Monthly Entomological Inoculation Rate Data for Studying the Seasonality of Malaria Transmission in Africa
Abstract
1. Introduction
2. Methods
2.1. Compiling Sources of Monthly EIR Data
2.2. Recording EIRm Data from Articles
2.3. Inclusion and Exclusion Criteria
- That the mosquito sampling activity at the location lasted for at least a year;
- That the mosquitoes were sampled monthly throughout the study period or the transmission season;
- That the biting rates were estimated from standard methods such as Pyrethrum Spray Catches (PSC), Light Trap Catches (LTC) and HLC;
- That the proportion of sporozoite-infected mosquitoes were determined using either dissection or Enzyme-Linked Immune Sorbent Assay (ELISA) methods;
- That the study took place at the time mosquito control operations were not in effect.
2.4. Study Location Information and Classification
3. Results
3.1. Data Records
3.2. Application/Case Use of the Data
3.2.1. EIR Seasonality
3.2.2. Vector Type
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PR | Parasite Rate |
| MAP | Malaria Atlas Project |
| CSPR | CircumSporozoite Protein Rate |
| EIR | Entomological Inoculation Rate |
| HBR | Human Biting Rate |
| HLC | Human Landing Catches |
| PSC | Pyrethrum Spray Catches |
| LTC | Light Trap Catches |
| PU | Peri-Urban |
| PWB | Permanent Water Body |
| GPDWv3 | Gridded Population Density data of the World, version 3 |
| AG | Anopheles gambiae |
| AF | Anopheles funestus |
| AA | Anopheles arabiensis |
| AN | Anopheles nili |
| AM | Anopheles moucheti |
References
- World Health Organization. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019. Available online: https://www.who.int/malaria/publications/world-malaria-report-2019/en/ (accessed on 12 February 2020).
- Zhao, J.; Lama, M.; Korenromp, E.; Aylward, P.; Shargie, E.; Filler, S.; Komatsu, R.; Atun, R. Adoption of rapid diagnostic tests for the diagnosis of malaria, a preliminary analysis of the global fund program data, 2005 to 2010. PLoS ONE 2012, 7, e43549. [Google Scholar] [CrossRef]
- Chaulagai, C.N.; Moyo, C.M.; Koot, J.; Moyo, H.B.; Sambakunsi, T.C.; Khunga, F.M.; Naphini, P.D. Design and implementation of a health management information system in Malawi: Issues, innovations and results. Health Policy Plan. 2005, 20, 375–384. [Google Scholar] [CrossRef]
- Abeku, T.A.; Kristan, M.; Jones, C.; Beard, J.; Mueller, D.H.; Okia, M.; Rapuoda, B.; Greenwood, B.; Cox, J. Determinants of the accuracy of rapid diagnostic tests in malaria case management: Evidence from low and moderate transmission settings in the East African highlands. Malar. J. 2008, 7, 202. [Google Scholar] [CrossRef]
- Smith, D.L.; Dushoff, J.; Snow, R.W.; Hay, S.I. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 2005, 438, 492–495. [Google Scholar] [CrossRef]
- Delley, V.; Bouvier, P.; Breslow, N.; Doumbo, O.; Sagara, I.; Diakite, M.; Mauris, A.; Dolo, A.; Rougemont, A. What does a single determination of malaria parasite density mean? A longitudinal survey in Mali. Trop. Med. Int. Health 2000, 5, 404–412. [Google Scholar] [CrossRef]
- Omumbo, J.A.; Hay, S.I.; Guerra, C.A.; Snow, R.W. The relationship between the Plasmodium falciparum parasite ratio in childhood and climate estimates of malaria transmission in Kenya. Malar J. 2004, 3, 17. [Google Scholar] [CrossRef][Green Version]
- Hay, S.I.; Snow, R.W. The malaria atlas project: Developing global maps of malaria risk. PLoS Med. 2006, 3, e473. [Google Scholar] [CrossRef]
- Hay, S.L.; Guerra, C.A.; Gething, P.W.; Patil, A.P.; Tatem, A.J.; Noor, A.M.; Kabaria, C.W.; Manh, B.H.; Elyazar, I.R.; Brooker, S.; et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009, 6, e1000048. [Google Scholar] [CrossRef]
- Durnez, L.; Van Bortel, W.; Denis, L.; Roelants, P.; Veracx, A.; Trung, H.D.; Sochantha, T.; Coosemans, M. False positive circumsporozoite protein ELISA: A challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar. J. 2011, 10, 195. [Google Scholar] [CrossRef]
- Lekweiry, K.M.; Salem, M.S.O.A.; Cotteaux-Lautard, C.; Jarjaval, F.; Marin-Jauffre, A.; Bogreau, H.; Basco, L.; Briolant, S.; Boukhary, A.O.M.S.; Brahim, K.O.; et al. Circumsporozoite protein rates, blood-feeding pattern and frequency of knockdown resistance mutations in Anopheles spp. in two ecological zones of Mauritania. Parasite Vectors 2016, 9, 268. [Google Scholar] [CrossRef]
- Hay, S.I.; Rogers, D.J.; Toomer, J.F.; Snow, R.W. Annual Plasmodium falciparum entomological inoculation rates(EIR) across Africa: Literature survey, internet access and review. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 113–127. [Google Scholar] [CrossRef]
- Ermert, V.; Fink, A.H.; Jones, A.E.; Morse, A.P. Development of a new version of the Liverpool Malaria Model. II. Calibration and validation for West Africa. Malar. J. 2011, 10, 62. [Google Scholar] [CrossRef]
- Poisot, T. The digitize package: Extracting numerical data from scatterplots. R J. 2011, 3, 25–26. [Google Scholar] [CrossRef]
- Macdonald, G. The Epidemiology and Control of Malaria; Oxford University Press: London, UK, 1957. [Google Scholar]
- Beier, J.C.; Killen, G.F.; Githure, J.I. A short report: Entomologic inoculation rate and plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg 1999, 61, 109–113. [Google Scholar] [CrossRef]
- Kelly-Hope, L.A.; McKenzie, F.E. The multiplicity of malaria transmission: A review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malaria J. 2009, 8, 19. [Google Scholar] [CrossRef]
- CIESIN-CIAT. Gridded Population of the World, Version 3 (GPWv3): Population Density Grid; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2005. [CrossRef]
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Atkinson, P.M.; Snow, R.W. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 2005, 3, 81–90. [Google Scholar] [CrossRef]
- Yamba, E.I.; Tompkins, A.M.; Fink, A.H.; Ermert, V.; Djouda, A.; Amekudzi, L.K.; Briët, O.J.T. Monthly entomological inoculation rates for studying malaria transmission seasonality in Africa. PANGAEA 2018. [Google Scholar] [CrossRef]
- Akogbeto, M.; Modiano, D.; Bosman, A. Malaria transmission in the lagoon area of Cotonou, Benin. Parassitologia 1992, 34, 147–154. [Google Scholar]
- Ilboudo-Sanogo, E.; Tiono, B.A.; Sagnon, N.; Cuzin Ouattara, N.; Nébié, I.; Sirima, S. Temporal dynamics of malaria transmission in two rural areas of Burkina Faso with two ecological differences. J. Med. Entomol. 2010, 47, 618–624. [Google Scholar]
