet, P.; Maincent, P.; Berthelot, X.; Kaltsatos, V. Bovine mastitis and intramammary drug delivery: Review and perspectives. Adv. Drug Deliv. Rev. 2001, 50, 245–259. [Google Scholar] [CrossRef]
- Gabai, G.; De Luca, E.; Miotto, G.; Zin, G.; Stefani, A.; Da Dalt, L.; Barberio, A.; Celi, P. Relationship between protein oxidation biomarkers and uterine health in dairy cows during the postpartum period. Antioxidants 2019, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Ruggerone, B.; Colombo, G.; Paltrinieri, S. Identification of Protein Carbonyls (PCOs) in Canine Serum by Western Blot Technique and Preliminary Evaluation of PCO Concentration in Dogs With Systemic Inflammation. Front. Vet. Sci. 2020, 7, 566402. [Google Scholar] [CrossRef] [PubMed]
- Rezamand, P.; Hoagland, T.A.; Moyes, K.M.; Silbart, L.K.; Andrew, S.M. Energy status, lipid-soluble vitamins, and acute phase proteins in periparturient Holstein and Jersey dairy cows with or without subclinical mastitis. J. Dairy Sci. 2007, 90, 5097–5107. [Google Scholar] [CrossRef]
- Singh, R.; Bhardwaj, R.K.; Azad, M.S.; Beigh, S.A. Effect of mastitis on haemato-biochemical and plasma mineral profile in crossbred cattle. Indian J. Anim. Res. 2014, 48, 63–66. [Google Scholar] [CrossRef]
