Cardiovascular and Respiratory Effects of Increased Intra-Abdominal Pressure with and without Dexmedetomidine in Anesthetized Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
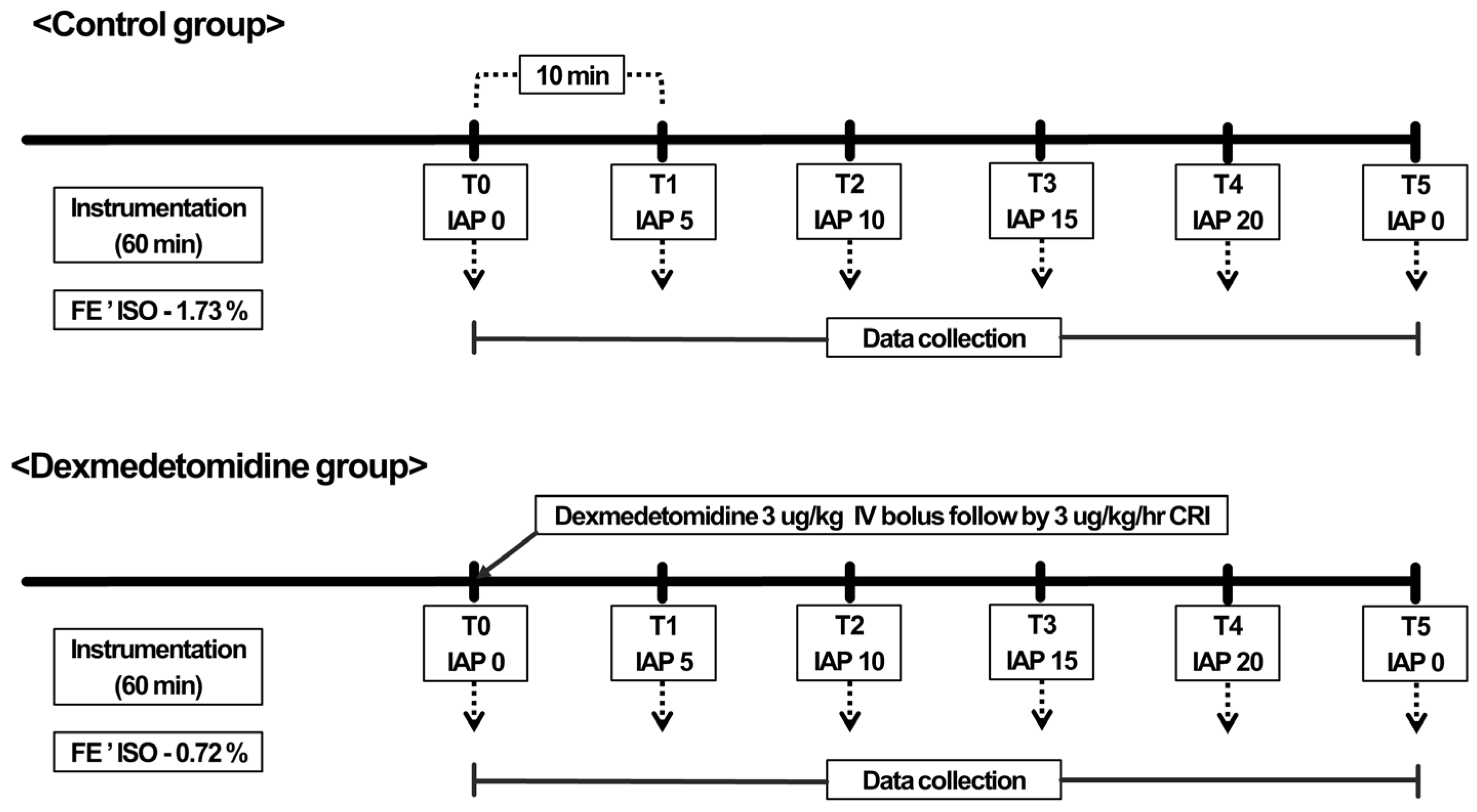
2.2. Anesthesia and Instrumentation
2.3. LiDCO™ Monitor Calibration
2.4. Veress Needle Placement
2.5. Experimental Design
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardner, A.K.; Schroeder, E.L. Pathophysiology of intraabdominal hypertension and abdominal compartment syndrome and relevance to veterinary critical care. J. Vet. Emerg. Crit. Care 2022, 32, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Fransson, B.A.; Grubb, T.L.; Perez, T.E.; Flores, K.; Gay, J.M. Cardiorespiratory changes and pain response of lift laparoscopy compared to capnoperitoneum laparoscopy in dogs. Vet. Surg. 2015, 44, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.T.; Okano, S. Influence of pneumoperitoneum and postural change on the cardiovascular and respiratory systems in dogs. J. Vet. Med. Sci. 2015, 77, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Fransson, B.A.; Mayhew, P.D. Small Animal Laparoscopy and Thoracoscopy; John Wiley & Sons: Hoboken, NJ, USA, 2021; p. 101. [Google Scholar]
- Bloor, B.C.; Frankland, M.; Alper, G.; Raybould, D.; Weitz, J.; Shurtliff, M. Hemodynamic and sedative effects of dexmedetomidine in dog. J. Pharmacol. Exp. Ther. 1992, 263, 690–697. [Google Scholar] [PubMed]
- Pascoe, P.J.; Raekallio, M.; Kuusela, E.; McKusick, B.; Granholm, M. Changes in the minimum alveolar concentration of isoflurane and some cardiopulmonary measurements during three continuous infusion rates of dexmedetomidine in dogs. Vet. Anaesth. Analg. 2006, 33, 97–103. [Google Scholar] [CrossRef]
- Hellebrekers, L.; Sap, R. Medetomidine as a premedicant for ketamine, propofol or fentanyl anaesthesia in dogs. Vet. Rec. 1997, 140, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Pypendop, B.H.; Verstegen, J.P. Hemodynamic effects of medetomidine in the dog: A dose titration study. Vet. Surg. 1998, 27, 612–622. [Google Scholar] [CrossRef]
- Vora, K.S.; Baranda, U.; Shah, V.R.; Modi, M.; Parikh, G.P.; Butala, B.P. The effects of dexmedetomidine on attenuation of hemodynamic changes and there effects as adjuvant in anesthesia during laparoscopic surgeries. Saudi J. Anaesth. 2015, 9, 386. [Google Scholar] [CrossRef]