- Robert, V.; Gazin, P.; Boudin, C.; Molez, J.F.; Oudreaogo, V.; Carnevale, P. La Transmission du paludisme en zone de savane arboree et en zone rizicole des environs de Bobo Dioulasso (Burkina Faso). Annales de la Societe Belge de Medecine Tropicale 1985, 65, 201–214. [Google Scholar]
- Rossi, P.; Belli, A.; Mancini, L.; Sabatinelli, G. [A longitudinal entomologic survey on the transmission of malaria in Ouagadougou (Burkina Faso)]. Parassitologia 1986, 28, 1–15. [Google Scholar] [PubMed]
- Robert, V.; Ouari, B.; Ouedraogo, V.; Carnevale, P. Etude écologique des Culicidae adultes et larvaires dans une rivière en vallée du Kou, Burkina Faso. Acta Trop. 1988, 45, 351–359. [Google Scholar] [PubMed]
- Robert, V.; Carnevale, P. Influence of deltamethrin treatment of bed nets on malaria transmission in the Kou valley, Burkina Faso. Bull. World Health Organ. 1991, 69, 735. [Google Scholar]
- Dabire, K.; Diabate, A.; Pare-Toe, L.; Rouamba, J.; Ouari, A.; Fontenille, D.; Baldet, T. Year to year and seasonal variations in vector bionomics and malaria transmission in a humid savannah village in west Burkina Faso. J. Vector Ecol. 2008, 33, 70–75. [Google Scholar] [CrossRef]
- Coosemans, M.H. Comparaison de l’endémie malarienne dans une zone de riziculture et dans une zone de culture de coton dans la plaine de la Rusizi, Burundi. Annales de la Société belge de Médecine Tropicale 1985, 65, 187–200. [Google Scholar]
- Fondjo, E.; Robert, V.; Le Goff, G.; Toto, J.; Carnevale, P.; Kalema, M.; Hughlett, S. Le paludisme urbain à Yaoundé (Cameroun). II: Etude entomologique dans deux quartiers peu urbanisés. Commentaire. Bulletin de la Société de Pathologie Exotique 1992, 85, 57–63. [Google Scholar]
- Njan Nloga, A.; Robert, V.; Toto, J.; Carnevale, P. Anopheles moucheti, vecteur principal du paludisme au sud-Cameroun. Bulletin de Liaison et de Documentation-OCEAC 1993, 26, 63–67. [Google Scholar]
- Meunier, J.Y.; Safeukui, I.; Fontenille, D.; Boudin, C. [Malaria transmission in an area of future vaccination in equatorial forest of south Cameroon]. Bulletin de la Societe de Pathologie Exotique (1990) 1999, 92, 309–312. [Google Scholar]
- Fils, E.M.B.; Ntonga, P.A.; Belong, P.; Messi, J. Contribution of mosquito vectors in malaria transmission in an urban district of southern Cameroon. J. Entomol. Nematol. 2010, 2, 13–17. [Google Scholar]
- Tanga, M.C.; Ngundu, W.I.; Tchouassi, P.D. Daily survival and human blood index of major malaria vectors associated with oil palm cultivation in Cameroon and their role in malaria transmission. Trop. Med. Int. Health 2011, 16, 447–457. [Google Scholar] [CrossRef]
- Bigoga, J.D.; Manga, L.; Titanji, V.P.K.; Coetzee, M.; Leke, R.G.F. Malaria vectors and transmission dynamics in coastal south-western Cameroon. Malaria J. 2007, 6, 5. [Google Scholar] [CrossRef]
- Tanga, M.C.; Ngundu, W.I. Ecological transition from natural forest to tea plantations: Effect on the dynamics of malaria vectors in the highlands of Cameroon. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 659–668. [Google Scholar] [CrossRef]
- Le Goff, G.; Carnevale, P.; Fondjo, E.; Robert, V. Comparison of three sampling methods of man-biting anophelines in order to estimate the malaria transmission in a village of south Cameroon. Parasite 1997, 4, 75–80. [Google Scholar] [CrossRef][Green Version]
- Manga, L.; Bouchite, B.; Toto, J.; Froment, A. Anopheles species and the transmission of malaria in the forest/savannah transition zone in central Cameroon. Bulletin de la Societe de Pathologie Exotique (1990) 1997, 90, 128–130. [Google Scholar]
- Cohuet, A.; Simard, F.; Wondji, C.S.; Antonio-Nkondjio, C.; Awono-Ambene, P.; Fontenille, D. High malaria transmission intensity due to Anopheles funestus (Diptera: Culicidae) in a village of savannah–forest transition area in Cameroon. J. Med. Entomol. 2004, 41, 901–905. [Google Scholar] [CrossRef]
- Antonio-Nkondjio, C.; Defo-Talom, B.; Tagne-Fotso, R.; Tene-Fossog, B.; Ndo, C.; Lehman, L.G.; Tchuinkam, T.; Kengne, P.; Awono-Ambene, P. High mosquito burden and malaria transmission in a district of the city of Douala, Cameroon. BMC Infect. Dis. 2012, 12, 275. [Google Scholar] [CrossRef]
- Manga, L.; Toto, J.; Carnevale, P. Malaria vectors and transmission in an area deforested for a new international airport in southern Cameroon. In Annales-Societe Belge de Medecine Tropicale; Institute of Tropical Medicine: Antwerpen, Belgium, 1995; Volume 75, p. 43. [Google Scholar]
- Carnevale, P.; Goff, G.; TOTO, J.C.; Robert, V. Anopheles nili as the main vector of human malaria in villages of southern Cameroon. Med. Vet. Entomol. 1992, 6, 135–138. [Google Scholar] [CrossRef]
- Antonio-Nkondjio, C.; Awono-Ambene, P.; Toto, J.C.; Meunier, J.Y.; Zebaze-Kemleu, S.; Nyambam, R.; Wondji, C.S.; Tchuinkam, T.; Fontenille, D. High malaria transmission intensity in a village close to Yaounde, the capital city of Cameroon. J. Med. Entomol. 2002, 39, 350–355. [Google Scholar] [CrossRef]
- Koum, G.; Yekel, A.; Ndifon, B.; Etang, J.; Simard, F. Design of a Two-level Adaptive Multi-Agent System for Malaria Vectors driven by an ontology. BMC Med. Inform. Decis. Mak. 2007, 7, 19. [Google Scholar] [CrossRef]
- Kerah-Hinzoumbé, C.; Péka, M.; Antonio-Nkondjio, C.; Donan-Gouni, I.; Awono-Ambene, P.; Samè-Ekobo, A.; Simard, F. Malaria vectors and transmission dynamics in Goulmoun, a rural city in south-western Chad. BMC Infect. Dis. 2009, 9, 71. [Google Scholar] [CrossRef]
- Richard, A.; Lallemant, M.; Trape, J.; Carnevale, P.; Mouchet, J. Malaria in the forest region of Mayombe, People’s Republic of the Congo. III. The role of malaria in general morbidity. Annales de la Societe belge de Medecine Tropicale 1988, 68, 317–329. [Google Scholar]
- Coene, J. Malaria in urban and rural Kinshasa: The entomological input. Med. Vet. Entomol. 1993, 7, 127–137. [Google Scholar] [CrossRef]
- Karch, S.; Garin, B.; Asidi, N.; Manzambi, Z.; Salaun, J.J.; Mouchet, J. Moustiquaires imprégnées contre le paludisme au Zaïre. Annales de la Société belge de Médecine Tropicale 1993, 73, 37–53. [Google Scholar]
