Variation in Diffusion Tensor Imaging Parameters in the Cervical and Thoracic Spinal Cord (C1-C5 and C6-T2) Segments of Normal Beagle Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Animals
2.2. MR Imaging Protocol
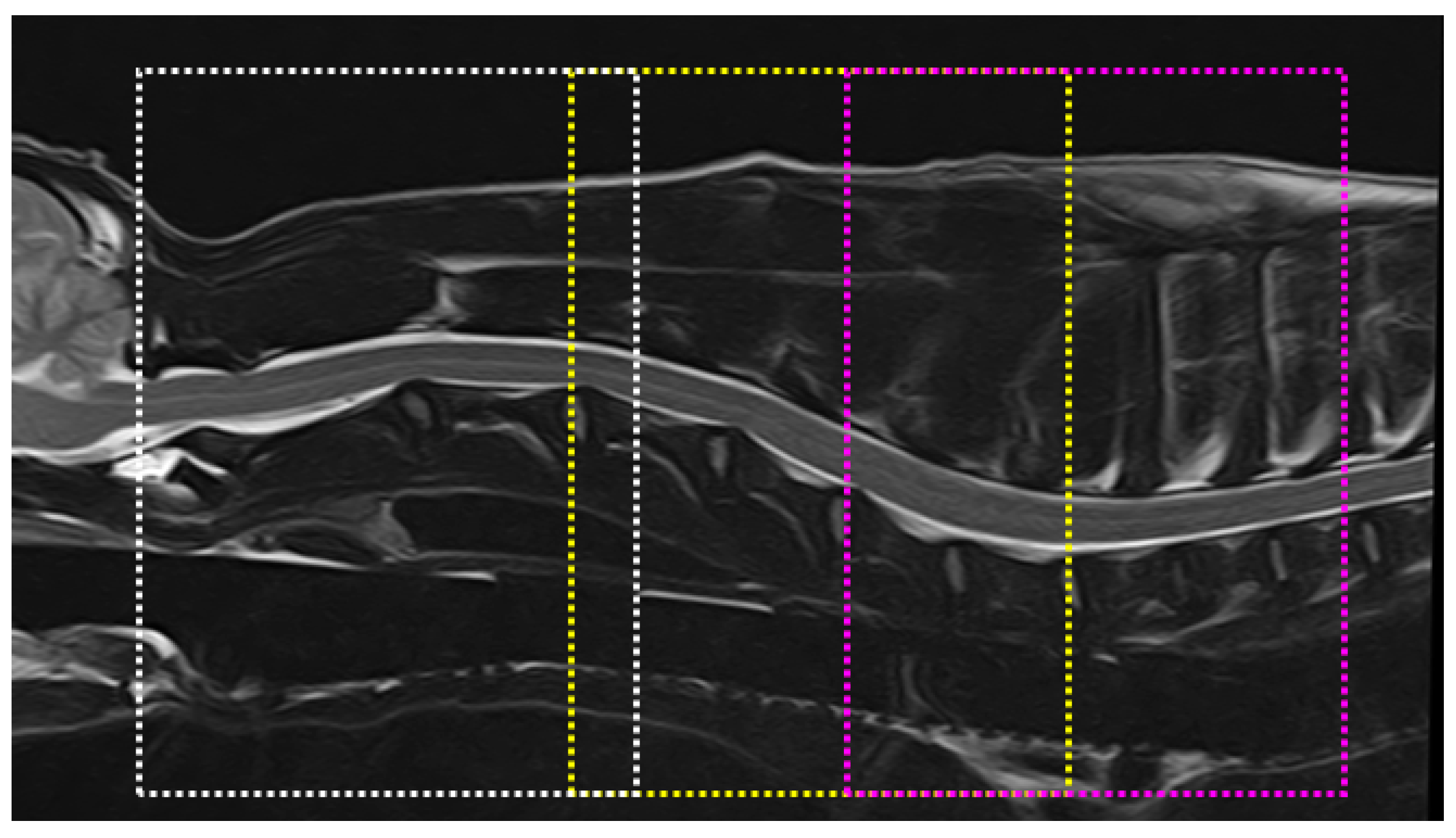
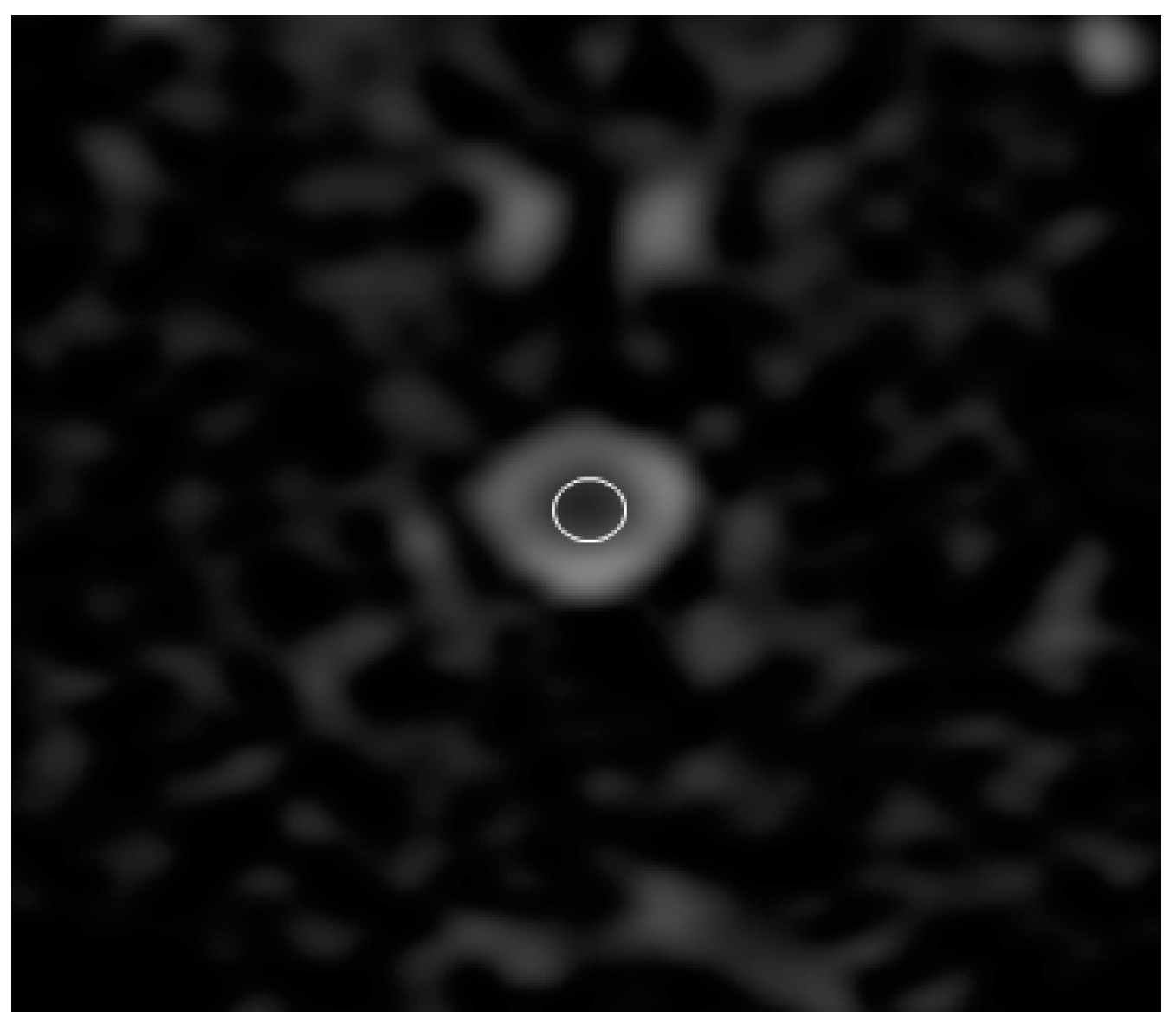
2.3. Image Analysis
2.4. Statistical Analysis
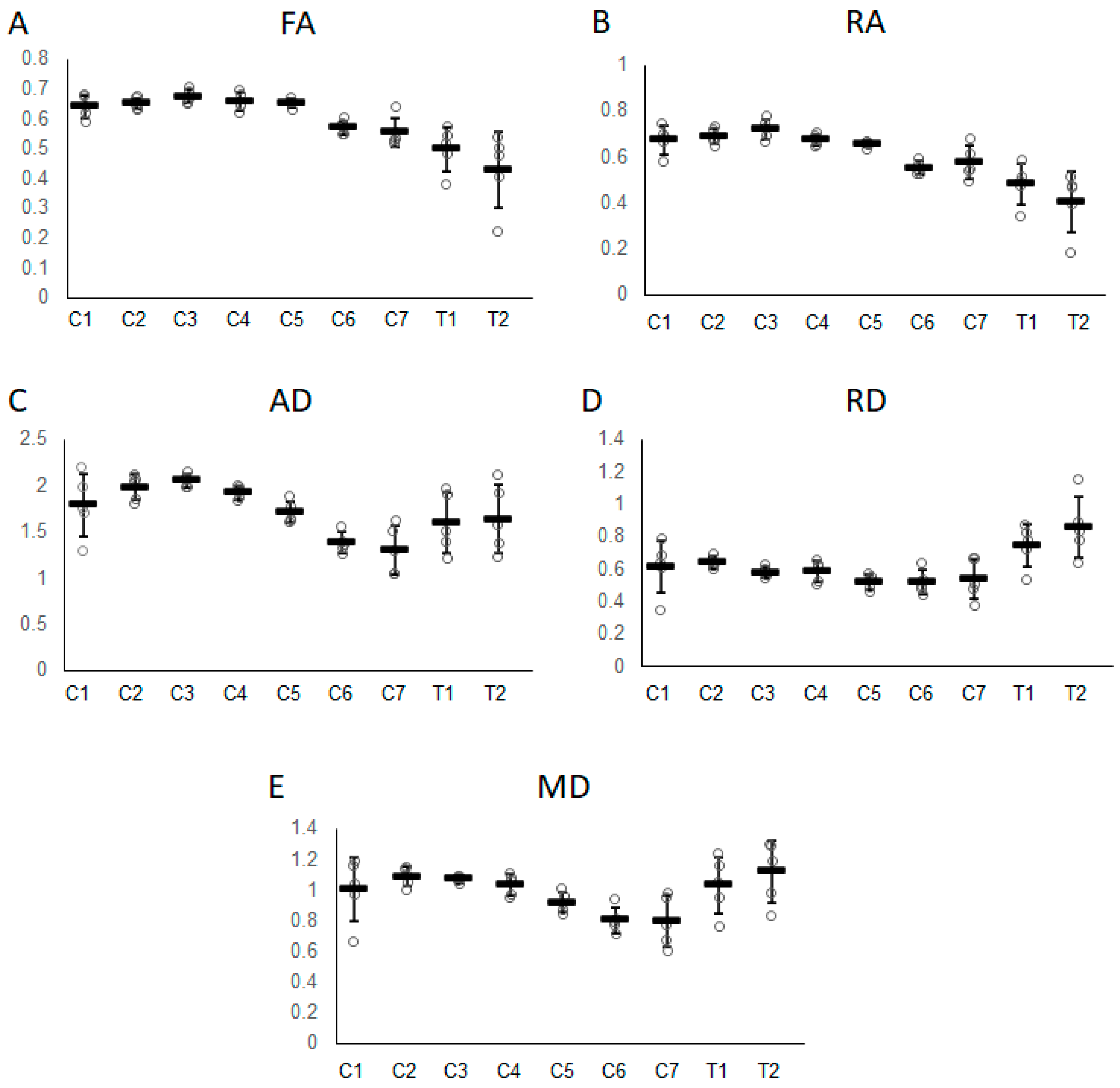
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shanmuganathan, K.; Gullapalli, R.P.; Zhuo, J.; Mirvis, S.E. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am. J. Neuroradiol. 2008, 29, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Ulmer, J.L.; Kurpad, S.N.; Schmit, B.D. Diffusion tensor MR imaging of the neurologically intact human spinal cord. AJNR Am. J. Neuroradiol. 2008, 29, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.; Mohamed, F.B.; Hunter, L.N.; Shah, P.; Faro, S.H.; Samdani, A.F.; Finsterbusch, J.; Betz, R.; Gaughan, J.; Mulcahey, M.J. Diffusion tensor imaging of the normal pediatric spinal cord using an inner field of view echo-planar imaging sequence. AJNR Am. J. Neuroradiol. 2012, 33, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y.; Li, X.; Mak, K.C.; Cheung, J.P.; Luk, K.D.; Hu, Y. Normal values of cervical spinal cord diffusion tensor in young and middle-aged healthy Chinese. Eur. Spine J. 2015, 24, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Saksena, S.; Middleton, D.M.; Krisa, L.; Shah, P.; Faro, S.H.; Sinko, R.; Gaughan, J.; Finsterbusch, J.; Mulcahey, M.J.; Mohamed, F.B. Diffusion tensor imaging of the normal cervical and thoracic pediatric spinal cord. AJNR Am. J. Neuroradiol. 