Abstract
This study aimed to assess the metal content of tannic extracts obtained from grapevine canes, to evaluate their suitability as wood shavings for direct infusion during the aging process of alcoholic beverages or vinegars. Traditional barrel aging is a slow and costly process that can be enhanced through direct infusion of wood chips. Our investigation focused on the pruning materials of two widely cultivated Lambrusco cultivars in the Modena (Italy) area, Ancellotta and Salamino. The grapevine chips underwent preliminary heat treatments at temperatures ranging from 120 °C to 260 °C. Tannic extracts were obtained by ethanol maceration at 80 °C for 2 h. The metal composition was determined using inductively coupled plasma optical emission spectroscopy. Generally, the metal content increased with the roasting temperature of the chips. Two exceptions were noted in the Salamino extracts, where the concentrations of Bi and Ni decreased as the roasting temperature increased. The levels of heavy metal, such as Pb and Cd, were low, posing no toxicity concerns for using infused grapevine chips during the beverage aging process. The Ni concentration slightly exceeded the limits imposed by certain regulations. Its content is likely derived from the type of soil in which the plant has grown.
1. Introduction
The circular economy is recognized as a promising solution to address the pressing challenges posed by the current environmental crisis, as highlighted in the Agenda 2030 for Sustainable Development [,,,]. By embracing the principles of “reduce, reuse, and recycle”, the circular economy strives to establish a closed-loop system that minimizes waste and optimizes resource utilization [,]. These approaches not only reduce reliance on virgin natural resources but also enhance product efficiency and contribute to better quality of life and environmental health.
In this context, the valorization of agricultural residues plays a crucial role in waste reduction and sustainability promotion [,,,,,,]. Agricultural residues are significant contributors to waste streams, and their improper disposal can have adverse environmental impacts [,]. However, by finding innovative ways to use these materials, a more sustainable and circular system can be established []. Apart from waste reduction, the valorization of agricultural residues also offers economic benefits, creating new value streams and job opportunities, and stimulating economic growth [,,]. Furthermore, these residues are often inexpensive and abundantly available [,].
The viticulture industry, primarily known for wine production, generates a substantial amount of organic residue as waste every year [,]. According to the Food and Agriculture Organization of the United Nations (FAO) [], in 2021, the global production of grapes amounted to approximately 74 million tons, resulting in significant residue generation, including grapevine canes. Vine pruning, an essential annual agricultural practice aimed at managing plant growth, optimizing fruit yield, and maintaining vine health, involves systematic removal of a significant portion of vine canes. This process ensures that the vines remain productive and balanced. The disposal of large quantities of these organic residues represents a challenge for the viticulture industry []. They are mainly subject to burning, burying, or abandoning in the field, practices that can have detrimental environmental effects and contribute to greenhouse gas emissions and soil degradation []. Therefore, alternative solutions that promote the valorization of these residues are urgently required [].
Italy is widely recognized as one of the world’s leading producers of wine and its derivatives, including alcoholic distillates, balsamic vinegar, balsamic sauces, and other condiments []. The country boasts a cultivated vineyard area of approximately 671,000 ha [], yielding an annual biomass production from pruning that exceeds one million tons. Consequently, the disposal of substantial amounts of biomass poses significant challenges. For Italy, this sector is particularly important, as products, such as Lambrusco wine and Modena balsamic vinegar, have gained recognition worldwide and occupy important positions in the market [,]. Traditionally, the aging process of alcoholic beverages and vinegars involves the use of oak barrels to enhance their sensory properties, such as color, flavor, and aroma [,,]. However, this approach can be both time-consuming and expensive, and certain products require several years of aging to achieve optimal results. Furthermore, the use of oak barrels can have adverse environmental effects, including deforestation and the associated carbon footprint resulting from transportation. In recent years, there has been growing interest in alternative methods to improve the aging process of alcoholic beverages and vinegars, with the aim of reducing time and cost while preserving or even enhancing the sensory qualities of the final product []. Two prominent methods include (i) direct infusion of wood chips during the aging process or (ii) addition of tannic extracts derived from wood [,,]. Notably, these alternative aging methods using wood chips have shown promising results, enabling producers to achieve desirable sensory profiles within a short timeframe. Additionally, the implementation of these techniques may have potential environmental benefits, as they minimize reliance on oak barrels and the associated environmental impacts.
The environmental benefits can be further enhanced by utilizing vine pruning as chips, thereby contributing positively to their disposal and providing them with a new purpose. Additionally, incorporating these prunings into the aging process can also improve the final products (i.e., aged beverages). Tannic extracts derived from vine wood contain significant amounts of antioxidants, such as resveratrol and its derivatives, which can enhance the nutritional value of aged alcoholic beverages [,,]. Typically, these extracts are obtained by macerative extraction in ethanol. Various classes of phenolic compounds can be obtained from vine shoots by varying grape variety, solvent, contact temperature, and solid particle size. Most importantly, the composition of the extracted fraction varies depending on the wood toasting temperature. While scientific evidence regarding the content of resveratrol and stilbenoids in vine pruning is available, limited information is available regarding the metal content of their tannic extracts. These data are crucial for assessing safety in terms of heavy metal content, which can be detrimental to human health, and the potential presence of essential mineral elements, such as K, Ca, Mg, and Fe. The safety and quality of the final product can be ensured by exploring the metal content of the tannic extracts derived from vine pruning. Furthermore, understanding the mineral composition of these extracts allows for comprehensive evaluation of their potential nutritional benefits in terms of essential mineral elements.