- Shililu, J.; Ghebremeskel, T.; Mengistu, S.; Fekadu, H.; Zerom, M.; Mbogo, C.; Githure, J.; Novak, R.; Brantly, E.; Beier, J.C. High seasonal variation in entomologic inoculation rates in Eritrea, a semi-arid region of unstable malaria in Africa. Am. J. Trop. Med. Hyg. 2003, 69, 607–613. [Google Scholar] [CrossRef]
- Shililu, J.; Ghebremeskel, T.; Seulu, F.; Mengistu, S.; Fekadu, H.; Zerom, M.; Asmelash, G.; Sintasath, D.; Mbogo, C.; Githure, J.; et al. Seasonal abundance, vector behavior, and malaria parasite transmission in Eritrea. J. Am. Mosq. Control. Assoc. 2004, 20, 155–164. [Google Scholar]
- Jaleta, K.T.; Hill, S.R.; Seyoum, E.; Balkew, M.; Gebre-Michael, T.; Ignell, R.; Tekie, H. Agro-ecosystems impact malaria prevalence: Large-scale irrigation drives vector population in western Ethiopia. Malaria J. 2013, 12, 350. [Google Scholar] [CrossRef]
- Massebo, F.; Lindtjørn, B. The effect of screening doors and windows on indoor density of Anopheles arabiensis in south-west Ethiopia: A randomized trial. Malaria J. 2013, 12, 319. [Google Scholar] [CrossRef]
- Animut, A.; Balkew, M.; Gebre-Michael, T.; Lindtjørn, B. Blood meal sources and entomological inoculation rates of anophelines along a highland altitudinal transect in south-central Ethiopia. Malaria J. 2013, 12, 76. [Google Scholar] [CrossRef]
- Krafsur, E.S. The bionomics and relative prevalence of Anopheles species with respect to the transmission of Plasmodium to man in western Ethiopia. J. Med. Entomol. 1977, 14, 180–194. [Google Scholar] [CrossRef]
- Elissa, N.; Migot-Nabias, F.; Luty, A.; Renaut, A.; Toure, F.; Vaillant, M.; Lawoko, M.; Yangari, P.; Mayombo, J.; Lekoulou, F.; et al. Relationship between entomological inoculation rate, Plasmodium falciparum prevalence rate, and incidence of malaria attack in rural Gabon. Acta Trop. 2003, 85, 355–361. [Google Scholar] [CrossRef]
- Badu, K.; Brenya, R.C.; Timmann, C.; Garms, R.; Kruppa, T.F. Malaria transmission intensity and dynamics of clinical malaria incidence in a mountaneous forest region of Ghana. Malaria World J. 2013, 4, 4–14. [Google Scholar]
- Dery, D.B.; Brown, C.; Asante, K.P.; Adams, M.; Dosoo, D.; Amenga-Etego, S.; Wilson, M.; Chandramohan, D.; Greenwood, B.; Owusu-Agyei, S. Patterns and seasonality of malaria transmission in the forest-savannah transitional zones of Ghana. Malaria J. 2010, 9, 314. [Google Scholar] [CrossRef]
- Appawu, M.; Owusu-Agyei, S.; Dadzie, S.; Asoala, V.; Anto, F.; Koram, K.; Rogers, W.; Nkrumah, S.; Hoffman, S.L.; Fryauff, D.J. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop. Med. Int. Health 2004, 9, 164–170. [Google Scholar] [CrossRef]
- Kasasa, S.; Asoala, V.; Gosoniu, L.; Anto, F.; Adjuik, M.; Tindana, C.; Smith, T.; Owusu-Agyei, S.; Vounatsou, P. Spatio-temporal malaria transmission patterns in Navrongo demographic surveillance site, northern Ghana. Malaria J. 2013, 12, 63. [Google Scholar] [CrossRef]
- Dossou-Yovo, J.; Doannio, J.; Riviere, F.; Chauvancy, G. Malaria in Cote d’Ivoire wet savannah region: The entomological input. Trop. Med. Parasitol. Off. Organ Dtsch. Tropenmedizinische Ges. Dtsch. Ges. Fur Tech. Zusammenarbeit (GTZ) 1995, 46, 263–269. [Google Scholar]
- Briët, O.J.T.; Dossou-Yovo, J.; Akodo, E.; Van De Giesen, N.; Teuscher, T.M. The relationship between Anopheles gambiae density and rice cultivation in the savannah zone and forest zone of Cote d’Ivoire. Trop. Med. Int. Health 2003, 8, 439–448. [Google Scholar] [CrossRef]
- Dossou-Yovo, J.; Doannio, J.; Diarrassouba, S.; Chauvancy, G. Impact d’aménagements de rizières sur la transmission du paludisme dans la ville de Bouaké, Côte d’Ivoire. Bull. Soc. Pathol. Exot. 1998, 91, 327–333. [Google Scholar]
- Adja, A.; N’Goran, K.; Kengne, P.; Koudou, G.; Toure, M.; Koffi, A.; Tia, E.; Fontenille, D.; Chandre, F. Vectorial transmission of malaria in shrubby Savannah area at Ganse, Ivory Coast. Medecine Tropicale: Revue du Corps de Sante Colonial 2006, 66, 449–455. [Google Scholar]
- Doannio, J.; Dossou-Yovo, J.; Diarrassouba, S.; Rakotondraibe, M.; Chauvancy, G.; Chandre, F.; Riviere, F.; Carnevale, P. Dynamics of malaria transmission in Kafine, a rice growing village in a humid savannah area of Cote d’Ivoire. Bulletin de la Societe de Pathologie Exotique (1990) 2002, 95, 11–16. [Google Scholar]
- Nzeyimana, I.; Henry, M.; Dossou-Yovo, J.; Doannio, J.; Diawara, L.; Carnevale, P. The epidemiology of malaria in the southwestern forests of the Ivory Coast (Tai region). Bulletin de la Societe de Pathologie Exotique (1990) 2002, 95, 89–94. [Google Scholar]
- Koudou, B.; Tano, Y.; Doumbia, M.; Nsanzabana, C.; Cissé, G.; Girardin, O.; Dao, D.; N’goran, E.; Vounatsou, P.; Bordmann, G.; et al. Malaria transmission dynamics in central Côte d’Ivoire: The influence of changing patterns of irrigated rice agriculture. Med. Vet. Entomol. 2005, 19, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Githeko, A.K.; Service, M.W.; Mbogo, C.M.; Atieli, F.K.; Juma, F.O. Plasmodium falciparum sporozoite and entomological inoculation rates at the Ahero rice irrigation scheme and the Miwani sugar-belt in western Kenya. Ann. Trop. Med. Parasitol. 1993, 87, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Koros, J.K.; Nduati, J.; Copeland, R.S.; Collins, F.H.; Brandling-Bennett, A.D. Plasmodium falciparum infection rates in Anopheles gambiae, An. arabiensis, and An. funestus in western Kenya. Am. J. Trop. Med. Hyg. 1990, 43, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Muriu, S.; Shililu, J.; Mwangangi, J.; Jacob, B.G.; Mbogo, C.; Githure, J.; Novak, R.J. Effect of rice cultivation on malaria transmission in central Kenya. Am. J. Trop. Med. Hyg. 2008, 78, 270–275. [Google Scholar] [CrossRef]
- Mbogo, C.N.; Snow, R.W.; Kabiru, E.W.; Ouma, J.H.; Githure, J.I.; Marsh, K.; Beier, J.C. Low-level Plasmodium falciparum transmission and the incidence of severe malaria infection on the Kenyan coast. Am. J. Trop. Med. Hyg. 1993, 49, 245–253. [Google Scholar] [CrossRef]
- Beier, J.C.; Perkins, P.V.; Koros, J.K.; Onyango, F.K.; Gargan, T.P.; Wirtz, R.A.; Koech, D.K.; Roberts, C.R. Malaria sporozoite detection by dissection and ELISA to assess infectivity of afrotropical Anopheles (Diptera: Culicidae). J. Med Entomol. 1990, 27, 377–384. [Google Scholar] [CrossRef]
- Aniedu, I. Dynamics of malaria transmission near two permanent breeding sites in Baringo district, Kenya. Indian J. Med Res. 1997, 105, 206–211. [Google Scholar]