2016, 37, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.W.; Kalsi-Ryan, S.; Lange, S.F.; Tetreault, L.; Nouri, A.; Crawley, A.; Mikulis, D.J.; et al. Clinically feasible microstructural MRI to quantify cervical spinal cord tissue injury using DTI, MT, and T2*-weighted imaging: Assessment of normative data and reliability. AJNR Am. J. Neuroradiol. 2017, 38, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Satoh, H.; Kuroiwa, Y.; Kusaka, M.; Yamashita, A.; Asada, Y.; Asanuma, T. Application of fiber tractography and diffusion tensor imaging to evaluate spinal cord diseases in dogs. J. Vet. Med. Sci. 2017, 79, 418–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naito, E.; Nakata, K.; Sakai, H.; Yamato, O.; Islam, M.S.; Maeda, S.; Kamishina, H. Diffusion tensor imaging-based quantitative analysis of the spinal cord in Pembroke Welsh Corgis with degenerative myelopathy. J. Vet. Med. Sci. 2022, 84, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Shinn, R.L.; Pancotto, T.E.; Stadler, K.L.; Werre, S.R.; Rossmeisl, J.H. Magnetization transfer and diffusion tensor imaging in dogs with intervertebral disk herniation. J. Vet. Intern. Med. 2020, 34, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Pease, A.; Miller, R. The use of diffusion tensor imaging to evaluate the spinal cord in normal and abnormal dogs. Vet. Radiol. Ultrasound. 2011, 52, 492–497. [Google Scholar] [CrossRef] [PubMed]