- Bhattacharjee, D.P.; Nayek, S.K.; Dawn, S.; Bandopadhyay, G.; Gupta, K. Effects of dexmedetomidine on haemodynamics in patients undergoing laparoscopic cholecystectomy—A comparative study. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 45–48. [Google Scholar] [CrossRef]
- Jun, G.-W.; Kim, M.-S.; Yang, H.-J.; Sung, T.-Y.; Park, D.-H.; Cho, C.-K.; Kwon, H.-U.; Kang, P.-S.; Moon, J.-I. Laparoscopic appendectomy under spinal anesthesia with dexmedetomidine infusion. Korean J. Anesthesiol. 2014, 67, 246–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eisenberg, P.R.; Jaffe, A.S.; Schuster, D.P. Clinical evaluation compared to pulmonary artery catheterization in the hemodynamic assessment of critically ill patients. Crit. Care Med. 1984, 12, 549–553. [Google Scholar] [CrossRef]
- Shih, A.; Maisenbacher, H.W.; Bandt, C.; Ricco, C.; Bailey, J.; Rivera, J.; Estrada, A. Assessment of cardiac output measurement in dogs by transpulmonary pulse contour analysis. J. Vet. Emerg. Crit. Care 2011, 21, 321–327. [Google Scholar] [CrossRef]
- Patel, C.; Laboy, V.; Venus, B.; Mathru, M.; Wier, D. Acute complications of pulmonary artery catheter insertion in critically ill patients. Crit. Care Med. 1986, 14, 195–197. [Google Scholar] [CrossRef]
- Linton, N.; Linton, R. Estimation of changes in cardiac output from the arterial blood pressure waveform in the upper limb. Br. J. Anaesth. 2001, 86, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Bleedorn, J.A.; Dykema, J.L.; Hardie, R.J. Minimally invasive surgery in veterinary practice: A 2010 survey of diplomates and residents of the American College of Veterinary Surgeons. Vet. Surg. 2013, 42, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Quandt, J.; Hofmeister, E. Determination of minimum alveolar concentration of isoflurane in dogs and cats using the up-and-down method. A Prelim. Study. Res. Vet. Sci. 2016, 106, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Diamant, M.; Benumof, J.L.; Saidman, L.J. Hemodynamics of increased intra-abdominal pressure: Interaction with hypovolemia and halothane anesthesia. Anesthesiology 1978, 48, 23–27. [Google Scholar] [CrossRef]
- Ivankovich, A.D.; Miletich, D.J.; Albrecht, R.F.; Heyman, H.J.; Bonnet, R.F. Cardiovascular effects of intraperitoneal insufflation with carbon dioxide and nitrous oxide in the dog. Anesthesiology 1975, 42, 281–287. [Google Scholar] [CrossRef]