- Shililu, J.; Maier, W.; Seitz, H.; Orago, A. Seasonal density, sporozoite rates and entomological inoculation rates of Anopheles gambiae and Anopheles funestus in a high altitude sugarcane growing zone in western Kenya. Trop. Med. Int. Health 1998, 3, 706–710. [Google Scholar] [CrossRef]
- Pull, J.; Grab, B. A simple epidemiological model for evaluating the malaria inoculation rate and the risk of infection in infants. Bull. World Health Organ. 1974, 51, 507. [Google Scholar]
- Lepers, J.P.; Fontenille, D.; Rason, M.D.; Chougnet, C.; Astagneau, P.; Coulanges, P.; Deloron, P. Transmission and epidemiology of newly transmitted falciparum malaria in the central highland plateaux of Madagascar. Ann. Trop. Med. Parasitol. 1991, 85, 297–304. [Google Scholar] [CrossRef]
- Andrianaivolambo, L.; Domarle, O.; Randrianarivelojosia, M.; Ratovonjato, J.; Le Goff, G.; Talman, A.; Ariey, F.; Robert, V. Anthropophilic mosquitoes and malaria transmission in the eastern foothills of the central highlands of Madagascar. Acta Trop. 2010, 116, 240–245. [Google Scholar] [CrossRef]
- Sogoba, N.; Doumbia, S.; Vounatsou, P.; Bagayoko, M.M.; Dolo, G.; Traoré, S.F.; Maïga, H.M.; Touré, Y.T.; Smith, T. Malaria transmission dynamics in Niono, Mali: The effect of the irrigation systems. Acta Trop. 2007, 101, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Sagara, I.; Sangaré, D.; Dolo, G.; Guindo, A.; Sissoko, M.; Sogoba, M.; Niambélé, M.B.; Yalcoué, D.; Kaslow, D.C.; Dicko, A.; et al. A high malaria reinfection rate in children and young adults living under a low entomological inoculation rate in a periurban area of Bamako, Mali. Am. J. Trop. Med. Hyg. 2002, 66, 310–313. [Google Scholar] [CrossRef]
- Zharov, A. Observations on malaria vectors in Mozambique. I. The status of Anopheles populations before the start of mosquito control. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 1992, 3, 23–28. [Google Scholar]
- Aranda, C.; Aponte, J.J.; Saute, F.; Casimiro, S.; Pinto, J.; Sousa, C.; Rosario, V.D.O.; Petrarca, V.; Dgedge, M.; Alonso, P. Entomological characteristics of malaria transmission in Manhica, a rural area in southern Mozambique. J. Med. Entomol. 2005, 42, 180–186. [Google Scholar] [CrossRef]
- Thomson, R.M. Studies on Anopheles gambiae and A. melas in and around Lagos. Bull. Entomol. Res. 1948, 38, 527–558. [Google Scholar] [CrossRef]
- Molineaux, L.; Gramiccia, G.; World Health Organization. The Garki Project: Research on the Epidemiology and Control of Malaria in the Sudan Savanna of West Africa; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- Vercruysse, J. Etude entomologique sur la transmission du paludisme humain dans le bassin du fleuve Sénégal (Sénégal). Annales de la Société belge de Médecine Tropicale 1985, 65, 171–179. [Google Scholar]
- Faye, O.; Gaye, O.; Faye, O.; Diallo, S. La transmission du paludisme dans des villages éloignés u situés en bordure de la mangrove au Sénégal. Bulletin de la Société de Pathologie Exotique 1994, 87, 157–163. [Google Scholar]
- Lemasson, J.J.; Fontenille, D.; Lochouarn, L.; Dia, I.; Simard, F.; Ba, K.; Diop, A.; Diatta, M.; Molez, J.F. Comparison of behavior and vector efficiency of Anopheles gambiae and An. arabiensis (Diptera: Culicidae) in Barkedji, a Sahelian area of Senegal. J. Med. Entomol. 1997, 34, 396–403. [Google Scholar] [CrossRef]
- Fontenille, D.; Lochouarn, L.; Diatta, M.; Sokhna, C.; Dia, I.; Diagne, N.; Lemasson, J.J.; Ba, K.; Tall, A.; Rogier, C.; et al. Four years entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and A. arabiensis. Am. J. Trop. Med. Hyg. 1997, 91, 647–652. [Google Scholar] [CrossRef]
- Robert, V.; Dieng, H.; Lochouarn, L.; Traore, S.; Trape, J.F.; Simondon, F.; Fontenille, D. La transmission du paludisme dans la zone de Niakhar, Sénégal. Trop. Med. Int. Health 1998, 3, 667–677. [Google Scholar] [CrossRef]
- Faye, O.; Fontenille, D.; Hervé, J.; Diack, P.; Diallo, S.; Mouchet, J. Malaria in the Saharan region of Senegal. 1. Entomological transmission findings. Annales de la Societe belge de Medecine Tropicale 1993, 73, 21–30. [Google Scholar]
- Vercruysse, J.; Jancloes, M.; van de Velden, L. Epidemiology of seasonal falciparum malaria in an urban area in Senegal. Bull. World Health Organ. 1983, 61, 821–831. [Google Scholar]
- Faye, O.; Gaye, O.; Fontenille, D.; Sy, N.; Konate, L.; Hébrard, G.; Hervé, J.P.; Trouillet, J.; Diallo, S.; Mouchet, J. Comparaison de la transmission du paludisme dans deux faciès épidémiologiques au Sénégal: La zone côtière sahélienne et la zone méridionale soudanienne. Dakar Méd. 1995, 40, 201–207. [Google Scholar]
- Bockarie, M.J.; Service, M.W.; Barnish, G.; Touré, Y.T. Vectorial capacity and entomological inoculation rates of Anopheles gambiae in a high rainfall forested area of southern Sierra Leone. Trop. Med. Parasitol. Off. Organ Dtsch. Tropenmedizinische Ges. Dtsch. Ges. Fur Tech. Zusammenarbeit (GTZ) 1995, 46, 164–171. [Google Scholar]
- Bockarie, M.J.; Service, M.W.; Barnish, G.; Maude, G.H.; Greenwood, B.M. Malaria in a rural area of Sierra Leone. III. Vector ecology and disease transmission. Ann. Trop. Med. Parasitol. 1994, 88, 251–262. [Google Scholar] [CrossRef]
- Shiff, C.J.; Minjas, J.N.; Hall, T.; Hunt, R.H.; Lyimo, S.; Davis, J.R. Malaria infection potential of anopheline mosquitoes sampled by light trapping indoors in coastal Tanzanian villages. Med. Vet. Entomol. 1995, 9, 256–262. [Google Scholar] [CrossRef]
- Ijumba, J.N.; Shenton, F.C.; Clarke, S.E.; Mosha, F.W.; Lindsay, S.W. Irrigated crop production is associated with less malaria than traditional agricultural practices in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 476–480. [Google Scholar] [CrossRef]
- Charlwood, J.D.; Smith, T.; Lyimo, E.; Kitua, A.Y.; Masanja, H.; Booth, M.; Alonso, P.L.; Tanner, M. Incidence of Plasmodium falciparum infection in infants in relation to exposure to sporozoite-infected anophelines. Am. J. Trop. Med. Hyg. 1998, 59, 243–251. [Google Scholar] [CrossRef]
- Bodker, R.; Akida, J.; Shayo, D.; Kisinza, W.; Msangeni, H.A.; Pedersen, E.M.; Lindsay, S.W. Relationship between altitude and intensity of malaria transmission in the Usambara Mountains, Tanzania. J. Med. Entomol. 2003, 40, 706–717. [Google Scholar] [CrossRef]
