Abstract
Here we present data on distinct stimuli as elicitors of substrate-borne vibrations performed by groups of termites belonging to the species Constrictotermes cyphergaster (Blattodea: Isoptera: Termitidae: Nasutitermitinae). The study consisted of assays where termite workers and soldiers were exposed to different airborne stimuli and the vibrations thereby elicited were captured by an accelerometer attached under the floor of the arena in which the termites were confined. A video camera was also used as a visual complement. The data provided here contribute to fill a gap currently existing in published datasets on termite communication.
DataSet: 10.5281/zenodo.2790686.
DataSet License: CC-BY 4.0
1. Summary
Vibration signaling is widespread in insects [1]. Such a communication channel can be used in a range of situations, e.g., to detect predators [2], prey [3], mates [4], and to recruit nestmates [5]. Furthermore, vibration can also be used as an important component to establish communication with another individual or a group of individuals.
Termites are social insects that are well known to use vibrations to convey information to their nestmates or to gather contextual information about the environment. For instance, by combining substrate-borne vibrations with chemical scents, termites communicate alarm [6]. They are also able to use the resonant frequency of a block of wood to assess its size, thereby choosing one food item over another [7]. Termites are also sensitive to heterospecific vibration, using that information for their own benefit: as recently demonstrated [8], termites can escape danger by eavesdropping the vibrational cues emitted by predatory ants. In spite of such an importance, vibratory communication in termites is relatively underexplored, and published datasets on the subject are definitely very rare.
To cover such a gap, here we present a large dataset (c.a. 13 million lines) containing the intensity of the substrate-borne vibration of termite groups confined in arenas specially designed to amplify such vibrations (see [9]). Vibrations were measured using an accelerometer and were triggered by airborne stimuli.
2. Data Descriptor
Here we provide the raw data describing the intensity of substrate-borne vibrations produced by groups of termites confined in arenas bearing a flexible floor. Such arenas (Figure 1; so-called “tympanic” in allusion to their flexible floor) were designed to amplify the feeble vibrations produced by the termites and are fully described and tested by Nunes et al. [9]. Vibrations were measured by an accelerometer whose sensor was attached to the outer surface of the arenas’ floor.
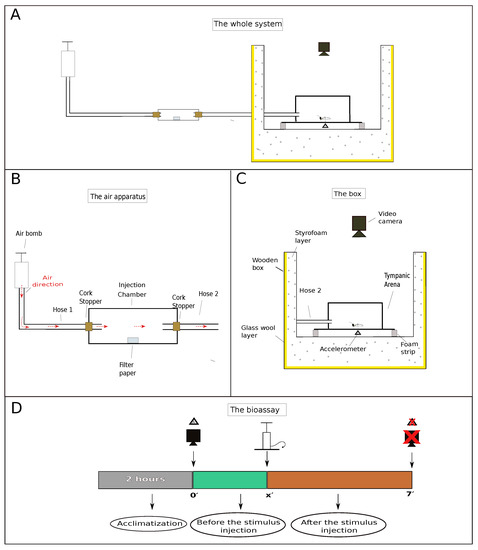
Figure 1.
Schematic depiction of the setup designed to measure termite vibrational responses when subjected to different stimuli. (A) Global view of the setup, showing an air pump connected by hoses through a injection chamber and to a tympanic arena housed in a wooden box. (B) Detailed view of the air pumping system, showing the injection chamber used to house the sources of stimuli. (C) Detailed view of the wooden box lined with styrofoam and glass wool to minimize external noise disturbance. (D) The chronology of each assay: (i) each termite group was allowed at least 2 h of acclimatization before the beginning of the assays; (ii) video and accelerometer recordings start about 2 min before the stimulus injection; (iii) after injecting the stimulus, recordings proceed for another 5 min, totaling 7 min of readings. “x” is the exactly time at which the stimulus was injected, which is given in Table 1.
Each termite group was composed of 12 workers and 3 soldiers from a given nest. Vibrations were triggered by subjecting these termites to distinct airborne stimuli gently pumped into the arena after termites were allowed to acclimatize for 2 h.