- Turk, R.; Piras, C.; Kovačić, M.; Samardžija, M.; Ahmed, H.; De Canio, M.; Urbani, A.; Zlata Meštrić, F.; Soggiu, A.; Bonizzi, L.; et al. Proteomics of inflammatory and oxidative stress response in cows with subclinical and clinical mastitis. J. Proteom. 2012, 75, 4412–4428. [Google Scholar] [CrossRef] [PubMed]
- Sadat, A.; Farag, A.M.; Elhanafi, D.; Awad, A.; Elmahallawy, E.K.; Alsowayeh, N.; El-khadrag, M.F.; Elshopakey, G.E. Immunological and Oxidative Biomarkers in Bovine Serum from Healthy, Clinical, and Sub-Clinical Mastitis Caused by Escherichia coli and Staphylococcus aureus Infection. Animals 2023, 13, 892. [Google Scholar] [CrossRef]
- Augustyniak, E.; Adam, A.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Willetts, R.; Korkmaz, A.; Atalay, M.; Weber, D.; Grune, T.; Borsa, C.; et al. Validation of protein carbonyl measurement: A multi-centre study. Redox Biol. 2015, 4, 149–157. [Google Scholar] [CrossRef]
- Shacter, E.; Williams, J.A.; Lim, M.; Levine, R.L. Differential susceptibility of plasma proteins to oxidative modification: Examination by western blot immunoassay. Free Radic. Biol. Med. 1994, 17, 429–437. [Google Scholar] [CrossRef]