This study presents a novel investigation of the potential of vine pruning chips as a mineral source for the aging of alcoholic beverages and vinegars, focusing on two distinctive Lambrusco cultivars, Salamino and Ancellotta, which are prevalent in the Modena region of Italy. All grapevine cane samples were collected following the grape harvest in autumn 2022. We evaluated the effectiveness of different roasting temperatures ranging from 120 to 240 °C, for the pre-treatment of vine pruning chips by considering the extraction yield and mineral content at different temperatures. Understanding the suitability of vine pruning chips as a mineral source is of great significance for the beverage industry, as it offers an alternative and sustainable approach to traditional methods. The utilization of vine pruning chips not only presents an opportunity for resource optimization but also aligns with the growing demand for environmentally friendly winemaking practices.
2. Materials and Methods
2.1. Reagents and Standards
Merck ICP standards (As, B, Ba, S) and multi-standards containing 22 elements (Ag, Al, Bi, Ca, Cd, Co, Cr, Cu, Fe, Ga, In, K, Mg, Mn, Na, Ni, P, Pb, Se, Si, Sr, Zn), at different concentrations (10–1000 mg/L) were used to prepare the reference solutions. All mineral acids and oxidants (HNO3 and H2O2) were of the highest purity (Suprapure, Merck; Darmstadt, Germany). Ethanol (96%) were obtained from Carlo Erba Reagents (Milano Italy). Deionized water is produced by a Milli-Q Plus (Millipore system, Merck KGaA, Darmstadt, Germany).
2.2. Samples Preparation
The woody stems of Vitis vinifera cv. Ancellotta (Anc) and Salamino (Sal) were obtained from a farm located in the Modena (Italy) district.
The cultivation conditions, including soil, climate, and water, were consistent for both the cultivars. In order to minimize potential contamination from pesticides and herbicides, the analysis focused solely on the internal parts of the grapevine canes. Pesticides and herbicides tend to accumulate on the outer bark; therefore, it was carefully removed from the samples. The sampling was conducted in September 2022, following the annual grape harvest.
All samples were manually dehulled and minced into 4–5 mm chips. They were then placed in glass vials and subjected to thermal treatment for 2 h at different temperatures (120, 140, 160, 180, 200, 220, 240, 260 °C) in an inert atmosphere (N2). Subsequently, the samples were manually ground into a powder and extracted using ethanol through maceration.
2.3. Proximate Composition
Moisture, ash, elemental, and crude protein contents were assessed following the methods recommended by the Association of Official Analytical Chemists. Moisture content was determined by drying the samples at 105 °C to a constant weight. The ash content was determined using a laboratory furnace at 550 °C and the temperature was gradually increased. Each measurement was performed on four replicates and the results were averaged.
2.4. Macerative Solvent Extraction
Two grams (±1%) of ground grapevine shoots were placed in an extraction vessel and covered with 96% v/v ethanol (10 mL/g of powdered material). The vessel was sealed with a PBT screw cap. In order to optimize the extraction yield, we employed a two-step process consisting of 1 h of sonication at 35 °C, followed by 24 h of maceration in an oven at 80 °C. The extracts were then filtered, and the solid residues were washed three times with 10 mL of ethanol. The extracts were subsequently dried using a rotary evaporator and further dried in an oven at 105 °C for 2 h prior to the analysis. The resulting products exhibited pitchy consistency and were stored at 4 °C until analysis.
2.5. ICP-OES Analysis
The dried extracts were subjected to microwave acid digestion to facilitate the complete dissolution (wet method). The element content was measured using inductively coupled plasma optical emission spectroscopy (ICP-OES), following established procedures documented in previous studies [,,,]. A Perkin-Elmer ICP-OES (model Optima 4200 DV) instrument equipped with an ultrasonic nebulizer (Cetac Technologies Inc.; Omaha, NE, USA) and a charge coupled device (CCD) area detector were used to determine the total element content. All analyses were performed on four replicates. The results are expressed as mean ± standard deviation of four replicates. For further comparison, the total metal content in the Anc and Sal grapevine wood samples, including the outer bark, was determined. After incinerating the pruning samples, the resulting ashes were treated with 2 mL of high-purity HNO3 for solubilization in 50 mL flasks (dry method). The clear solutions were then analyzed using an ICP-OES instrument, following the same standardized procedures applied to all the samples.
2.6. Statistical Analysis
The experimental data were compared by conducting an analysis of variance (one-way ANOVA) with Tukey–Kramer honestly significant difference (HSD) post hoc testing, by using the Matlab® 2023a environment (Mathworks Inc., Natick, MA, USA). The level of significance was determined at p < 0.05 to see whether there were statistical differences between the mean values.
3. Results and Discussion
Table 1 presents the workplan of the sampling and analysis procedures applied to the grapevine cane samples (Anc and Sal) sampled in 2022. This workplan outlines the systematic approach followed to collect and analyze the samples and provide a clear understanding of the procedures adopted in this study.

Table 1.