Readings thereby produced are presented here in a series of comma-separated values (.csv) files. Each file corresponds to a full assay on a single termite group from a single nest and with a single stimulus. Each line in the the file presents the readings of termite vibrations captured by the accelerometer at the “x” (horizontal), “y” (horizontal), and “z” (vertical) axes (Figure 2) at a given time. The accelerometer was configured at high gain. Readings are expressed in “counts” units. There are 13,108 counts per g (the acceleration due to gravity) in high gain mode. Each set of 512 lines corresponds to 1 s (more details are given in Section 3.5.
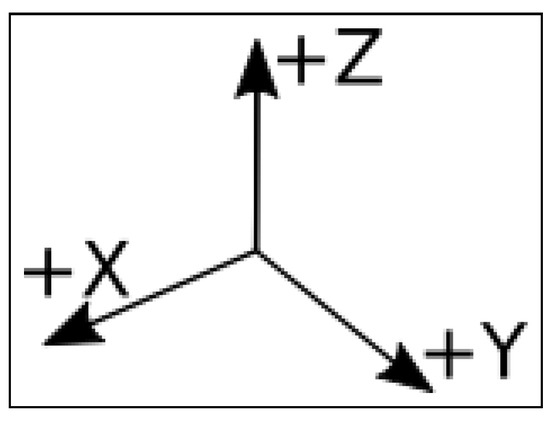
Figure 2.
The orientation of the X, Y, and Z axes from which vibrations were captured by the accelerometer.
The columns in the datafiles are:
| time: | the moment, from the beginning of the recording session, when vibration was recorded |
| Ax: | counts read by the accelerometer at the horizontal x axis |
| Ay: | counts read by the accelerometer at the horizontal y axis |
| Az: | counts read by the accelerometer at the vertical z axis |
| NestID: | the field identification of the nest from which the termite group was collected. Codes within NestID column are built as nnncccyyyy, where nnn = the nest sequential number used as a field label; ccc = the initials of the collector (name and surname); yyyy = the year in which the nest was taken from the field to the lab. |
| TypeStimulus: | the type of stimulus that was deposited within the injection chamber to be carried by the air injected into the arena to trigger termite vibrations. Codes within TypeStimulus are: air = only air; air_paper = air plus a piece of filter paper was deposited into the injection chamber and the air therein was injected into the arena; air_paper_hexane = a piece of filter paper onto which hexane was applied was deposited into the injection chamber and the air therein was injected into the arena; air_paper_extract = a piece of filter paper onto which a hexane extract from soldiers’ heads was applied was deposited into the injection chamber and the air therein was injected into the arena. |
| TermiteGroup: | the identification of the nest from which the termite group was collected and the stimulus they were exposed to. Codes within the TermiteGroup column are built as gnns, where: g = “group”; nn = the nest sequential number at which the termite group was collected; s = the stimulus that the termite group was exposed to. |
3. Methods
3.1. Ethical Statement
The current study is in compliance with the relevant regulations of Brazil, including collection and transportation permits from The Brazilian Institute for the Environment and Renewable Natural Resources (IBAMA), and permission from The Brazilian Enterprise for Agricultural Research (EMBRAPA, CNPMS) to conduct the study on their site. O. DeSouza holds a permanent collecting and transporting permit (# 10014-1) from IBAMA. Tacit approval from the Brazilian Government is implied by the authors being hired as scientific researchers. The species collected for the present study are neither endangered nor protected, and thus no specific permits were required for laboratory experiments. No genetic information was accessed.
3.2. Termite Material
Assays were conducted using termites from 15 wild colonies of C. cyphergaster (Silvestri, 1901) collected in April 2017 in the Brazilian “cerrado”, near the town of Sete Lagoas (2719 S, 1444 W; altitude 800–900 m above sea level), Minas Gerais State, Southeastern Brazil. The colonies were transported to Viçosa (Minas Gerais, Brazil), where they were kept in laboratory in room-level conditions of humidity, temperature, and light. As food, the bark of trumpet trees (Tabebuia aurea, Bignoniaceae) was offered ad libitum to all nests. Water was also offered ad libitum through a piece of cotton attached to the opening of a test tube full of water.
3.3. Experimental Procedures
The assays were conducted from April to mid-July 2017. They were designed to measure the vibrational reaction exhibited by workers and soldiers of C. cyphergaster when they are subjected to different stimuli.