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Sordillo, L.M. Production of 15-F2t-isoprostane as an assessment of oxidative stress in dairy cows at different stages of lactation. J. Dairy Sci. 2018, 101, 9287–9295. [Google Scholar] [CrossRef]
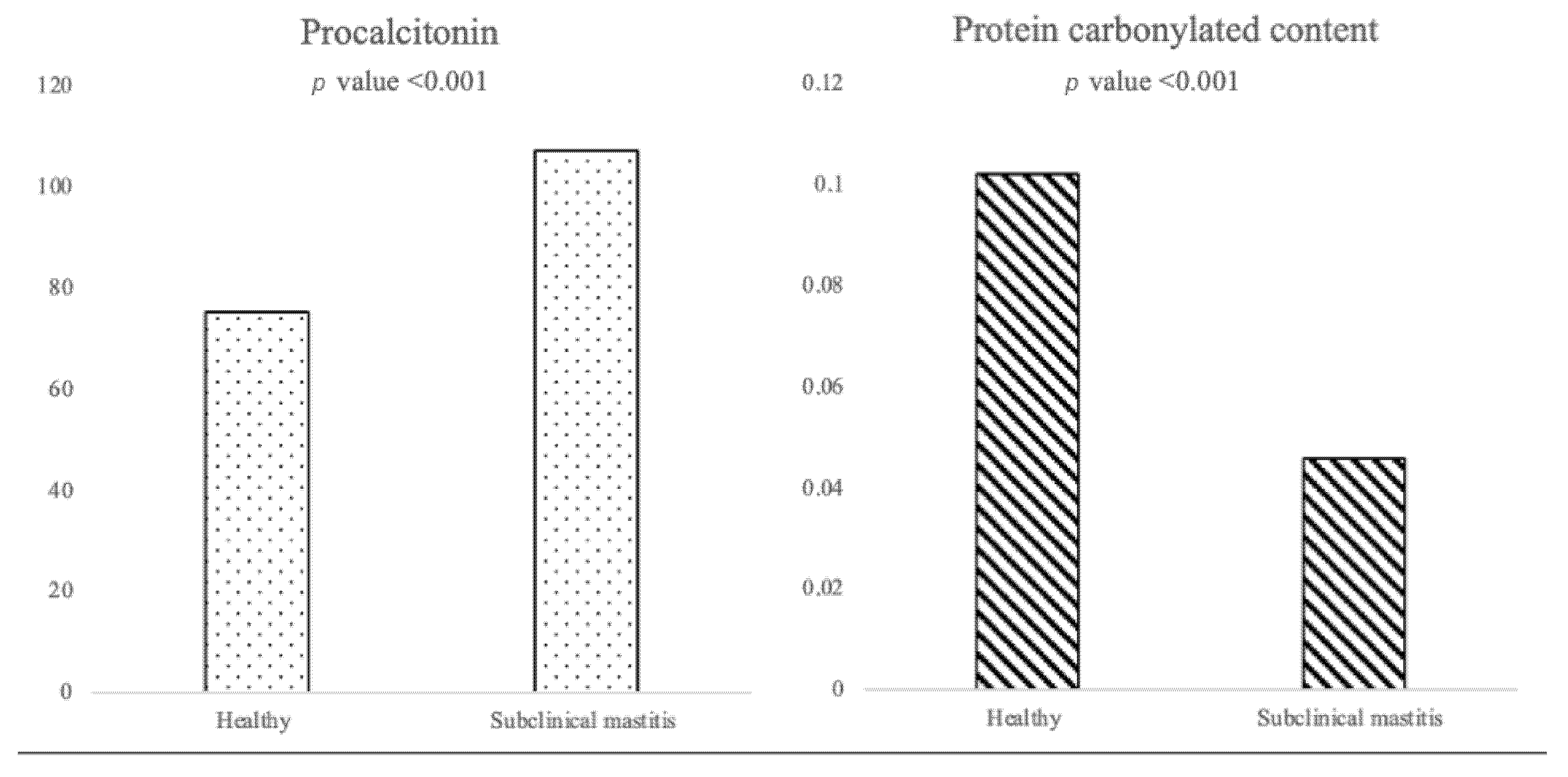
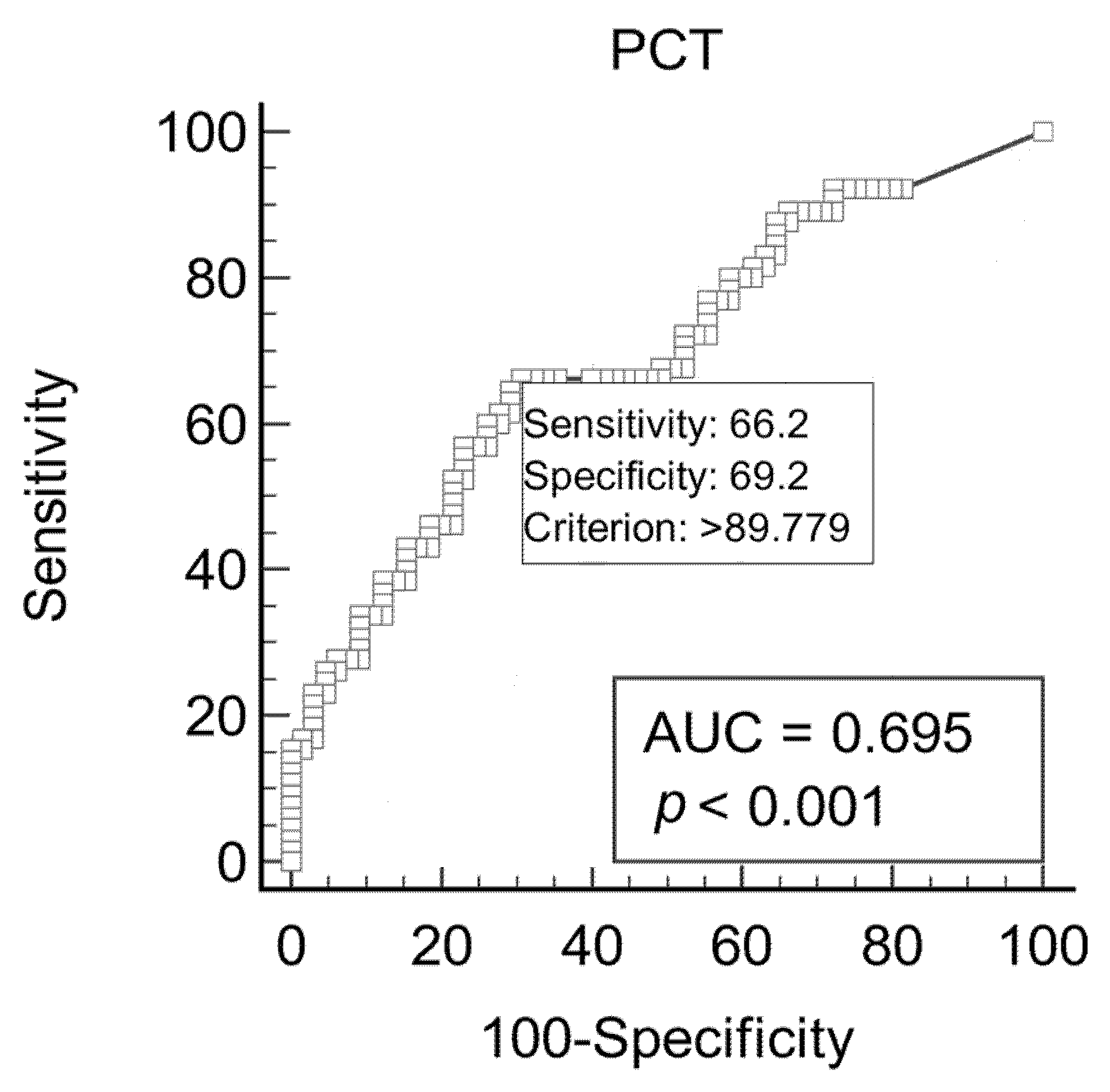
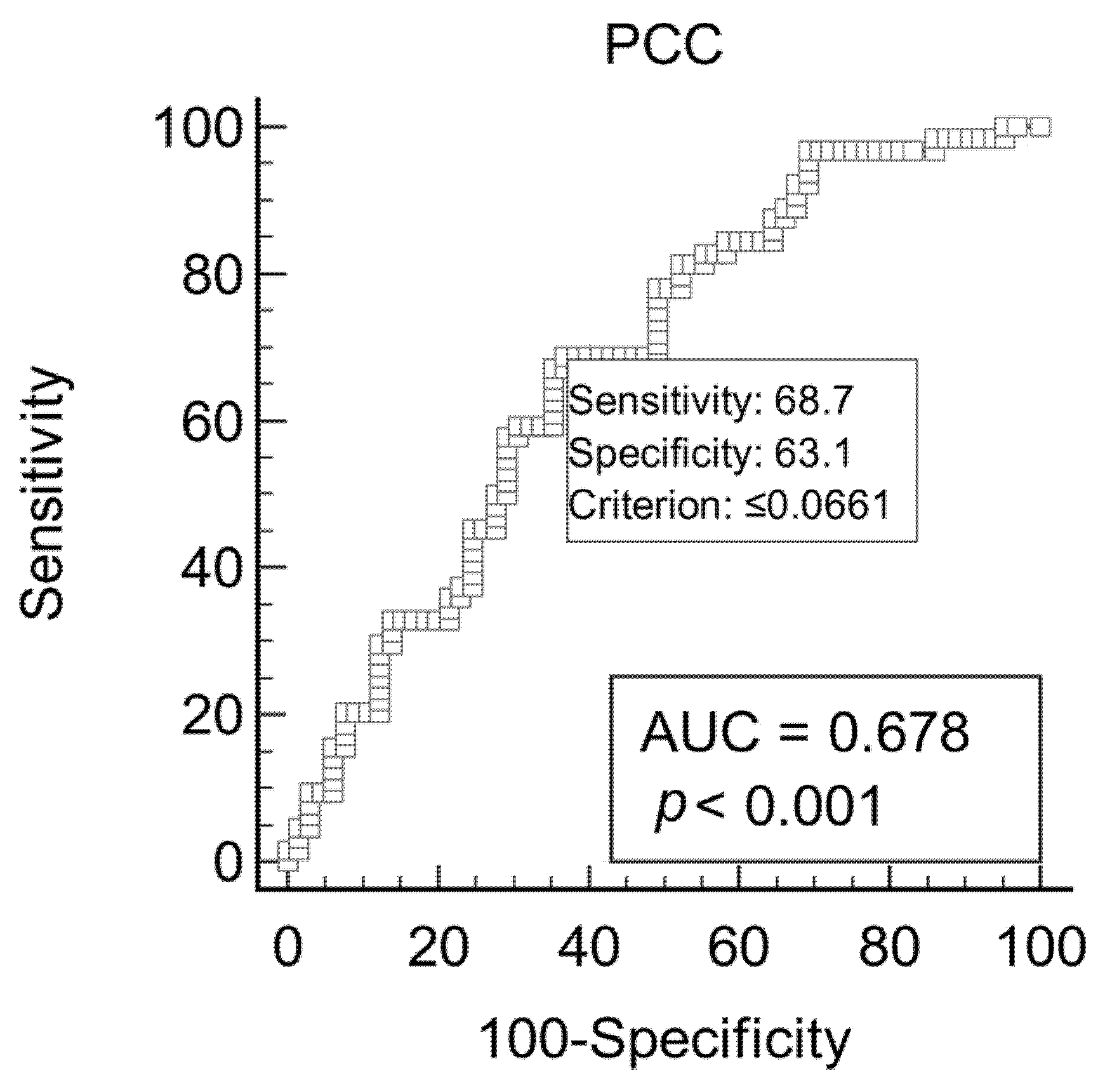
| PCT (pg/mL) * | PCC (nmol/mL/mg) * | DIM (Days) * | |
|---|---|---|---|
| Healthy | 75.36 (37.44–99.14) | 0.102 (0.038–0.192) | 60.00 (15.00–150.00) |
| Subclinical mastitis | 107.27 (68.63–253.25) | 0.046 (0.021–0.095) | 170.00 (58.75–290.75) |
| Healthy | Subclinical Mastitis | |
|---|---|---|
| BCS | ||
| 2 | 1 (1.5%) | / |
| 2.5 | 1 (1.5%) | / |
| 2.75 | 7 (10.8%) | 9 (13.8%) |
| 3 | 35 (53.9%) | 30 (46.2%) |
| 3.25 | 12 (18.5%) | 12 (18.5%) |
| 3.5 | 6 (9.2%) | 8 (12.3%) |
| 3.75 | 3 (4.6%) | 5 (7.7%) |
| 4 | / | 1 (1.5%) |
| Cow parity * | ||
| 1 | 30 (46.2%) | 21 (32.3%) |
| 2 | 30 (46.2%) | 14 (21.5%) |