- Richardson, J.D.; Trinkle, J.K. Hemodynamic and respiratory alterations with increased intra-abdominal pressure. J. Surg. Res. 1976, 20, 401–404. [Google Scholar] [CrossRef]
- Duke, T.; Steinacher, S.L.; Remedios, A.M. Cardiopulmonary effects of using carbon dioxide for laparoscopic surgery in dogs. Vet. Surg. 1996, 25, 77–82. [Google Scholar] [CrossRef]
- De Wilde, R.; Schreuder, J.; Van Den Berg, P.; Jansen, J. An evaluation of cardiac output by five arterial pulse contour techniques during cardiac surgery. Anaesthesia 2007, 62, 760–768. [Google Scholar] [CrossRef]
- Hadian, M.; Kim, H.K.; Severyn, D.A.; Pinsky, M.R. Cross-comparison of cardiac output trending accuracy of LiDCO, PiCCO, FloTrac and pulmonary artery catheters. Crit. Care 2010, 14, R212. [Google Scholar] [CrossRef]
- Park, K.; Yeon, S.; Lee, H. Comparative evaluation of cardiac output using echocardiography in beagle dogs. J. Vet. Clin. 2012, 29, 384–390. [Google Scholar]
- Beck, R.; Halberthal, M.; Zonis, Z.E.; Shoshani, G.; Hayari, L.; Bar-Joseph, G. Abdominal compartment syndrome in children. Pediatr. Crit. Care Med. 2001, 2, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Steffey, E.; Howland, D., Jr. Isoflurane potency in the dog and cat. Am. J. Vet. Res. 1977, 38, 1833–1836. [Google Scholar] [PubMed]
- Hirshman, C.; McCullough, R.; Cohen, P.; Weil, J. Depression of hypoxic ventilatory response by halothane, enflurane and isoflurane in dogs. Br. J. Anaesth. 1977, 49, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Maney, J.K.; Shepard, M.K.; Braun, C.; Cremer, J.; Hofmeister, E.H. A comparison of cardiopulmonary and anesthetic effects of an induction dose of alfaxalone or propofol in dogs. Vet. Anaesth. Analg. 2013, 40, 237–244. [Google Scholar] [CrossRef]
- Jang, M.; Son, W.G.; Jo, S.M.; Kim, H.; Shin, C.W.; Lee, I. A novel balloon technique to induce intra-abdominal hypertension and its effects on cardiovascular parameters in a conscious dog model. J. Vet. Emerg. Crit. Care 2018, 28, 326–333. [Google Scholar] [CrossRef]
| T0 (IAP 0 mmHg) | T1 (IAP 5 mmHg) | T2 (IAP 10 mmHg) | T3 (IAP 15 mmHg) | T4 (IAP 20 mmHg) | T5 (IAP 0 mmHg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| HR (/min) | Control | 103.0 | 13.7 | 111.2 | 13.5 | 110.8 | 15.9 | 114.8 | 11.9 | 116.0 | 14.4 | 117.0 | 14.6 |