- Lyimo, E.O.K. The Bionomics of the Malaria Mosquito Anopheles Gambiae Sensu lato in Southeast Tanzania: Adult Size Variation and Its Effect on Female Fecundity, Survival and Malaria Transmission. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, December 1993. [Google Scholar]
- Smith, T.; Charlwood, J.D.; Kihonda, J.; Mwankusye, S.; Billingsley, P.; Meuwissen, J.; Lyimo, E.; Takken, W.; Teuscher, T.; Tanner, M. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop. 1993, 54, 55–72. [Google Scholar] [CrossRef]
- Iyengar, R. The bionomics of salt-water Anopheles gambiae in East Africa. Bull. World Health Organ. 1962, 27, 223. [Google Scholar]
- Okello, P.E.; Van Bortel, W.; Byaruhanga, A.M.; Correwyn, A.; Roelants, P.; Talisuna, A.; D’Alessandro, U.; Coosemans, M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am. J. Trop. Med. Hyg. 2006, 75, 219–225. [Google Scholar] [CrossRef]
- Lindblade, K.A.; Walker, E.D.; Onapa, A.W.; Katungu, J.; Wilson, M.L. Highland malaria in Uganda: Prospective analysis of an epidemic associated with El Nino. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 480–487. [Google Scholar] [CrossRef]
- Kent, R.J.; Thuma, P.E.; Mharakurwa, S.; Norris, D.E. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am. J. Trop. Med. Hyg. 2007, 76, 267–274. [Google Scholar] [CrossRef]
- Bomblies, A.; Duchemin, J.B.; Eltahir, E.A. Hydrology of malaria: Model development and application to a Sahelian village. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Novella, N.S.; Thiaw, W.M. African Rainfall Climatology Version 2 for Famine Early Warning Systems. J. Appl. Meteorol. Climatol. 2013, 52, 588–606. [Google Scholar] [CrossRef]
- Dee, D.P.; Uppala, S.M.; Simmons, A.; Berrisford, P.; Poli, P.; Kobayashi, S.; Andrae, U.; Balmaseda, M.; Balsamo, G.; Bauer, d.P.; et al. The ERA-Interim reanalysis: Configuration and performance of the data assimilation system. Q. J. R. Meteorol. Soc. 2011, 137, 553–597. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and Middle East: Occurrence data, distribution maps and bionomic precis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef]
- Coetzee, M.; Craig, M.; le Sueur, D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol. Today 2000, 16, 149–151. [Google Scholar] [CrossRef]
- Lindsay, S.W.; Parson, L.; Thomas, C.J. Mapping the ranges and relative abundance of the two principal Africa malaria vectors, Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proc. R. Soc. Lond. B 1998, 265, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Charlwood, J.D.; Thompson, R.; Madsen, H. Observations on the swarming and mating behaviour of Anopheles funestus from Southern Mozambique. Malar. J. 2003, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Nkuo-Akenji, T.; Ntonifor, N.N.; Ndukum, M.B.; Kimbi, H.K.; Abongwa, E.L.; Nkwescheu, A.; Anong, D.N.; Songmbe, M.; Boyo, M.G.; Ndamukong, K.N.; et al. Environmental factors affecting malaria parasite prevalence in rural Bolifamba, South-West Cameroon. Afr. J. Health Sci. 2006, 13, 40–46. [Google Scholar] [CrossRef]
- Adja, A.M.; N’Goran, E.K.; Koudou, B.G.; Dia, I.; Kengne, P.; Fontenille, D.; Chandre, F. Contribution of An. funestus, An. gambiae, and An. nili (Diptera:Culicidae) to the perenial malaria transmission in the southern and western forest areas of Cote d’Ivoire. Ann. Trop. Med. Parasitol. 2011, 105, 13–24. [Google Scholar] [CrossRef]
- Fontenille, D.; Meunier, J.Y.; Nkondjio, C.A.; Tchuinkam, T. Use of circumsporozoite protein enzyme-linked immunosorbent assay compared with microscopic examination of salivary glands for calculation of malaria infectivity rates in mosquitoes (Diptera: Culicidae) from Cameroon. J. Med. Entomol. 2001, 38, 451–454. [Google Scholar] [CrossRef]
- Kilama, M.; Smith, D.L.; Hutchinson, R.; Kigozi, R.; Yeka, A.; Lavoy, G.; Kamya, M.R.; Staedke, S.G.; Donnelly, M.J.; Drakeley, C.; et al. Estimating the annual entomological inoculation rate for plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malaria J. 2014, 13, 111. [Google Scholar] [CrossRef]
- Tusting, L.S.; Bousema, T.; Smith, D.L.; Drakeley, C. Measuring changes in Plasmodium falciparum transmission: Precision, accuracy and costs of metrics. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 84, pp. 151–208. [Google Scholar]
- Cairns, M.; Roca-Feltrer, A.; Garske, T.; Wilson, A.L.; Diallo, D.; Milligan, P.J.; Ghani, A.C.; Greenwood, B.M. Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nat. Commun. 2012, 3, 1–9. [Google Scholar] [CrossRef]
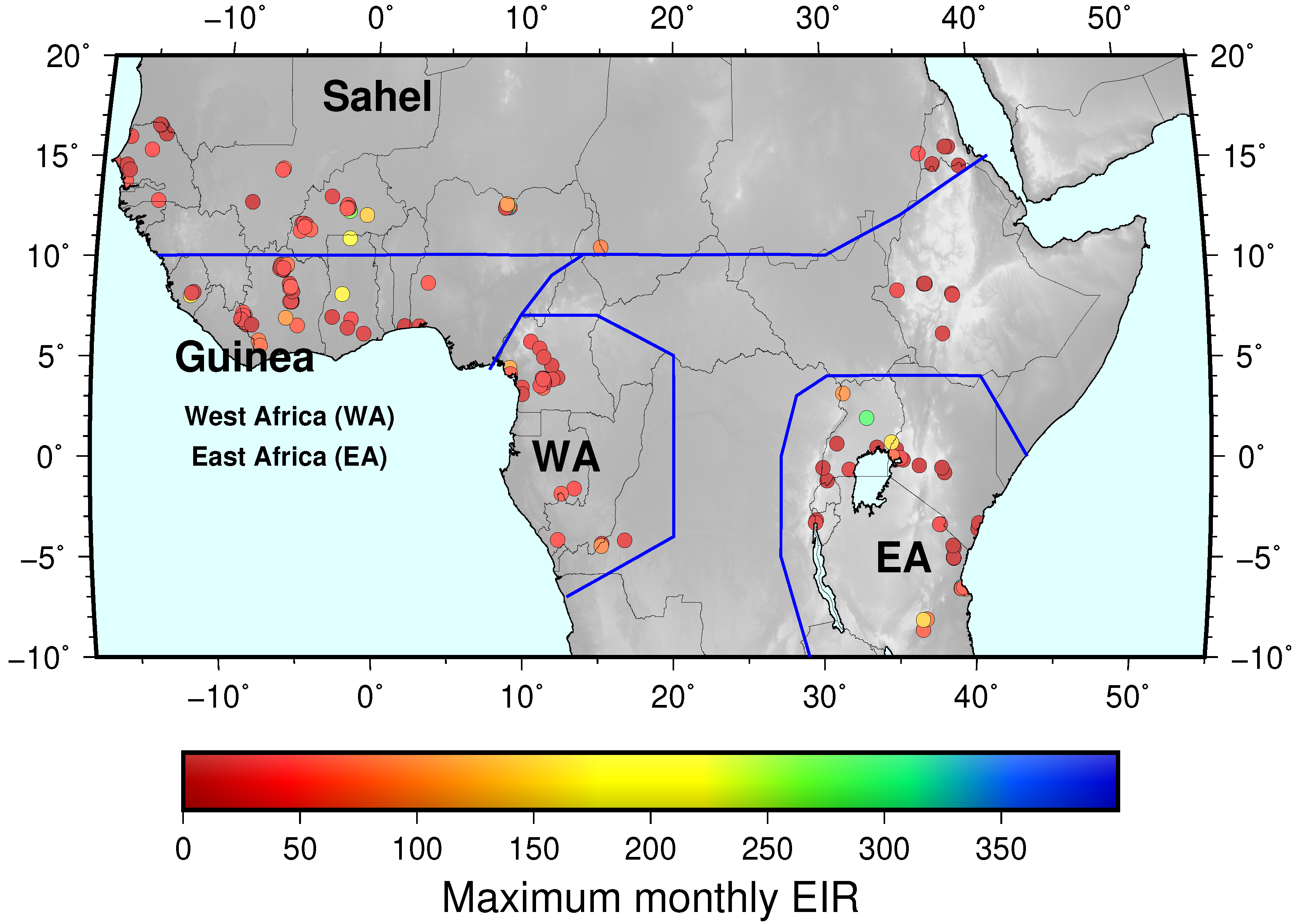
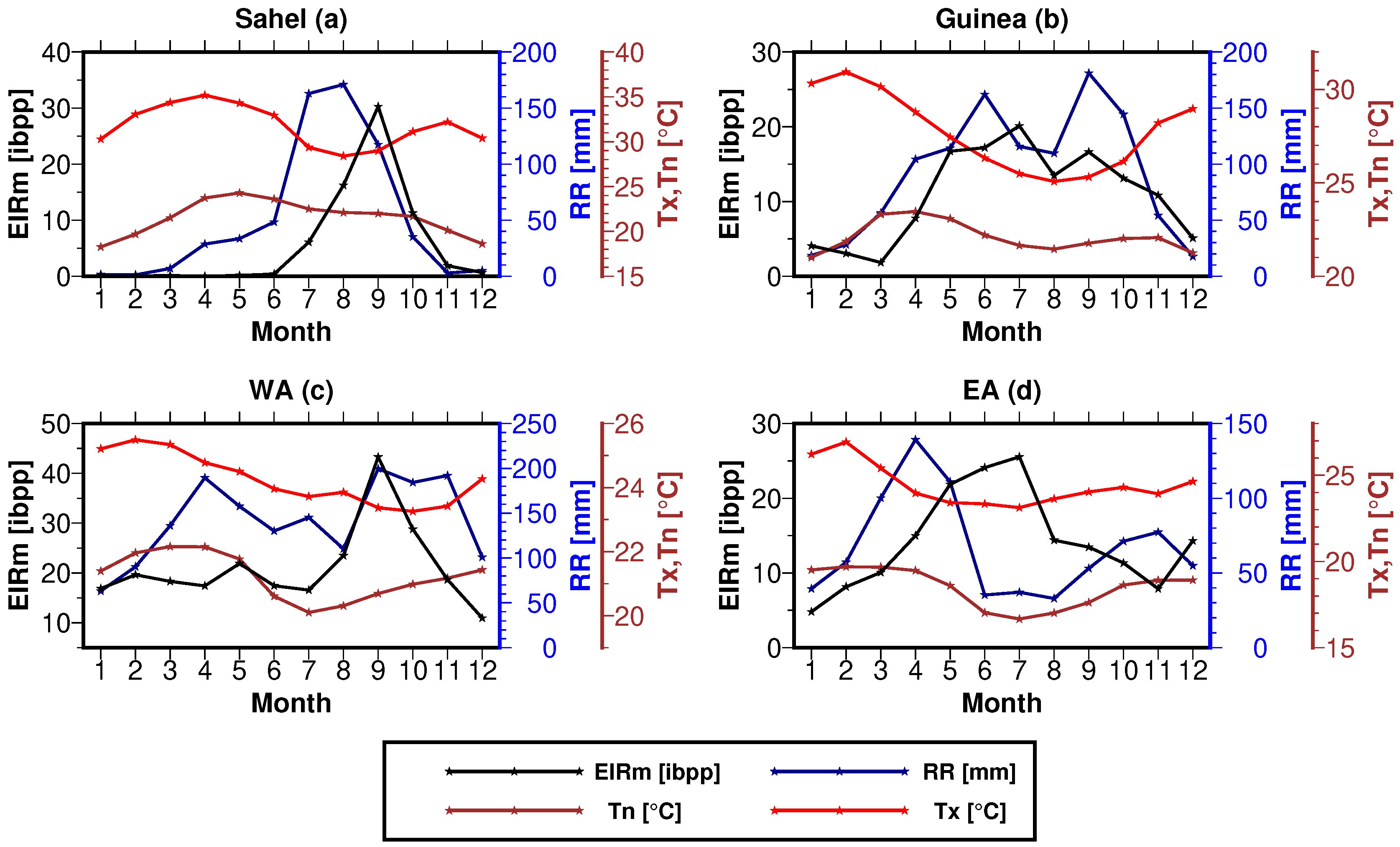

| ZONE | 1 Year | 2 Years | 3 Years | 4 Years | 5 Years | Total |
|---|---|---|---|---|---|---|
| SAHEL | 36 | 18 | 4 | 1 | 1 | 60 |
| GUINEA | 31 | 28 | 2 | 0 | 0 | 61 |
| WA | 22 | 4 | 0 | 0 | 0 | 26 |
| EA | 38 | 10 | 2 | 0 | 0 | 50 |
| Country | Site | Lon | Lat | Elevation | Pd | Hydrology | Vector Species | SY | SM | EY | EM | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benin | Gbegame | 2.41 | 6.36 | 6 | U | N | AG | 1987 | 01 | 1987 | 12 | [21] |
| Benin | Ladji | 2.43 | 6.39 | 2 | U | N | AG | 1987 | 01 | 1987 | 12 | [21] |
| Benin | St. Ritha Nord | 2.40 | 6.38 | 4 | U | N | AG | 1987 | 01 | 1987 | 12 | [21] |
| Benin | Ganvie | 2.417 | 6.467 | 2 | PU | PWB | AG | 1994 | 01 | 1995 | 12 | [21] |
| Burkina Faso | Balonguen | −1.621 | 12.403 | 343 | PU | PWB | AF, AG | 2000 | 12 | 2001 | 11 | [22] |
| Burkina Faso | Dande | −4.557 | 11.582 | 275 | R | N | AF, AG | 1983 | 01 | 1984 | 12 | [23] |
| Burkina Faso | Guinghin Nord | −1.54 | 12.35 | 305 | U | N | AG | 1984 | 03 | 1985 | 02 | [24] |
| Burkina Faso | Karangaso | −4.64 | 11.21 | 366 | R | PWB | AF, AG, AN | 1985 | 03 | 1986 | 02 | [25] |
| Burkina Faso | Kologh Naba | −1.54 | 12.38 | 292 | U | N | AG | 1984 | 03 | 1985 | 02 | [24] |
| Burkina Faso | Kongodjan | −4.45 | 11.58 | 480 | R | PWB | AF, AG | 1983 | 01 | 1984 | 12 | [23] |
| Burkina Faso | Kou | −4.27 | 11.52 | 288 | R | I | AG | 1985 | 05 | 1986 | 04 | [26] |
| Burkina Faso | Koubri | −1.406 | 12.198 | 289 | R | N | AGSL | 1984 | 03 | 1985 | 02 | [24] |
| Burkina Faso | Lena | −3.98 | 11.28 | 307 | R | N | AF, AG | 1999 | 01 | 2001 | 12 | [27] |
| Burkina Faso | Nongremassm | −1.56 | 12.40 | 310 | U | N | AG | 1984 | 03 | 1985 | 02 | [24] |
| Burkina Faso | Pabre | −1.57 | 12.505 | 303 | R | N | AGSL | 1984 | 03 | 1985 | 02 | [24] |