Workplan of the sampling and analysis procedures applied to the vine cane samples (Anc and Sal) collected in 2022.
We emphasize that the pesticide and contaminant contents were minimized by removing the outer bark of vine pruning samples. Moreover, in Europe, the use of pesticides in grape cultivation is regulated by Regulation (EC) No. 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placement of plant protection products [,]. This regulation mandates adherence to safety intervals between the last pesticide application and the fruit harvest. Therefore, we can assume that the collection of vine canes after grape harvest ensures greater phytosanitary safety and assumes the absence or presence of trace amounts of pesticides.
3.1. Proximate Composition of Grapevine Canes
Figure 1 shows the grapevine cane chips roasted at 180 °C (a), 220 °C (b), and 260 °C (c).

Figure 1.
Grapevine cane chips roasted at 180 °C (a), 220 °C (b), and 260 °C (c).
Grapevine canes, which are regularly removed during pruning, account for approximately 90–95% of vineyard waste. Typically, they reach their peak productive maturity within the first year of growth. However, if left on the parent plant for extended periods, their productivity decreases, necessitating their removal during the annual winter pruning cycle. Grapevine canes are composed of soft and sufficiently elastic wood and primarily consist of cellulose (40–42%), hemicellulose (24–26%), lignin (18–21%), extractable components (8–10%), and ash (3–4%) []. Table 2 shows the proximal chemical composition of the grapevine cane samples analyzed in this study. All values are expressed as the mean ± standard deviation of four replicates.

Table 2.
Proximate composition of grapevine cane samples of the selected Lambrusco cultivars, Ancellotta and Salamino.
The last column of Table 2 presents some of the literature data regarding the elemental and proximate composition of grapevine pruning samples from 14 cultivars of Vitis vinifera, collected in a specific district in the La Rioja region of Spain []. For each parameter, the range of minimum–maximum values is presented, and the mean value derived from the entire dataset is indicated in parentheses. In terms of elemental composition, the Lambrusco cultivars generally exhibited values similar to those reported by Mendívil et al. [], with the exception of nitrogen content, which consistently fell below the minimum value of the Spanish cultivars. Furthermore, there were significant differences in the moisture content between the Lambrusco cultivar samples and the Spanish cultivars. A more effective comparison can be made by examining the mass loss values obtained through forced drying at 120 °C, which eliminated all the water from the wood. However, even in this case, the differences remained substantial, as the Ancellotta and Salamino cultivars were approximately 48% and 25% lower than the Spanish average (51.7%), respectively. Regarding the peculiar behavior observed in our samples, no additional evidence has been collected thus far to explain this experimental trend.
3.2. Effects of Roasting on Sample Mass Loss
Table 3 shows the mass loss (Δm%) values obtained from the roasting of grapevine cane samples of two Lambrusco cultivars, Ancellotta and Salamino, at different temperatures. Values are expressed as the mean ± standard deviation of four replicates.

Table 3.
Mass variation of roasted grapevine shoots at different temperatures for the two selected Lambursco cultivars, Ancellotta and Salamino.
Table 3 highlights the significant differences in the behavior of wood from the two cultivars after roasting under an inert atmosphere (N2). The Salamino cultivar exhibited an average mass loss that was approximately 10% higher than that of the Ancellotta variety. This suggests that the wood of the Ancellotta vine is denser and more thermoresistant than that of the Salamino vine. Upon moisture removal at 105 °C, along with the highly volatile organic compounds (VOCs) responsible for the characteristic woody aroma, noticeable mass loss was observed up to 120 °C. At this temperature, the process of eliminating residual water present in the wood matrix, including tightly bound fractions and those retained by mineral components, such as crystallization water, was completed. Along with water traces, VOCs continued to be lost, and the color of the wood did not significantly change, while the samples became increasingly compact and rigid. Notably, wood toasted at 120 °C, despite having a pleasant scent, did not emit specific aromas. As the roasting temperature was increased further, there was a gradual increase in mass loss due to the elimination of semi-volatile organic compounds (SVOCs) until it reached approximately 180 °C. The effects of heating on wood mass became significant at approximately 160 °C, resulting in progressive stiffening and the loss of characteristic elasticity. The aromatic profile of the toasted wood varied progressively with contact temperature as the composition of the resulting gas phase changed [].
The observed changes in aroma profiles resulting from roasting at different temperatures have significant implications for flavor development in aged beverages, including spirits and vinegars. Wood is well-known for its contribution to complex aromatic compounds, particularly phenolic compounds, during the beverage aging process. A comprehensive understanding of the temperature-dependent release of volatile compounds from roasted wood can greatly assist the enological and beverage industry in selecting the desired flavor characteristics for their products. Furthermore, a deeper understanding of the thermal properties of different vine cultivars could provide valuable guidance for selecting suitable wood sources for beverage aging. By optimizing the roasting temperature and duration, it is possible to finely tailor the sensory characteristics of aged beverages and introduce unique flavor profiles derived from specific wood sources. Analyzing the results presented in Table 3, it is evident that a minimum temperature of 160 °C is required to initiate a substantial release of VOCs responsible for imparting characteristic aromas. Notably, the Salamino cultivar exhibited significant mass loss, even at lower temperatures, and demonstrated higher VOC emissions.