To perform the assays, we designed an experimental setup to minimize noise and vibrations from human traffic and other activities in nearby labs (Figure 1A). This setup consisted of a wooden box lined with (approx.) a 5-cm thick layer of glass wool plus an 8-cm thick styrofoam layer.
In order to amplify the feeble vibrations exhibited by the termites, we used an arena bearing a flexible floor (so as to mimic a tympanum) as described and tested by Nunes et al. [9]. The tympanic arena was placed inside the wooden box described above, over a pair of egg crate foam strips lying on a hollowed, cubic styrofoam structure (Figure 1C). Groups of 15 termite individuals (12 workers + 3 soldiers) were taken from their colonies and placed inside the arena (one group at a time). At least 2 h were allowed for termites to acclimatize before the beginning of the assays. The number of termites and caste ratio of the groups were chosen according to natural proportions found in field nests (4.5 workers: 1 soldier) [10] and within the range of densities known to improve interindividual interactions and survival [11].
Stimuli were offered to termites by gently pumping air through a hose to an injection chamber and from there through another hose to the arena (Figure 1A). This injection chamber (internal space: ⌀ 18 mm × 80 mm long) was used to house any source of stimulus in addition to air (Figure 1B). Both ends of the injection chamber were sealed with cork stoppers. A small hole was made in the cork stoppers to connect the ends of the two hoses (⌀ = 4 mm). The other end of the first hose (Figure 1B, hose 1; ≈1190 mm long) was coupled to an air pump and the end of the other hose (Figure 1B, hose 2; ≈650 mm long), which flowed directly into the tympanic arena. A light touch was given to the air pump lever, which descended by weight and gravity, blowing the stimulus from the injection chamber to the arena where the individuals were confined (Figure 1C). A total of c.a. 230 cm of air was injected into the system by the air pump.
The stimuli consisted of injecting into the arena:
- the air present in the injection chamber;
- the air present in the injection chamber after it had contact with a piece of filter paper (7 × 2 cm) previously deposited therein;
- the air present in the injection chamber after it had contact with a known amount of hexane that was loaded onto a piece of filter paper deposited in the injection chamber;
- the air present in the injection chamber after it had contact with hexane extracts of termite soldier heads. These extracts were loaded onto a piece of filter paper deposited in the injection chamber.
Each assay was recorded on video and registered by the accelerometer for a total of 7 min divided into “time before the stimulus injection” and “time after the stimulus injection” (Figure 1D). This was done to differentiate the activity of the groups before the stimulus injection from the activity after the stimulus injection, in order to obtain only the real effect of each stimulus on the termites’ behavior.
Due to operational reasons, both the time of the stimulus injection and the volume of stimulus applied onto the filter paper varied among assays. These are specified in Table 1. Each nest provided four termite groups to be independently assayed with a given stimulus. Each group was assayed only once.

Table 1.
Overview of stimuli at which termites were exposed to. Nest—the identification of the nest from which the termite group was collected. The nest identity code followed the sequence “nnncccyyyy”, where nnn = nest sequential number used as field label; ccc = initials of collector (name and surname); yyyy = year in which the nest was taken from the field to the lab. Stimulus—the kind of stimulus applied to each termite group, where “A” is air; “AP” is air + filter paper; “APH” is air + paper + hexane, and “APE” is air + paper + extract. Injection time—the time (s) at which the stimulus was injected. Aliquot applied—the proportional volume of hexane or extract applied at each assay. Head equivalence—how many heads of soldiers the aliquot corresponds to. Missing values are indicated by a dash.
3.4. Extract Preparation
The soldiers of philogenetic advanced termites species have a secretory epithelium located inside a large sac in their head known as a frontal gland. This gland is responsible for the production of a blend of chemicals that is related to alarm situations [12,13]. In C. cyphergaster, such chemicals are composed of (1S)--pinene, myrcene, and (E)--ocimene [6], which are known to be soluble in hexane. To provide assayed termites with this type of stimulus, we prepared hexane extracts from soldiers’ heads, following Cristaldo et al., 2015 [6], as described below and depicted in Figure 3.