| Dex | 81.0 † | 11.0 | 71.2 † | 10.0 | 70.0 † | 17.7 | 69.8 † | 9.2 | 67.8 † | 9.09 | 64.8 † | 4.8 | |
| SAP (mmHg) | Control | 97.0 | 18.9 | 94.4 | 9.0 | 91.2 | 7.6 | 112.6 | 16.4 | 122.6 | 24.6 | 95.0 | 14.3 |
| Dex | 154.2 † | 14.0 | 146.0 † | 11.1 | 140.8 † | 10.4 | 134.6 | 8.7 | 135.2 | 11.0 | 131.4 † | 12.1 | |
| MAP (mmHg) | Control | 68.4 | 17.5 | 68.0 | 8.2 | 66.6 | 7.7 | 84.8 | 15.1 | 90.8 | 22.2 | 67.4 | 13.9 |
| Dex | 124.0 † | 11.8 | 114.0 † | 9.8 | 103.8 † | 14.7 | 98.4 | 8.6 | 97.8 | 13.1 | 96.8 † | 8.2 | |
| DAP (mmHg) | Control | 53.6 | 17.6 | 53.6 | 9.8 | 53.0 | 8.6 | 72.6 | 16.0 | 80.2 | 20.6 | 56.4 | 12.9 |
| Dex | 111.0 † | 11.1 | 98.4 † | 12.5 | 88.6 † | 13.2 | 84.2 | 9.2 | 84.0 | 14.1 | 83.0 † | 10.0 | |
| CO (L/min) | Control | 1.5 | 0.4 | 1.5 | 0.4 | 1.5 | 0.4 | 1.3 | 0.3 | 1.2 | 0.4 | 1.6 | 0.3 |
| Dex | 0.7 † | 0.2 | 0.6 † | 0.2 | 0.7 † | 0.2 | 0.8 † | 0.2 | 0.6 † | 0.2 | 0.7 † | 0.1 | |
| SV (mL) | Control | 13.7 | 2.7 | 13.7 | 2.5 | 13.0 | 2.6 | 11.1 | 2.1 | 11.1 | 2.1 | 13.4 | 2.7 |
| Dex | 7.8 † | 1.4 | 8.2 † | 1.6 | 9.1 † | 1.9 | 9.8 | 1.6 | 10.1 | 1.4 | 10.4 | 1.0 | |
| SVR (Dynes/ s/cm−5) | Control | 3621.1 | 1416.7 | 3467.5 | 1272.1 | 3563.2 | 1267.3 | 5043.2 | 1972.0 | 5962.9 | 2286.7 | 3436.2 | 1302.9 |
| Dex | 17,577.4 † | 5397.8 | 18,031.9 † | 8199.3 | 13,097.5 † | 3921.4 | 10,535.2 † | 2745.0 | 12,548.2 † | 4613.3 | 11,417.9 † | 2510.2 | |
| T0 (IAP 0 mmHg) | T1 (IAP 5 mmHg) | T2 (IAP 10 mmHg) | T3 (IAP 15 mmHg) | T4 (IAP 20 mmHg) | T5 (IAP 0 mmHg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| RR (/min) | Control | 13.2 | 6.3 | 14.6 | 5.9 | 17.4 | 7.8 | 17.2 | 5.4 | 20.2 | 6.5 | 17.4 | 4.5 |
| Dex | 12.4 | 2.7 | 14.2 | 4.2 | 18.8 | 6.8 | 21.0 | 7.2 | 21.0 | 6.9 | 15.4 | 2.1 | |
| EtCO2 (mmHg) | Control | 55.4 | 5.4 | 58.0 | 7.3 | 56.0 | 6.5 | 59.2 | 8.6 | 56.4 | 11.5 | 53.4 | 7.0 |
| Dex | 51.0 | 4.5 | 50.6 | 5.0 | 51.0 | 5.2 | 53.6 | 7.0 | 53.2 | 8.4 | 44.8 | 3.0 | |
| TV (ml/min) | Control | 139.6 | 27.8 | 136.0 | 24.9 | 136.8 | 32.4 | 129.6 | 14.5 | 125.6 | 17.5 | 155.6 | 32.1 |
| Dex | 146.0 | 17.7 | 155.6 | 6.6 | 130.8 | 4.9 | 117.8 | 10.2 | 113.4 | 12.2 | 158.8 | 10.9 | |
| MV (L/min) | Control | 1.9 | 0.7 | 1.7 | 0.8 | 2.2 | 0.6 | 2.0 | 0.8 | 2.5 | 1.1 | 2.7 | 0.5 |