Roasting plays a fundamental role in wood transformation. Specifically, it can cause the degradation of thermolabile bioactive molecules, while simultaneously generating compounds harmful to human health, such as furan compounds. Increasing the roasting temperature promotes significant degradation and facilitates the release of furans from grapevine canes [,]. The Food and Drug Administration (FDA) [] and European Food Safety Authority (EFSA) [] have established guidelines and regulations to monitor the presence of furanic compounds in food products to ensure their safety. Therefore, it is important to select a roasting temperature that allows for the release and improvement of the aromatic profile without exceeding the limits imposed by food safety regulations. In the context of enology, vinegar, and alcoholic beverages, the use of tannic extracts generally involves significantly low concentrations that remain within the established limits for furanic compounds. Therefore, from a health perspective, the use of tannic extracts in winemaking and related products is not considered a significant risk factor []. Regarding our vine pruning samples, we believe that the temperatures of 240 and 260 °C are excessively high, as they result in a significant modification of the wood composition. This leads to a strong release of VOCs and poses a greater health risk along with an unpleasant aromatic profile (Table 3).
3.3. EtOH Extraction Yield
Table 4 presents the data related to the yields of solvent extraction in EtOH at 80 °C for all the grapevine shoot samples. All values are expressed as the mean ± standard deviation. Statistical analysis indicated a significant difference in the extraction yield as the roasting temperature of the grapevine cane samples varied (p < 0.05).

Table 4.
Extraction yield (EtOH) relative to the grapevine cane samples of the two Lambrusco cultivars toasted at different temperatures.
The scientific literature reports the identification of numerous highly bioactive compounds in grapevine canes and their tannic extracts. These include caffeic, gallic acid, p-coumaric acids [], catechin, epicatechin [], luteolin, trans-resveratrol, trans-ε-viniferin, piceatannol, ampelopsin A, and miyabenol C [,], among many others. However, these molecules are heat-labile, and their concentrations typically decrease with increasing roasting temperature. For instance, there was a decrease in the content of the main stilbenoids by 55–60% when passing from a roasting temperature of 180 °C to 240 °C. This explains the trend observed in the extraction yield with increasing roasting temperature. In particular, a significant decrease was observed when the temperature was increased from 220 to 240 °C. These findings align with the results presented in Table 3; Table 4, where greater changes in color, aromatic profile, and mass loss were observed starting from a temperature of 240 °C. These changes indicate strong degradation of the woody matrix and its constituent components, including the loss of valuable stilbenoids. Understanding the impact of roasting temperature on extraction yield is crucial for industries and researchers interested in utilizing grapevine canes for their bioactive properties. It allows for the optimization of roasting conditions to preserve and maximize the retention of stilbenoids, thereby enhancing the potential health benefits and sensory characteristics of the derived products. By carefully selecting the appropriate roasting temperature, it is possible to strike a balance between aromatic development and stilbenes preservation in the processed grapevine pruning.
During the ethanol extraction process, not only organic compounds but also minerals and metals can be extracted from grapevine canes. Extraction of inorganic components occurs because of the solubility of certain mineral salts in ethanol, allowing them to be carried along during the extraction process. The presence of minerals and metals in tannic extracts is of great importance from both the food safety and nutritional perspectives. Heavy metals, such as lead, cadmium, mercury, and arsenic, are of particular concern because of their potential toxicity and adverse health effects when consumed at high concentrations. Therefore, it is crucial to evaluate their concentration to ensure compliance with food safety regulations and to safeguard consumer health. However, extraction of macroelements, including potassium, magnesium, phosphorus, and iron, can provide added nutritional value to tannic extracts. These macroelements play essential roles in various physiological processes in the human body, such as maintaining proper muscle function, supporting bone health, and participating in energy metabolism.
3.4. ICP-OES Analysis of Debarked Grapevine Cane Samples
ICP-OES analysis allowed for the measurement of the metal content in the analyzed extracts. The experimental results are presented in Table 5 (Ancellotta) and Table 6 (Salamino). Samples derived from chips roasted at 260 °C were not included in the analysis due to their excessively roasted attributes. This decision was based on the properties of the extract, which exhibited unpleasant and overly tarry aromatic profiles. All results are reported based on 100 g of dried extract. Statistical analysis showed significant differences in the mineral content of the ethanolic extracts when the roasting temperature of the grapevine cane samples was varied (p < 0.05).

Table 5.
Concentration of some elements (mg/100 g on a dry matter basis) * in the EtOH extracts of grapevine shoots from Ancellotta cultivar, roasted at different temperatures from 120 to 240 °C.

Table 6.
Concentration of some elements (mg/100 g on a dry matter basis) * in the EtOH extracts of grapevine shoots from Salamino cultivar, toasted at different temperatures from 120 to 240 °C.