| Burkina Faso | St Camille | −1.50 | 12.36 | 299 | U | N | AG | 1984 | 03 | 1985 | 02 | [24] |
| Burkina Faso | St Leon | −1.522 | 12.361 | 306 | U | N | AGSL | 1984 | 03 | 1985 | 02 | [24] |
| Burkina Faso | Tago | −2.643 | 12.932 | 308 | R | N | AF, AG | 1983 | 01 | 1983 | 12 | [23] |
| Burkina Faso | Tensobtenga | −0.267 | 12.00 | 295 | R | PWB | AF, AG | 2000 | 12 | 2001 | 11 | [22] |
| Burkina Faso | VK4 | −4.37 | 11.41 | 288 | R | I | AG | 1984 | 01 | 1984 | 12 | [23] |
| Burkina Faso | VK6 | −4.37 | 11.41 | 288 | R | I | AF, AG | 1983 | 01 | 1984 | 12 | [23] |
| Burkina Faso | Zagtouli | −1.625 | 12.329 | 310 | U | N | AGSL | 1984 | 03 | 1985 | 02 | [24] |
| Burundi | Gihanga | 29.29 | −3.19 | 827 | R | I | AG | 1982 | 01 | 1983 | 12 | [28] |
| Burundi | Katumba | 29.237 | −3.317 | 776 | R | N | AF, AG | 1982 | 01 | 1982 | 12 | [28] |
| Cameroon | Nkol Bikok | 11.48 | 3.87 | 728 | U | PWB | AG | 1989 | 03 | 1990 | 02 | [29] |
| Cameroon | Nkol Bisson | 11.44 | 3.87 | 760 | R | PWB | AG | 1989 | 04 | 1990 | 03 | [29] |
| Cameroon | Ebogo | 11.47 | 3.40 | 659 | R | N | AG, AMO | 1991 | 04 | 1992 | 03 | [30] |
| Cameroon | Ebolakounou | 12.44 | 3.91 | 701 | R | N | AF, AG, AMO | 1997 | 06 | 1998 | 05 | [31] |
| Cameroon | Ekombite | 11.83 | 3.12 | 693 | R | PWB | AG, AME, AF | 2007 | 01 | 2007 | 12 | [32] |
| Cameroon | Esuke camp | 9.31 | 4.10 | 279 | R | N | AF, AG, AHAN, AN | 2004 | 10 | 2005 | 09 | [33] |
| Cameroon | Idenau | 9.05 | 4.21 | 359 | R | N | AG, AF, AN | 2001 | 08 | 2002 | 07 | [34] |
| Cameroon | Koundou | 12.12 | 3.90 | 705 | R | N | AF, AG, AMO | 1997 | 06 | 1998 | 05 | [31] |
| Cameroon | Likoko | 9.31 | 4.39 | 1933 | R | N | AF, AG, AHAN | 2002 | 10 | 2003 | 09 | [35] |
| Cameroon | Limbe | 9.18 | 4.03 | 185 | R | N | AF, AG, AN | 2001 | 08 | 2002 | 07 | [34] |
| Cameroon | Mbebe | 10.12 | 3.41 | 70 | R | PWB | AF, AG, AN | 1989 | 04 | 1990 | 03 | [36] |
| Cameroon | Nditam | 11.26 | 5.36 | 712 | R | PWB | AG | 1995 | 05 | 1996 | 04 | [37] |
| Cameroon | Nkoteng | 12.05 | 4.5 | 587 | R | N | AF, AG | 1999 | 02 | 2001 | 01 | [38] |
| Cameroon | Ndogpassi | 10.13 | 3.08 | 72 | R | N | ALL | 2011 | 01 | 2011 | 12 | [39] |
| Cameroon | Nsimalen Ekoko | 12.12 | 3.82 | 699 | R | PWB | AME, AG | 1991 | 04 | 1992 | 03 | [39] |
| Cameroon | Nsimalen Mefou | 11.58 | 3.63 | 680 | R | PWB | AME | 1991 | 04 | 1992 | 03 | [39] |
| Cameroon | Nsimalen (deforested) | 11.553 | 3.723 | 679 | R | PWB | AMO | 1991 | 04 | 1992 | 03 | [40] |
| Cameroon | Nsimalen (forested) | 11.553 | 3.723 | 679 | R | PWB | AMO, AG | 1991 | 04 | 1992 | 03 | [40] |
| Cameroon | Sanaga river villages | 11.52 | 4.92 | 474 | R | PWB | AN, AG | 1989 | 04 | 1990 | 03 | [41] |
| Cameroon | Simbock-Block6 | 11.30 | 3.50 | 717 | R | PWB | AF, AG, AME, AN | 1999 | 01 | 1999 | 12 | [42] |
| Cameroon | Simbock | 11.5 | 3.83 | 625 | U | N | AF, AG, AMO AN | 1998 | 10 | 1999 | 09 | [43] |
| Cameroon | Tiko | 9.36 | 4.08 | 182 | R | N | AG, AF, AN | 2001 | 08 | 2002 | 07 | [34] |
| Chad | Goulmoun | 15.30 | 10.39 | 324 | R | I | AA, AF, AP, AZ | 2006 | 06 | 2007 | 05 | [44] |
| Congo | Kulila | 12.43 | −4.17 | 400 | R | PWB | AG | 1981 | 12 | 1982 | 11 | [45] |
| DRC | Kimbangu | 15.31 | −4.36 | 295 | U | N | AG | 1988 | 09 | 1990 | 08 | [46] |
| DRC | Kwamutu | 15.28 | −4.47 | 346 | U | N | AB, AF, AG, AN | 1988 | 09 | 1990 | 08 | [46] |
| DRC | Mbansale | 16.80 | −4.19 | 289 | R | PWB | AG | 1990 | 05 | 1991 | 04 | [47] |
| Eritrea | Adibosqual | 38.39 | 15.42 | 1482 | R | N | AG | 1999 | 01 | 1999 | 12 | [48] |
| Eritrea | Anseba Adibosqual | 38.39 | 15.42 | 894 | R | N | AA | 1999 | 10 | 2000 | 09 | [49] |
| Eritrea | Anseba Hagaz | 37.39 | 15.42 | 894 | R | N | AA | 1999 | 10 | 2000 | 09 | [49] |
| Eritrea | Dasse | 37.29 | 14.55 | 916 | R | N | AG | 1999 | 01 | 1999 | 12 | [48] |
| Eritrea | Debub Maiaini | 39.06 | 14.48 | 1809 | R | PWB | AA | 1999 | 10 | 2000 | 09 | [49] |
| Eritrea | Gash Barka Dasse | 37.29 | 14.55 | 610 | R | N | AA | 1999 | 10 | 2000 | 09 | [49] |
| Eritrea | Gash Barka Hiletsidi | 36.39 | 15.07 | 610 | R | N | AA | 1999 | 10 | 2000 | 09 | [49] |
| Eritrea | Hagaz | 38.17 | 15.42 | 883 | R | N | AG | 1999 | 01 | 1999 | 12 | [48] |
| Eritrea | Hiletsidi | 36.39 | 15.07 | 586 | R | N | AG | 1999 | 01 | 1999 | 12 | [48] |
| Eritrea | Maiaini | 39.09 | 14.49 | 1554 | R | N | AG | 1999 | 01 | 1999 | 12 | [48] |
| Ethiopia | Baka-Boro | 36.52 | 8.58 | 1316 | R | I | AG | 2010 | 02 | 2011 | 01 | [50] |
| Ethiopia | Chano | 37.58 | 6.10 | 1211 | R | N | AA | 2009 | 05 | 2010 | 04 | [51] |
| Ethiopia | Dirama | 38.25 | 8.10 | 2031 | PU | PWB | AA | 2008 | 07 | 2010 | 06 | [52] |
| Ethiopia | Gambela town | 34.67 | 8.25 | 551 | R | PWB | AF, AG | 1968 | 01 | 1968 | 12 | [53] |
| Ethiopia | Gambela villages | 34.67 | 8.25 | 551 | R | PWB | AF, AG | 1968 | 01 | 1968 | 12 | [53] |
| Ethiopia | Hobe | 38.29 | 8.02 | 1834 | PU | PWB | AA, AP | 2008 | 07 | 2010 | 06 | [52] |
| Ethiopia | Machara | 36.42 | 8.58 | 1351 | R | N | AG | 2010 | 02 | 2011 | 01 | [50] |
| Ethiopia | Wama Kusaye | 36.49 | 8.59 | 1319 | R | I | AG | 2010 | 02 | 2011 | 01 | [50] |
| Gabon | Benguia | 13.52 | −1.63 | 37 | R | N | AG | 2003 | 05 | 2004 | 04 | [54] |
| Gabon | Dienga | 12.68 | −1.87 | 772 | R | N | AG | 2003 | 05 | 2004 | 04 | [54] |
| Ghana | Abotanso | −0.26 | 6.09 | 374 | R | N | AF, AG | 2004 | 09 | 2005 | 08 | [55] |
| Ghana | Gyidim | −1.11 | 6.81 | 408 | R | N | AG | 2003 | 11 | 2005 | 10 | [55] |
| Ghana | Hwidiem | −2.35 | 6.93 | 186 | R | N | AG | 2003 | 11 | 2005 | 10 | [55] |
| Ghana | Kintampo | −1.73 | 8.05 | 354 | R | N | AF, AG | 2003 | 11 | 2006 | 10 | [56] |
| Ghana | KND Irrigated | −1.33 | 10.84 | 212 | R | I | AF, AG | 2001 | 06 | 2002 | 05 | [57] |
| Ghana | KND Lowland | −1.33 | 10.84 | 212 | R | N | AF, AG | 2001 | 06 | 2002 | 05 | [57] |
| Ghana | KND Rocky Highland | −1.33 | 10.84 | 212 | R | N | AF, AG | 2001 | 06 | 2002 | 05 | [57] |
| Ghana | LowCost | −1.33 | 6.38 | 250 | U | N | AG | 2003 | 11 | 2005 | 10 | [55] |
| Ghana | NHDSS | −1.33 | 10.84 | 287 | R | PWB | AF, AG | 2001 | 11 | 2004 | 10 | [58] |
| Country | Site | Lon | Lat | Elevation | Pd | Hydrology | Vector Species | SY | SM | EY | EM | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ivory Coast | Alloukoukro | −5.15 | 7.69 | 334 | R | PWB | AF, AG | 1991 | 01 | 1992 | 12 | [59] |