- De Lahunta, A.; Glass, E. Veterinary Neuroanatomy and Clinical Neurology, 3rd ed.; Elsevier Saunders: St. Louis, MO, USA, 2009; pp. 243–284. [Google Scholar]
- Fletcber, T.E. Spinal Cord and Meninges. In Miller’s Anatomy of the Dog, 4th ed.; Evams, E.H., Lahunta, A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 589–610. [Google Scholar]
- Yoon, H.; Park, N.W.; Ha, Y.M.; Kim, J.; Moon, W.J.; Eom, K. Diffusion tensor imaging of white and grey matter within the spinal cord of normal Beagle dogs: Sub-regional differences of the various diffusion parameters. Vet. J. 2016, 215, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gatto, R.G.; Amin, M.Y.; Deyoung, D.; Hey, M.; Mareci, T.H.; Magin, R.L. Ultra-high field diffusion MRI reveals early axonal pathology in spinal cord of ALS mice. Transl. Neurodegener. 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Mogatadakala, K.V.; Narayana, P.A. In vivo diffusion tensor imaging of thoracic and cervical rat spinal cord at 7 T. Magn. Reson. Imaging 2009, 27, 1236–1241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saliani, A.; Perraud, B.; Duval, T.; Stikov, N.; Rossignol, S.; Cohen-Adad, J. Axon and myelin morphology in animal and human spinal cord. Front. Neuroanat. 2017, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R.; Stroman, P.W. Investigation of human cervical and upper thoracic spinal cord motion: Implications for imaging spinal cord structure and function. Magn. Reson. Med. 2007, 58, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Summers, P.; Staempfli, P.; Jaermann, T.; Kwiecinski, S.; Kollias, S. A preliminary study of the effects of trigger timing on diffusion tensor imaging of the human spinal cord. AJNR Am. J. Neuroradiol. 