Significant differences were observed in the tannic extracts, primarily attributed to the effects of increasing roasting temperature. The concentration of some elements, including Al, Cd, Cr, Cu, Fe, Pb, and Zn, increases with roasting temperature, while others such as B, Ba, Co, Mn, and Sr, showed a decrease, regardless of the specific cultivar. Additionally, concentrations of elements, such as Ba, Co, Cu, Fe, Mn, Sr, and Zn, are higher in the extracts obtained from Salamino samples compared to Ancellotta samples. Conversely, elements, such as Al, B, Cd, Cr, and Pb, were found in higher concentrations in the Ancellotta cultivar. Bi and Ni exhibited opposite trends, with an increase in the Ancellotta samples and a decrease in the Salamino samples with increasing temperature. The concentration of Zn in both cultivars increased by a factor of approximately 10–20 times when passing from samples roasted at 120 °C to those roasted at 240 °C. Conversely, B displayed a decreasing trend with roasting temperature for both varieties. Notably, the Ancellotta cultivar showed a significant decrease (−51%) in B concentration in samples roasted at 240 °C compared to those roasted at 120 °C. Similar trends were observed for the Co and Mn contents. Notably, the Salamino cultivar experienced the most significant loss in Mn concentration.
Several hypotheses can be formulated to explain the decrease in the metal content of the extracts resulting from the increase in the roasting temperature of grapevine cane samples.
- i.
- These metals are generally involved in protein–enzyme systems that ensure the physiological viability of the initial plant structures. The elevated roasting temperature is likely responsible for disrupting the original metal complex–chelate system present in the woody matrix, leading to the release of poorly soluble metal oxides and a subsequent reduction in their concentrations, as detected by the ICP-OES analysis of the extracts.
- ii.
- It is important to highlight the significance of B in this context, as its concentration reduction with increasing roasting temperature may be attributed to the covalent molecular characteristics of metal oxides and other compounds, which render them highly volatile. Consequently, boron can be lost through thermal stress during roasting.
- iii.
- It is worth noting that the release of metal oxides during roasting can be influenced not only by temperature but also by the presence of other compounds and the chemical environment. For instance, the interaction between metals and polyphenolic compounds, such as tannins, can affect the stability and solubility of the metal species. These interactions can be complex and depend on factors, such as pH, oxidation-reduction potential, and the specific polyphenolic profile of grapevine cane extracts. At elevated temperatures, tannins undergo various reactions, including disruption of their molecular structures and the formation of degradation compounds. These changes can affect the ability of tannins to bind metal ions and form stable complexes. The breakdown of tannin–metal complexes may facilitate the release of metal ions and subsequent formation of less soluble metal oxides or hydroxides.
- iv.
- Additionally, the two metal elements Ni and Bi exhibited opposing trends in the two cultivars, as depicted in Figure 2.
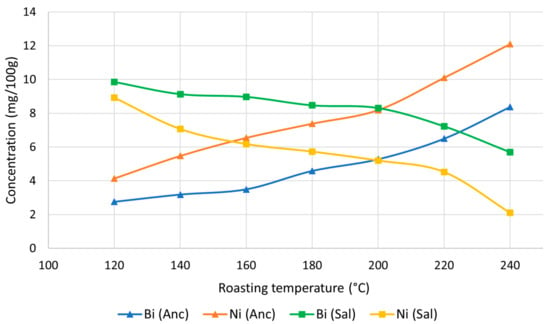 Figure 2. Bi and Ni content (mg/100 g on a dry matter basis) of the EtOH extracts obtained from Salamino and Ancellotta grapevine canes toasted at different temperatures.
Figure 2. Bi and Ni content (mg/100 g on a dry matter basis) of the EtOH extracts obtained from Salamino and Ancellotta grapevine canes toasted at different temperatures.
It is evident that these cultivars exhibit different affinities for accumulating the heavy metals Bi and Ni, as indicated by variations in their concentrations. The concentration of Bi in the extracts from Salamino chips treated at 120 °C was approximately 3.5 times higher compared of Ancellotta chips. An increase in roasting temperature reduced the solubility of Ni compounds in EtOH in the Salamino samples, whereas it increased in the Ancellotta samples. These observations can also be extended to other analytes that exhibit a similar trend with increasing roasting temperatures. The opposing trend represented by the Salamino cultivar can be explained by a reduction in the solubility of compounds containing the analytes of interest.
- v.
- The main mineral elements detected in the roasted grapevine canes were K, Mg, and Ca, which exhibited an increasing concentration with roasting temperature. These minerals are essential for maintaining the optimal health and functioning of the human body. Potassium is involved in various physiological processes, including fluid balance, nerve function, and muscle contraction [,]. Its ability to regulate blood pressure by counteracting the effects of sodium promotes cardiovascular health. In addition, potassium contributes to bone health and may reduce the risk of kidney stones. Magnesium plays a vital role in more than 300 enzymatic reactions in the body, making it vital for numerous physiological functions [,,]. It is involved in energy production, nerve function, muscle relaxation, and the synthesis of DNA and proteins. Magnesium also supports a healthy heart rhythm, promotes bone health, and helps regulate blood sugar levels. Calcium is known for its role in building and maintaining strong bones and teeth []. They are also involved in muscle function, nerve transmission, and blood clotting []. Calcium plays a crucial role in maintaining a normal heart rhythm and blood pressure. Additionally, it has been linked to a reduced risk of colorectal cancer and may help in weight management. Therefore, the high content of these minerals in ethanolic extracts allows for the enrichment of beverages and enological products aged with infused chips from a nutritional perspective. By incorporating these minerals into the aging process, the resulting beverages can offer health benefits beyond their flavor and aroma profiles. It meets the increasing demand of consumers to incorporate high nutrient levels with an adequate amount of essential minerals into their normal diet, preferably from plant sources [].