| Ivory Coast | Batouapleu | −8.32 | 6.79 | 243 | R | I | AF, AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Beoue | −7.87 | 6.55 | 268 | R | N | AG | 1998 | 04 | 1999 | 03 | [60] |
| Ivory Coast | Bepleu | −8.05 | 6.99 | 285 | R | I | AG | 1998 | 04 | 1999 | 03 | [60] |
| Ivory Coast | Bietouo | −8.13 | 6.90 | 283 | R | I | AF, AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Binguebougou | −5.81 | 9.53 | 357 | R | I | AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Bouake Dar es Salam | −5.04 | 7.69 | 325 | PU | N | AG | 1991 | 01 | 1992 | 12 | [61] |
| Ivory Coast | Bouake Kennedy | −5.01 | 7.69 | 351 | PU | N | AG | 1991 | 01 | 1992 | 12 | [61] |
| Ivory Coast | Bouake Sokoura | −5.01 | 7.90 | 361 | PU | N | AG | 1991 | 01 | 1992 | 12 | [61] |
| Ivory Coast | Bouake Tolakouadiokro | −5.02 | 7.68 | 331 | PU | I | AG | 1991 | 01 | 1992 | 12 | [61] |
| Ivory Coast | Bouake Zone | −5.02 | 7.71 | 367 | PU | I | AG | 1991 | 01 | 1992 | 12 | [61] |
| Ivory Coast | Bouenneu | −8.23 | 6.93 | 251 | R | I | AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Danta | −8.16 | 7.02 | 272 | R | N | AG | 1998 | 04 | 1999 | 03 | [60] |
| Ivory Coast | Douandrou | −7.92 | 6.54 | 237 | R | N | AG | 1998 | 04 | 1999 | 03 | [60] |
| Ivory Coast | Douedy-Guezon | −7.75 | 6.57 | 266 | R | N | AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Fapaha | −5.83 | 9.49 | 361 | R | I | AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Finneu | −8.15 | 6.99 | 274 | R | I | AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Folofonkaha | −5.21 | 8.58 | 328 | R | N | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Ganse | 3.9 | 8.617 | 392 | R | N | AF, AGSS, AN | 2000 | 07 | 2002 | 06 | [62] |
| Ivory Coast | Gbahouakaha | −5.41 | 9.50 | 345 | R | I | AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Gbontegleu | −8.24 | 6.97 | 257 | R | I | AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Glopaoudy | −7.63 | 6.55 | 234 | R | N | AG | 1998 | 04 | 1999 | 03 | [60] |
| Ivory Coast | Kabolo | −4.99 | 8.19 | 268 | R | N | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Kafine | −5.67 | 9.27 | 322 | R | N | AG | 1995 | 01 | 1995 | 12 | [63] |
| Ivory Coast | Kaforo | −5.67 | 9.29 | 329 | R | N | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Kombolokoura | −5.88 | 9.33 | 366 | R | N | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Meantouo | −8.14 | 6.89 | 277 | R | I | AG | 1998 | 04 | 1999 | 03 | [60] |
| Ivory Coast | Nanbekaha | −5.69 | 9.29 | 320 | R | I | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Nombolo | −5.83 | 9.41 | 379 | R | I | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Nongotchenekaha | −5.40 | 9.52 | 332 | R | I | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Ounandiekaha | −5.17 | 8.36 | 286 | R | PWB | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Pepleu | −8.20 | 6.95 | 256 | R | I | AF, AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Petionara | −5.12 | 8.43 | 277 | R | N | AF, AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Pohan | −7.93 | 6.54 | 249 | R | N | AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Seileu | −8.17 | 7.10 | 337 | R | N | AG | 1998 | 04 | 1999 | 03 | [60] |
| Ivory Coast | Tai | −7.12 | 5.75 | 218 | R | N | AF, AG | 1995 | 07 | 1996 | 06 | [64] |
| Ivory Coast | Tiemelekro | −4.617 | 6.5 | 91 | R | N | AF, AG | 2002 | 01 | 2003 | 12 | [65] |
| Ivory Coast | Tioroniaradougou | −5.70 | 9.36 | 361 | R | N | AG | 1996 | 12 | 1997 | 11 | [60] |
| Ivory Coast | Vetouo | −8.12 | 6.96 | 280 | R | I | AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Yotta | −8.19 | 7.15 | 340 | R | I | AF, AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Zaïpobly and Gahably | -7.0 | 5.5 | 180 | R | N | AF, AG | 1995 | 07 | 1997 | 06 | [64] |
| Ivory Coast | Zatta | −5.39 | 6.88 | 188 | R | I | AF | 2002 | 01 | 2003 | 12 | [65] |
| Ivory Coast | Zeale | −8.16 | 6.99 | 265 | R | I | AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Ziglo | −7.80 | 6.57 | 256 | R | N | AF, AG | 1998 | 04 | 2000 | 03 | [60] |
| Ivory Coast | Zoleu | −8.31 | 6.81 | 236 | R | I | AG | 1998 | 04 | 2000 | 03 | [60] |
| Kenya | Ahero | 34.92 | −0.18 | 1152 | PU | I | AF, AG | 1989 | 08 | 1990 | 07 | [66] |
| Kenya | Asembo | 34.40 | −0.18 | 1148 | PU | N | AF AG | 1988 | 03 | 1989 | 02 | [67] |
| Kenya | Kameichiri | 37.62 | −0.82 | 1188 | PU | N | AA | 2004 | 04 | 2005 | 03 | [68] |
| Kenya | Kilifi | 39.85 | −3.62 | 18 | PU | N | AG | 1990 | 12 | 1991 | 11 | [69] |
| Kenya | Kisian | 34.67 | −0.07 | 1246 | PU | PWB | AF, AG | 1985 | 10 | 1988 | 09 | [70] |
| Kenya | Loboi | 35.98 | −0.47 | 2285 | PU | PWB | AF, AG | 1994 | 01 | 1994 | 12 | [71] |
| Kenya | Mbuinjeru | 37.62 | −0.82 | 1141 | PU | I | AA | 2004 | 04 | 2005 | 03 | [68] |
| Kenya | Mumias | 34.49 | 0.34 | 1311 | PU | N | AF, AG | 1995 | 05 | 1996 | 04 | [72] |
| Kenya | Murinduko | 37.45 | −0.57 | 1311 | PU | N | AA | 2004 | 04 | 2005 | 03 | [68] |
| Kenya | Nyanza | 34.76 | −0.09 | 1170 | U | PWB | AF, AG | 1972 | 08 | 1973 | 07 | [73] |
| Kenya | Perkerra | 35.98 | −0.47 | 2285 | R | I | AG | 1994 | 01 | 1994 | 12 | [71] |
| Kenya | Saradidi | 34.24 | −0.02 | 1221 | R | PWB | AF, AG | 1985 | 10 | 1988 | 09 | [70] |
| Kenya | Sokoke | 39.88 | −3.33 | 125 | R | N | AG | 1990 | 12 | 1991 | 11 | [69] |
| Madagascar | Manarintsoa | 47.42 | −19.00 | 1290 | R | I | AF, AG | 1988 | 10 | 1989 | 09 | [74] |
| Madagascar | Saharevo | 48.10 | −18.82 | 873 | R | I | AA, AF, AG, AMA | 2003 | 10 | 2004 | 09 | [75] |
| Madagascar | St Marie Ambodifotatra | 49.88 | −17.00 | 3 | R | PWB | AF, AG | 1988 | 11 | 1990 | 10 | [74] |
| Mali | Ndebougou Sector | −5.96 | 14.327 | 280 | R | N | AGSL | 1999 | 04 | 2000 | 03 | [76] |
| Mali | Molodo Sector | −6.03 | 14.257 | 280 | R | N | AGSL | 1999 | 04 | 2000 | 03 | [76] |
| Mali | Sotuba | −7.91 | 12.66 | 323 | R | N | AG | 1998 | 01 | 1998 | 12 | [77] |
| Mozambique | CdSLCMPC | 32.57 | −25.92 | 35 | PU | N | AA, AF | 1985 | 01 | 1985 | 12 | [78] |
| Mozambique | Manhica | 32.48 | −25.44 | 20 | R | PWB | AF, AG | 2001 | 10 | 2002 | 09 | [79] |
| Nigeria | Apapa | 3.37 | 6.46 | 5 | U | PWB | AGSS, AME | 1945 | 06 | 1946 | 05 | [80] |
| Nigeria | Ajura | 8.94 | 12.48 | 390 | R | N | AF, AG | 1970 | 11 | 1973 | 10 | [81] |