| >3 | 5 (7.6%) | 30 (46.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sala, G.; Orsetti, C.; Meucci, V.; De Marchi, L.; Sgorbini, M.; Bonelli, F. Case–Control Study: Endogenous Procalcitonin and Protein Carbonylated Content as a Potential Biomarker of Subclinical Mastitis in Dairy Cows. Vet. Sci. 2023, 10, 670. https://doi.org/10.3390/vetsci10120670
Sala G, Orsetti C, Meucci V, De Marchi L, Sgorbini M, Bonelli F. Case–Control Study: Endogenous Procalcitonin and Protein Carbonylated Content as a Potential Biomarker of Subclinical Mastitis in Dairy Cows. Veterinary Sciences. 2023; 10(12):670. https://doi.org/10.3390/vetsci10120670
Chicago/Turabian StyleSala, Giulia, Chiara Orsetti, Valentina Meucci, Lucia De Marchi, Micaela Sgorbini, and Francesca Bonelli. 2023. "Case–Control Study: Endogenous Procalcitonin and Protein Carbonylated Content as a Potential Biomarker of Subclinical Mastitis in Dairy Cows" Veterinary Sciences 10, no. 12: 670. https://doi.org/10.3390/vetsci10120670
APA StyleSala, G., Orsetti, C., Meucci, V., De Marchi, L., Sgorbini, M., & Bonelli, F. (2023). Case–Control Study: Endogenous Procalcitonin and Protein Carbonylated Content as a Potential Biomarker of Subclinical Mastitis in Dairy Cows. Veterinary Sciences, 10(12), 670. https://doi.org/10.3390/vetsci10120670