Table 7 shows a comparison between our samples toasted at 120 °C and the data from Sánchez-Gómez et al. [], which pertains to a specific grape variety in the Castilla-La Mancha region of Spain. These values correspond to the extractions conducted on grapevine chips dried at room temperature to achieve a humidity level of 6.5%. The extractions were performed using water as a solvent and various extraction techniques, including conventional liquid–solid extraction and dynamic solid–liquid extraction. It is worth noting that the solubility of metallic compounds varies between ethanol and water, partially accounting for the observed differences based on solvent characteristics.

Table 7.
Concentration of some metals in EtOH extracts (mg/100 g on a dry matter basis) * of grapevine canes: comparison with the literature data.
Regarding macroelements, the concentration of K in the aqueous extracts was approximately five times higher than that in the ethanol extracts. A similar trend was observed for P, with higher concentrations in the aqueous extracts than in the ethanolic extracts. However, the Ca and Mg contents follow the order of magnitude reported by Sánchez-Gómez et al., although our values are lower and under average than those reported in the literature [].
Among the micronutrients, the Fe and Zn values in our samples were consistent with those extracted using water as the solvent. However, the B, Cu, and Mn concentrations in the ethanolic extracts were approximately 100 times higher for B, 50 times higher for Cu, and 5 times higher for Mn than the corresponding values obtained with an aqueous solvent [].
The differences in metal concentrations observed between the water and ethanol extractions can be attributed to variations in the solubility of metals in different solvents. Some metals may have higher solubility in water, resulting in greater extraction, while others may exhibit better solubility in ethanol. For example, specific metal species may form more stable complexes with water than with ethanol, thereby impacting the extraction efficiency. Furthermore, the composition of grapevine canes can vary depending on factors, such as grape variety, geographical region, and agricultural practices.
When using grapevine chips for aging vinegars or alcoholic beverages, it is essential to monitor the concentration of heavy metals to ensure the safety of the end products. High concentrations of metals, such as lead (Pb) and cadmium (Cd), can pose health risks to humans. Therefore, it is crucial to ensure that their concentrations remain within allowable limits established by food regulations to ensure food safety and to protect consumer health. International guidelines and food regulatory bodies, such as the European Food Safety Authority (EFSA), have established maximum allowable concentrations of heavy metals in food and beverages.
For ease of discussion, we examined only the maximum allowable values of metals for wine. To assess the safety in terms of heavy metal content of vine wood chips used to accelerate the aging process of wine, it is important to consider the amount of wood used per liter of beverage. The quantity of wood chips may vary, but the general dosages for white and red wines range from 0.5 to 2 g/L and 2 to 6 g/L, respectively, depending on the chip size and contact time []. These dosage recommendations are typically applied to oak wood, which has been primarily studied and used for this purpose. Although these quantities may differ when grapevine wood is used, preliminary considerations can be made based on these general guidelines. Considering the extraction yield values in Table 4, approximately 5–9 kg of vine wood is required to obtain 100 g of dry extract. Consequently, the data in Table 5; Table 6 must be reduced by a factor of 50–90 to obtain the amount of metal in 100 g of wood. This is crucial for determining whether the concentration values obtained comply with the limits imposed by the regulations.
European Union (EU) regulations have set a limit of less than 30 mg/L for iron (Fe) in wine, whereas the International Organization of Vine and Wine (OIV) specifies a limit of less than 10 mg/L []. The concentration of Fe detected in our samples, which did not exceed 0.69 mg/100 g of EtOH extract (on a dry basis), was significantly lower than these limits. This value is even lower when considering the very low amount of wood used as chips during the beverage aging process. Iron is an essential mineral in the human body that plays an important biological role. However, excessive iron concentrations in wine may indicate a potential contamination or inadequate production processes. High levels of Fe can result from factors, such as the use of rusty winemaking equipment or unintended sources during wine processing. Therefore, the low concentration found in our samples was not surprising, as it is consistent with the natural content of this metal in grapevine pruning, as confirmed by the literature data in Table 7 [].
The EU limits for copper (Cu) and zinc (Zn) concentrations in wine are set at less than 0.5 mg/L and 30 mg/L, respectively, while the OIV specifies limits of less than 1.0 mg/L for Cu and 5 mg/L for Zn []. Phytosanitary treatments using a Bordeaux mixture or zinc (Zn) fungicides are often used in vineyards, which increase soil Cu and Zn content. The concentration of Zn in our samples, which did not exceed 4.15 mg/100 g on a dry basis, was significantly lower than the established limits. The concentration of Cu reaches a maximum value of 9.19 mg/100 g on a dry basis in Sal240 sample. Considering a quantity of 6 g of chips per liter of wine, which is the maximum value suggested by the literature, and an extraction yield of 1.66 g/100 g of dried wood (Table 4), a Cu concentration of approximately 9 μg/L of wine is obtained, well below the imposed limits. The presence of Cu and Zn in wine is a matter of concern because of their potential toxicity to humans. Both are essential trace elements that are required for various biological functions in the human body. However, excessive intake of these metals can lead to health risks. Cu and Zn toxicity can cause gastrointestinal symptoms, such as nausea, vomiting, and abdominal pain.