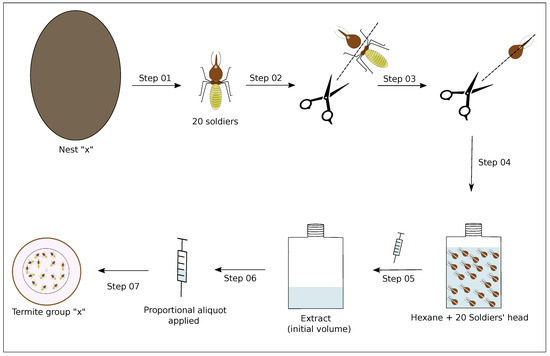
Figure 3.
Overview of steps used to prepare the soldiers’ head extracts. Step 01: 20 termites soldiers were taken from their respective colonies; Step 02: the soldiers were anesthetized on ice and dissected into the head and rest of body; Step 03: the soldiers’ heads were cut from the base of the neck to the nasus; Step 04: the heads were placed into hexane (10 L per head) for 24 h at ≈2.4 C; Step 05: the resulting extract was separated from the termite heads with the help of a microsyringe; Step 06: a known aliquot of this extract was collected from its initial volume, the aliquot being proportional to the amount of soldier heads we wanted to apply; Step 07: the aliquot was offered to a given termite group in a given assay. Assays are detailed in Table 1.
A total of 20 soldiers were taken from their respective nest and anesthetized on ice to have their heads severed and cut so as to expose the frontal gland. The heads were then immersed in hexane (10 L per head) and left for 24 h in a freezer at ≈2.4 C, after which the resulting extract was separated from the termite heads with the help of a microsyringe and again stored in the freezer at ≈2.4 C until used in the assays. From each termite nest, we prepared only one extract.
Because termite heads vary in the amount of hexane they absorb, each termite group from a given nest produced a distinct initial volume of extract. For each nest, a given aliquot of extract would thus represent a particular amount of termite heads. Due to this, we kept track of the precise volume of extract offered to termites in a given assay so that we could know how many “head equivalents” this volume represented. For comparability, this same volume was used in the assays using only hexane. The volumes used in each assay, as well as their head equivalences, are listed in Table 1.
3.5. Behavioral Response and Parameters Measured
Substrate-borne vibrations produced by the assayed termites were recorded using a USB accelerometer (Gulf Coast Data Concepts, LLCTM model X2-2 logger) equipped with a Kionix KXRB5-2050TM sensor at 2.5 volts, which results in a sensitivity factor of 500 mv/g. These electrical stimuli are recorded by the accelerometer independently in three axes (x, y, and z, Figure 2) as “counts”. The number of counts is recorded 512 times in each second. Therefore, each line in the files produced by the accelerometer (henceforth referred to as a “reading”) contains the number of counts read in s. These files form the basis on which we have built the datafile here presented (Section 2). To convert these counts into g (gravity acceleration), it suffices to divide the number of counts by 13,108 because this is a correcting factor corresponding to the high gain mode in which we operated the accelerometer (Section 2). To facilitate the assay, the sensor was removed from the accelerometer’s case while keeping it connected to the recording unit by electric wires. In doing so, we could attach this sensor directly to the external bottom surface of the arenas. This setup allowed us to record a series of 215,040 readings (512 readings × 60 s × 7 min) per assayed termite group, totaling about 12,840,000 readings = (215,040 readings × 4 stimuli × 15 assays) − (2 missing assays × 215,040 readings) for the whole experiment. As a visual complement, we also recorded the termite group’s activity in each assay using a SONY HDR-CX405TM digital video camera set to record 30 frames per second at Full HD (1920 × 1080 60p). The camera positioning and the chronology of this footage are explained in detail in Figure 1.
Author Contributions
“Conceptualization”: L.F.N., O.D. and P.F.C.; “Data curation”: D.M.R., L.F.N. and O.D.; “Funding acquisition”: L.F.N. and O.D.; “Investigation”: L.F.N. and O.D.; “Methodology”: L.F.N., O.D., D.M.R., L.B.F. and P.S.S.; “Project administration”: L.F.N.; “Resources”: L.F.N. and O.D.; “Supervision”: L.F.N. and O.D.; “Validation”: L.F.N., O.D. and P.F.C.; “Visualization”: L.F.N., O.D., D.M.R., L.B.F. and P.S.S.; “Writing—original draft”: L.F.N. and O.D.; “Writing—review & editing”: L.F.N., O.D., D.M.R., L.B.F. and P.S.S.