| Nigeria | Jaya | 9.18 | 12.38 | 380 | R | N | AF, AG | 1970 | 11 | 1972 | 10 | [81] |
| Nigeria | Matsari | 9.08 | 12.38 | 382 | R | N | AF, AG | 1970 | 11 | 1972 | 10 | [81] |
| Nigeria | Nasakar | 9.12 | 12.47 | 379 | R | N | AF, AG | 1970 | 11 | 1972 | 10 | [81] |
| Nigeria | Rafin Marke | 9.03 | 12.48 | 382 | R | N | AF, AG | 1970 | 11 | 1972 | 10 | [81] |
| Nigeria | Sungungun | 8.97 | 12.32 | 389 | R | N | AF, AG | 1970 | 11 | 1972 | 10 | [81] |
| Nigeria | Ungua Gaiya Kuwaru | 8.89 | 12.36 | 395 | R | N | AF, AG | 1970 | 11 | 1973 | 10 | [81] |
| Nigeria | Unguwar Bako | 8.99 | 12.55 | 388 | R | N | AF, AG | 1970 | 11 | 1972 | 10 | [81] |
| Senegal | Aere Lao | −14.32 | 16.4 | 13 | R | N | AA | 1982 | 05 | 1983 | 04 | [82] |
| Senegal | Affiniam Diagobel Tendimane | −16.24 | 14.28 | 12 | R | N | AG | 1985 | 01 | 1986 | 12 | [83] |
| Senegal | Barkedji | −14.88 | 15.28 | 349 | R | N | AA, AG | 1994 | 06 | 1996 | 05 | [84] |
| Senegal | Boke Dialllobe | −14 | 16.07 | 28 | R | N | AG | 1982 | 05 | 1983 | 04 | [82] |
| Senegal | Dielmo | −16.42 | 13.73 | 32 | R | PWB | AF, AG, AA | 1990 | 04 | 1995 | 03 | [85] |
| Senegal | Diohine | −16.50 | 14.50 | 8 | R | PWB | AG | 1995 | 01 | 1995 | 12 | [86] |
| Country | Site | Lon | Lat | Elevation | Pd | Hydrology | Vector Species | SY | SM | EY | EM | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Senegal | Diomandou Dieri | −14.43 | 16.52 | 10 | R | I | AG | 1990 | 06 | 1992 | 05 | [87] |
| Senegal | Diomandou Walo | −14.43 | 16.52 | 10 | R | I | AG | 1990 | 06 | 1992 | 05 | [87] |
| Senegal | Kotiokh | −16.53 | 14.48 | 7 | R | PWB | AG | 1995 | 01 | 1995 | 12 | [86] |
| Senegal | Ndiop | −16.36 | 15.95 | 6 | R | N | AG, AA | 1993 | 01 | 1996 | 12 | [85] |
| Senegal | Ngayokheme | −16.43 | 14.53 | 11 | R | N | AG | 1995 | 01 | 1995 | 12 | [86] |
| Senegal | Pikine | −17.40 | 14.75 | 10 | U | PWB | AA | 1979 | 10 | 1981 | 01 | [88] |
| Senegal | Takeme and Ousseuk | −16.24 | 14.28 | 21 | R | N | AG | 1985 | 01 | 1986 | 12 | [83] |
| Senegal | Toulde Galle | −14.48 | 16.53 | 11 | R | N | AG | 1990 | 06 | 1992 | 05 | [87] |
| Senegal | Wassadou | −14.33 | 12.75 | 26 | R | PWB | AG | 1992 | 09 | 1993 | 08 | [89] |
| Sierra Leone | Bayama | −11.67 | 8.00 | 102 | R | PWB | AG | 1990 | 11 | 1991 | 10 | [90] |
| Sierra Leone | Mendewa | −11.48 | 8.17 | 325 | R | N | AG | 1990 | 01 | 1990 | 12 | [91] |
| Sierra Leone | Nyandeyama | −11.62 | 8.12 | 118 | R | N | AG | 1990 | 01 | 1990 | 12 | [91] |
| Tanzania | Bagamoyo | 38.26 | −5.04 | 1093 | R | N | AF, AG | 1995 | 10 | 1996 | 09 | [91] |
| Tanzania | Balangai | 38.28 | −4.56 | 1230 | R | N | AF, AG | 1995 | 10 | 1996 | 09 | [91] |
| Tanzania | Chasimba | 38.82 | −6.58 | 36 | R | N | AF, AG | 1992 | 01 | 1992 | 12 | [92] |
| Tanzania | Chekereni | 37.36 | −3.38 | 763 | PU | I | AA | 1994 | 07 | 1995 | 06 | [93] |
| Tanzania | Idete | 36.42 | −8.66 | 295 | R | PWB | AF, AG | 1992 | 07 | 1994 | 06 | [94] |
| Tanzania | Kerege | 39.05 | −6.59 | 36 | R | PWB | AF, AG | 1992 | 01 | 1992 | 12 | [92] |
| Tanzania | Kisangasangeni | 37.39 | −3.39 | 759 | PU | N | AA | 1994 | 07 | 1995 | 06 | [93] |
| Tanzania | Kongo | 38.83 | −6.53 | 19 | R | PWB | AF, AG | 1992 | 01 | 1992 | 12 | [92] |
| Tanzania | Kwameta | 38.29 | −5.08 | 671 | R | N | AF, AG | 1995 | 10 | 1996 | 09 | [95] |
| Tanzania | Kwamhanya | 38.28 | −5.04 | 596 | R | N | AF, AG | 1995 | 10 | 1996 | 09 | [95] |
| Tanzania | Magundi | 38.28 | −5.04 | 671 | R | N | AF, AG | 1995 | 10 | 1996 | 09 | [95] |
| Tanzania | Mapinga | 39.07 | −6.60 | 59 | R | N | AF, AG | 1992 | 01 | 1992 | 12 | [92] |
| Tanzania | Matimbwa | 38.87 | −6.50 | 21 | R | PWB | AF, AG | 1992 | 01 | 1992 | 12 | [92] |
| Tanzania | Michenga | 36.63 | −8.12 | 258 | R | PWB | AG | 1990 | 01 | 1990 | 12 | [96] |
| Tanzania | Milungui | 38.23 | −4.45 | 1636 | R | N | AF, AG | 1995 | 10 | 1996 | 09 | [95] |
| Tanzania | Mvuleni | 37.33 | −3.39 | 786 | PU | N | AA | 1994 | 07 | 1995 | 06 | [93] |
| Tanzania | Namawala | 36.40 | −8.15 | 289 | R | I | AF, AG | 1990 | 08 | 1991 | 07 | [97] |
| Tanzania | Pemba Island | 39.75 | −5.18 | 36 | PU | N | AGSL | 1958 | 01 | 1958 | 12 | [98] |
| Tanzania | Yombo | 38.85 | −6.59 | 36 | R | N | AF, AG | 1992 | 01 | 1992 | 12 | [97] |
| Tanzania | Zinga | 38.99 | −6.52 | 22 | R | N | AF, AG | 1992 | 01 | 1992 | 12 | [97] |
| Uganda | Apac-Olami | 32.56 | 1.89 | 1053 | R | N | AF, AG | 2001 | 06 | 2002 | 05 | [99] |
| Uganda | Arua-Cilio | 31.02 | 3.11 | 976 | PU | N | AF, AG | 2001 | 06 | 2002 | 05 | [99] |
| Uganda | Jinja School | 33.21 | 0.43 | 1166 | U | N | AF, AG | 2001 | 06 | 2002 | 05 | [99] |
| Uganda | Kabale villages | 29.98 | −1.22 | 1888 | PU | N | AG | 1997 | 10 | 1998 | 09 | [100] |
| Uganda | Kanungu Kihihi | 29.70 | 0.59 | 758 | R | N | AF, AG | 2001 | 06 | 2002 | 05 | [99] |
| Uganda | Kyenjojo Kasiina | 30.65 | 0.62 | 1361 | R | N | AF, AG | 2001 | 06 | 2002 | 05 | [99] |
| Uganda | Tororo-Namwaya | 34.18 | 0.68 | 1143 | PU | N | AF, AG | 2001 | 06 | 2002 | 05 | [99] |
| Zambia | Chidakwa | 26.791 | −16.393 | 1000 | R | N | AA | 2005 | 11 | 2006 | 10 | [101] |
| Zambia | Lupata | 26.791 | −16.393 | 1000 | R | N | AA | 2005 | 11 | 2006 | 10 | [101] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamba, E.I.; Tompkins, A.M.; Fink, A.H.; Ermert, V.; Amelie, M.D.; Amekudzi, L.K.; Briët, O.J.T. Monthly Entomological Inoculation Rate Data for Studying the Seasonality of Malaria Transmission in Africa. Data 2020, 5, 31. https://doi.org/10.3390/data5020031
Yamba EI, Tompkins AM, Fink AH, Ermert V, Amelie MD, Amekudzi LK, Briët OJT. Monthly Entomological Inoculation Rate Data for Studying the Seasonality of Malaria Transmission in Africa. Data. 2020; 5(2):31. https://doi.org/10.3390/data5020031
Chicago/Turabian StyleYamba, Edmund I., Adrian M. Tompkins, Andreas H. Fink, Volker Ermert, Mbouna D. Amelie, Leonard K. Amekudzi, and Olivier J. T. Briët. 2020. "Monthly Entomological Inoculation Rate Data for Studying the Seasonality of Malaria Transmission in Africa" Data 5, no. 2: 31. https://doi.org/10.3390/data5020031
APA StyleYamba, E. I., Tompkins, A. M., Fink, A. H., Ermert, V., Amelie, M. D., Amekudzi, L. K., & Briët, O. J. T. (2020). Monthly Entomological Inoculation Rate Data for Studying the Seasonality of Malaria Transmission in Africa. Data, 5(2), 31. https://doi.org/10.3390/data5020031