2006, 27, 1952–1961. [Google Scholar] [PubMed]
- Wang, K.; Song, Q.; Zhang, F.; Chen, Z.; Hou, C.; Tang, Y.; Chen, S.; Hao, Q.; Shen, H. Age-related changes of the diffusion tensor imaging parameters of the normal cervical spinal cord. Eur. J. Radiol. 2014, 83, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Duval, T.; Saliani, A.; Nami, H.; Nanci, A.; Stikov, N.; Leblond, H.; Cohen-Adad, J. Axons morphometry in the human spinal cord. Neuroimage 2019, 185, 119–128. [Google Scholar] [CrossRef] [PubMed]
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | T1 | T2 | |
|---|---|---|---|---|---|---|---|---|---|
| ADC (×10−3 mm2/s) | 1.01 ± 0.20 | 1.10 ± 0.06 | 1.07 ± 0.02 | 1.02 ± 0.08 | 0.91 ± 0.08 | 0.80 ± 0.12 | 0.75 ± 0.17 | 1.00 ± 0.18 | 1.10 ± 0.21 |
| FA | 0.64 ± 0.04 | 0.66 ± 0.02 | 0.68 ± 0.03 | 0.66 ± 0.03 | 0.65 ± 0.05 | 0.58 ± 0.02 | 0.55 ± 0.05 | 0.49 ± 0.07 | 0.41 ± 0.13 |
| RA | 0.67 ± 0.06 | 0.70 ± 0.03 | 0.73 ± 0.05 | 0.68 ± 0.02 | 0.66 ± 0.06 | 0.56 ± 0.03 | 0.58 ± 0.07 | 0.47 ± 0.09 | 0.39 ± 0.13 |
| AD (×10−3 mm2/s) | 1.81 ± 0.34 | 2.01 ± 0.12 | 2.07 ± 0.08 | 1.92 ± 0.10 | 1.69 ± 0.19 | 1.38 ± 0.16 | 1.22 ± 0.26 | 1.52 ± 0.33 | 1.57 ± 0.37 |
| RD (×10−3 mm2/s) | 0.61 ± 0.16 | 0.64 ± 0.05 | 0.58 ± 0.04 | 0.58 ± 0.07 | 0.51 ± 0.04 | 0.51 ± 0.10 | 0.51 ± 0.13 | 0.74 ± 0.13 | 0.87 ± 0.19 |