Regarding cadmium (Cd) and lead (Pb), the OIV sets the maximum limits for their concentration in wines at 0.01 mg/L and 0.15 mg/L, respectively. Prolonged Pb exposure can result in neurological and developmental issues, particularly in young children [,], whereas Cd ingestion is associated with kidney damage and impaired bone health [,]. The highest concentration of Cd was observed in the Anc240 sample, with a value of 0.7 mg/100 g in the EtOH extract on a dry basis. When considering the recommended amount of 6 g of chips per liter of wine and the extraction yield of 1.48 g/100 g of Anc240 (Table 4), the resulting Cd concentration in wine is approximately 0.62 μg/L, which is significantly below the limits imposed by the OIV. The same considerations can be applied to Pb. Cadmium and Pb concentration decreased with an increase in roasting temperature. This result suggests the use of a lower amount of wood chips per liter of wine and avoiding excessive roasting temperatures to control their content. Pb primarily comes from fungicide treatments and atmospheric pollution, for example, in vines located near roads with heavy traffic, and only in small quantities from the soil []. Cd probably originates mainly from atmospheric pollution and has a remarkable capacity to accumulate in the soil [].
EU law does not specify a limit for nickel (Ni) concentration in wine, whereas the OIV sets the limit at 0.01 mg/L. The concentrations in our samples remained within the limits set by the OIV, except for the Anc240 sample, which slightly exceeded the threshold. Specifically, considering the extraction yield and the Ni concentration of 12.1 mg/100 g in the EtOH extract on a dry basis, the resulting Ni concentration in wine is calculated to be 0.011 mg/L. This suggests the need to restrict the roasting temperature, particularly for the Ancellotta cultivar, to mitigate the Ni concentration in the final product. Furthermore, this concentration pertains to the maximum suggested quantity of 6 g of chips per liter of wine. Consequently, using a smaller quantity of chips would not pose any concerns regarding Ni content. The average Ni content in plants varies between 0.05 and 8.00 μg/g dry weight [,]. Ni concentrations can vary significantly in ambient air, with the highest values reported in highly industrialized areas []. It can enter ambient air through various sources, including the combustion of coal, diesel oil, and fuel oil, the incineration of waste and sewage, and other miscellaneous sources. In soil, it can exist in various forms, including inorganic crystalline minerals, complexed or adsorbed on organic cation surfaces or inorganic cation exchange surfaces, as water-soluble free ions, or chelated metal complexes in soil solutions. The concentration of Ni in soil can be influenced by factors, such as soil pH, clay content, iron-manganese minerals, and soil organic matter. Decreased soil pH, often caused by the reduced use of soil liming in agriculture or increased acid rain, can lead to increased solubility and mobility of Ni in the soil []. The uptake of Ni by plants can differ depending on the species, with some species exhibiting a higher tolerance and an ability to take up metals from the soil. It is difficult to precisely identify the reason for the Ni content in our samples, as it could be due to pollution, soil type, or genotypic factors that increase its uptake, as shown Figure 2.
3.5. ICP-OES Analysis of Grapevine Wood Samples
In this section, we present the results of ICP-OES analysis conducted on vine wood samples of Ancellotta and Salamino cultivars. Wood samples, including the outer bark, were analyzed using the dry method. To ensure the reliability and validity of our findings, we compared them with those reported in the existing literature. This allowed us to contextualize our data within the broader body of knowledge and assess the consistency of our observations with those of previous studies. By doing so, we can gain a deeper understanding of the mineral composition of grapevine wood samples and identify any notable variations or similarities.
The mineral fraction of vine shoots from 10 Turkish cultivars grown in the same experimental field was evaluated by Çetin et al. []. The study design ensured that the composition of the plant growth soil and meteorological conditions remained consistent for all samples analyzed. This control over environmental factors eliminated exogenous variability, allowing the data obtained in this study to primarily reflect the genetic specificity of individual cultivars. The analysis revealed significant concentrations of K, P, Ca, Fe, Mg, and Zn in the vine shoots. The authors proposed that vine shoot extracts have potential nutraceutical applications in food supplements.
In another study, researchers examined the composition of vine shoots from Spanish cultivars in the La Rioja region []. They selected eight red-berried cultivars and six white-berried varieties for analysis. In our study, we considered all 14 cultivars without distinguishing the grape color.
Sanchez-Gómez et al. [] focused on the Aires cultivar, the most commonly cultivated variety in Spain, primarily in the Castilla-La Mancha region. They found that the major elements, apart from C, H, and O, included N, S, Al, K, and Ca. Based on their findings, they suggested that grapevine pruning could serve as an excellent fertilizer, as these minerals are essential nutrients for plants.
For our Ancellotta and Salamino shoot samples, we determined the total metal content in the entire vine wood, including the rind, using the dry method. The experimental results are presented in Table 8. For comparison purposes, the last two columns of the table present the literature data, showing the dispersion range (min–max values) and the average value indicated within brackets.

Table 8.
Concentration of some metals in wood (mg/100 g on a dry matter basis) * of grapevine canes: comparison with the literature data.