| Dex | 1.9 | 0.6 | 2.1 | 0.7 | 2.6 | 1.0 | 2.4 | 1.0 | 2.4 | 0.9 | 2.5 | 0.5 | |
| pH | Control | 7.4 | 0.1 | 7.3 | 0.0 | 7.3 | 0.1 | 7.3 | 0.1 | 7.3 | 0.1 | 7.3 | 0.1 |
| Dex | 7.4 | 0.1 | 7.3 | 0.1 | 7.3 | 0.1 | 7.3 | 0.1 | 7.3 | 0.1 | 7.4 | 0.1 | |
| PaO2 (mmHg) | Control | 447.4 | 60.9 | 472.6 | 70.5 | 477.2 | 113.4 | 503.6 | 73.7 | 516.8 | 93.6 | 508.6 | 81.1 |
| Dex | 481.2 | 62.3 | 520.2 | 29.9 | 521.2 | 48.9 | 522.6 | 25.7 | 525.0 | 56.4 | 538.8 | 53.9 | |
| PaCO2 (mmHg) | Control | 53.6 | 6.8 | 56.9 | 9.0 | 56.3 | 8.5 | 59.9 | 9.5 | 57.4 | 7.0 | 54.7 | 8.2 |
| Dex | 47.6 | 5.1 | 52.0 | 4.4 | 54.6 | 5.6 | 53.9 | 5.9 | 56.4 | 10.0 | 51.1 | 8.8 | |
| HCO3− (mmol/L) | Control | 30.1 | 2.9 | 29.4 | 2.7 | 29.5 | 3.0 | 28.9 | 4.3 | 28.5 | 4.4 | 27.8 | 3.6 |
| Dex | 26.5 | 4.9 | 26.8 | 4.9 | 27.4 | 5.4 | 27.0 | 5.8 | 28.1 | 6.0 | 28.2 | 5.0 | |
| BE (mmol/L) | Control | 5.1 | 3.0 | 4.0 | 2.4 | 4.0 | 3.1 | 3.0 | 4.6 | 2.7 | 4.5 | 2.2 | 3.8 |
| Dex | 1.6 | 5.6 | 1.5 | 5.6 | 1.9 | 6.0 | 1.5 | 6.4 | 2.4 | 6.4 | 3.1 | 5.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Seo, M.; Choi, G.; Lee, S.-K.; Lee, S.; Lee, W.-J.; Yun, S.-H.; Kwon, Y.-S.; Jang, M. Cardiovascular and Respiratory Effects of Increased Intra-Abdominal Pressure with and without Dexmedetomidine in Anesthetized Dogs. Vet. Sci. 2023, 10, 634. https://doi.org/10.3390/vetsci10110634
Kim D, Seo M, Choi G, Lee S-K, Lee S, Lee W-J, Yun S-H, Kwon Y-S, Jang M. Cardiovascular and Respiratory Effects of Increased Intra-Abdominal Pressure with and without Dexmedetomidine in Anesthetized Dogs. Veterinary Sciences. 2023; 10(11):634. https://doi.org/10.3390/vetsci10110634
Chicago/Turabian StyleKim, Dongseok, Minjun Seo, Geonho Choi, Sang-Kwon Lee, Sungin Lee, Won-Jae Lee, Sung-Ho Yun, Young-Sam Kwon, and Min Jang. 2023. "Cardiovascular and Respiratory Effects of Increased Intra-Abdominal Pressure with and without Dexmedetomidine in Anesthetized Dogs" Veterinary Sciences 10, no. 11: 634. https://doi.org/10.3390/vetsci10110634
APA StyleKim, D., Seo, M., Choi, G., Lee, S.-K., Lee, S., Lee, W.-J., Yun, S.-H., Kwon, Y.-S., & Jang, M. (2023). Cardiovascular and Respiratory Effects of Increased Intra-Abdominal Pressure with and without Dexmedetomidine in Anesthetized Dogs. Veterinary Sciences, 10(11), 634. https://doi.org/10.3390/vetsci10110634