Funding
This work was supported by the Brazilian Council for Research (CNPq), Minas Gerais State Agency for Research Support (FAPEMIG), and Coordination for the Improvement of Higher Education Personnel (CAPES). ODS holds a CNPq fellowship (# 307990/2017-6).
Acknowledgments
Many thanks to Octavio Miramontes (Institute of Physics—UNAM, Mexico) for providing the accelerometer and to Alex Koone (Gulf Coast Data Concepts engineer) who made the necessary modifications to the accelerometer for the study. We also thank Sidney G. Alves (Department of Physics and Mathematics at UFSJ) who came up with the idea of using an embroidery hoop as a tympanum. Our thanks also go to Ivan Cruz and Fernando Valicente from EMPRAPA-CNPMS for logistics support. This work was carried out using free software, especially, but not restricted to, LATEX + kyle, Ubuntu + Debian, Inkscape, Libreoffice, JabRef, Chrome, and custom-bib, as well as the free searching site Google Scholar, to which we are profoundly thankful. This is contribution no. 78 from the Termitology Laboratory at UFV, Brazil (http://www.isoptera.ufv.br) and derives from LFN’s MSc thesis defended at the UFV Graduate Program in Entomology (http://www.pos.entomologia.ufv.br).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hill, P.S. Vibration and animal communication: A review. Am. Zool. 2001, 41, 1135–1142. [Google Scholar] [CrossRef]
- Castellanos, I.; Barbosa, P. Evaluation of predation risk by a caterpillar using substrate-borne vibrations. Anim. Behav. 2006, 72, 461–469. [Google Scholar] [CrossRef]
- Fertin, A.; Casas, J. Orientation towards prey in antlions: Efficient use of wave propagation in sand. J. Exp. Biol. 2007, 210, 3337–3343. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Birch, M.C.; Wyatt, T.D. Mate location in the deathwatch beetle, Xestobium Rufovillosum De Geer (Anobiidae): Orientat. Substrate Vib. Anim. Behav. 1994, 47, 899–907. [Google Scholar] [CrossRef][Green Version]
- Schneider, S.S.; Stamps, J.A.; Gary, N.E. The vibration dance of the honey bee. I. Communication regulating foraging on two time scales. Anim. Behav. 1986, 34, 377–385. [Google Scholar] [CrossRef]
- Cristaldo, P.F.; Jandák, V.; Kutalová, K.; Rodrigues, V.B.; Brothánek, M.; Jiříček, O.; DeSouza, O.; Šobotník, J. The nature of alarm communication in Constrictotermes cyphergaster (Blattodea: Termitoidea: Termitidae): The integration of chemical and vibroacoustic signals. Biol. Open 2015, 4, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.A.; Lai, J.C.; Toledano, E.; McDowall, L.; Rakotonarivo, S.; Lenz, M. Termites assess wood size by using vibration signals. Proc. Natl. Acad. Sci. USA 2005, 102, 3732–3737. [Google Scholar] [CrossRef] [PubMed]
- Oberst, S.; Bann, G.; Lai, J.; Evans, T.A. Cryptic termites avoid predatory ants by eavesdropping on vibrational cues from their footsteps. Ecol. Lett. 2017, 20, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.F.; Roxinol, J.A.; Cristaldo, P.F.; Marinho, R.; DeSouza, O. The use of tympanic arena as an alternative for behavioral vibroacoustic essays in termites (Blattodea: Isoptera). Sociobiology 2018, 65, 101–107. [Google Scholar] [CrossRef]
- Cunha, H.F.D.; Andrade Costa, D.; Espirito Santo Filho, K.D.; Silva, L.O.; Brandão, D. Relationship between Constrictotermes Cyphergaster Inquiline Termit. Cerrado (Isoptera: Termitidae). Sociobiology 2003, 42, 761–770. [Google Scholar]
- DeSouza, O.; Miramontes, O.; Santos, C.; Bernardo, D. Social facilitation affecting tolerance to poisoning in termites (Insecta, Isoptera). Insectes Sociaux 2001, 48, 21–24. [Google Scholar] [CrossRef]
- Šobotník, J.; Jirošová, A.; Hanus, R. Chemical warfare in termites. J. Insect Physiol. 2010, 56, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Costa-Leonardo, A.M.; Haifig, I. Pheromones and exocrine glands in Isoptera. Vitam. Horm. 2010, 83, 521–549. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).