| MD (×10−3 mm2/s) | 1.01 ± 0.21 | 1.10 ± 0.06 | 1.08 ± 0.02 | 1.03 ± 0.07 | 0.91 ± 0.08 | 0.80 ± 0.12 | 0.75 ± 0.17 | 1.00 ± 0.18 | 1.10 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, K.; Itoi, T.; Akashi, N.; Miyabe, M.; Sugimoto, K.; Matsuda, A.; Maeta, N.; Kanda, T.; Kutara, K. Variation in Diffusion Tensor Imaging Parameters in the Cervical and Thoracic Spinal Cord (C1-C5 and C6-T2) Segments of Normal Beagle Dogs. Vet. Sci. 2023, 10, 31. https://doi.org/10.3390/vetsci10010031
Arai K, Itoi T, Akashi N, Miyabe M, Sugimoto K, Matsuda A, Maeta N, Kanda T, Kutara K. Variation in Diffusion Tensor Imaging Parameters in the Cervical and Thoracic Spinal Cord (C1-C5 and C6-T2) Segments of Normal Beagle Dogs. Veterinary Sciences. 2023; 10(1):31. https://doi.org/10.3390/vetsci10010031
Chicago/Turabian StyleArai, Kiyotaka, Takamasa Itoi, Natsuki Akashi, Masahiro Miyabe, Keisuke Sugimoto, Akira Matsuda, Noritaka Maeta, Teppei Kanda, and Kenji Kutara. 2023. "Variation in Diffusion Tensor Imaging Parameters in the Cervical and Thoracic Spinal Cord (C1-C5 and C6-T2) Segments of Normal Beagle Dogs" Veterinary Sciences 10, no. 1: 31. https://doi.org/10.3390/vetsci10010031
APA StyleArai, K., Itoi, T., Akashi, N., Miyabe, M., Sugimoto, K., Matsuda, A., Maeta, N., Kanda, T., & Kutara, K. (2023). Variation in Diffusion Tensor Imaging Parameters in the Cervical and Thoracic Spinal Cord (C1-C5 and C6-T2) Segments of Normal Beagle Dogs. Veterinary Sciences, 10(1), 31. https://doi.org/10.3390/vetsci10010031