It is worth noting that despite the unknown compositional characteristics of the soils, similar orders of magnitude were observed for each element, irrespective of the geographical origin of the cultivars (Italy, Turkey, and Spain). For example, the Ca and P contents in the woods of Ancellotta and Salamino fell approximately in the middle of the average values reported for both Turkish and Spanish cultivars, although they were closer to the lower limits (Ca: 450 mg/100 g []; P: 42 mg/100 g []). The data for K were closely aligned with the minimum values reported in the literature.
Magnesium (Mg) deserves special consideration. Table 8 clearly demonstrates the significant differences in Mg content among the three groups of pruning woods. The group comprising the two Italian vines (~32 mg/100 g on dry basis) fell in between the average value of Turkish cultivars (~4.7 mg/100 g on dry basis) and the maximum value of Spanish grape varieties (~159 mg/100 g on dry basis). Likely, both genotypic and external factors play a role, including the soil type, in which the vines are cultivated, and meteorological conditions. Currently, we do not have further explanation for this experimental observation.
Regarding the minor analytes, it is important to highlight that Al, B, and Cu are more prevalent in Lambrusco woods than in Spanish woods, with multiplication factors ranging approximately 5–6 times. Conversely, the Ni content was at least 50 times higher. Other trace elements, such as Fe, Cr, Mn, and Zn, aligned well with the concentration values reported by Mendivil et al. [].
These trace elements, including Cu and other analytes, play a crucial role in vital plant processes, contributing to the formation of metal enzymes, organometallic complexes, and chelates based on tannic compounds, polyphenols, catechins, and nucleic acids. These compounds have significant implications for redox processes and act as catalysts for electronic transport []. The presence of certain heavy metals in vine shoots has also been identified in other studies [,,]. In addition to studying the composition of woody matrices, these authors extended their investigations to analyze the metal content in wines treated with chips obtained from pruning. The results of these studies are particularly promising, supporting and encouraging the use of vine pruning chips and tannic extracts in winemaking practices as alternatives to traditional tannins derived from oak and chestnut wood, among others.
4. Conclusions
The concentrations of various metals were determined in samples of roasted grapevine canes from the Lambrusco cultivars Ancellotta and Salamino, with the aim of assessing the potential and safety of using these materials as chips for infusion to accelerate the aging process of alcoholic beverages and vinegars.
The main metals detected were K, Ca, and Mg. Their concentrations increased as roasting temperature increased, regardless of the cultivar. This finding is encouraging because these mineral elements play various beneficial roles in the human body and can enhance the nutritional value of aged beverages.
The levels of metals, such as iron, copper, zinc, lead, and cadmium were found to be below the regulatory limits, considering the typical quantity of chips used for beverage aging. The concentrations of these metals increased as roasting temperature increased. This suggests that caution should be exercised when minimizing potential contamination. To mitigate this risk, it is advisable to select a roasting temperature that is not excessively high (160–180 °C). By carefully controlling the roasting process and using moderate temperatures, the potential for elevated metal concentrations can be reduced, ensuring the safety and quality of the final product.
Some attention has been paid to the Ni content. The origin of this metal can be manifold; however, the level of pollution and the type of soil in which the plant has grown play a fundamental role. Genotypic factors also affect the uptake of Ni by grapevines. In particular, we observed that the Ni content, as for Bi, in tannic extracts from EtOH vs. roasting temperature followed a countertrend for the two selected Lambrusco cultivars, increasing in Ancellotta and decreasing in Salamino.
Based on the experimental evidence, we can conclude that the processes of release from the woody matrix to the wine were of minimal significance, as no toxic elements were detected, and no health risks were identified for wines and derived enological products aged with infused chips
Author Contributions
Conceptualization, V.D. and L.M.; methodology, V.D. and A.M.; software, V.D. and F.G.; validation, L.T., A.M., and V.D.; formal analysis, V.D. and L.M.; investigation, V.D., F.G., and L.M.; resources, A.M. and L.T.; data curation, V.D. and F.G.; writing—original draft preparation, V.D. and L.T.; writing—review and editing, V.D. and L.M.; visualization, V.D. and F.G.; supervision, A.M.; project administration, A.M. and L.T.; funding acquisition, L.T. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khajuria, A.; Atienza, V.A.; Chavanich, S.; Henning, W.; Islam, I.; Kral, U.; Liu, M.; Liu, X.; Murthy, I.K.; Oyedotun, T.D.T.; et al. Accelerating Circular Economy Solutions to Achieve the 2030 Agenda for Sustainable Development Goals. Circ. Econ. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Rodriguez-Anton, J.M.; Rubio-Andrada, L.; Celemín-Pedroche, M.S.; Alonso-Almeida, M.D.M. Analysis of the Relations between Circular Economy and Sustainable Development Goals. Int. J. Sustain. Dev. World Ecol. 2019, 26, 708–720. [Google Scholar] [CrossRef]
- Belmonte-Ureña, L.J.; Plaza-Úbeda, J.A.; Vazquez-Brust, D.; Yakovleva, N. Circular Economy, Degrowth and Green Growth as Pathways for Research on Sustainable Development Goals: A Global Analysis and Future Agenda. Ecol. Econ. 2021, 185, 107050. [Google Scholar] [CrossRef]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; UN: Geneva, Switzerland, 2015. [Google Scholar]
- Geisendorf, S.; Pietrulla, F. The Circular Economy and Circular Economic Concepts—A Literature Analysis and Redefinition. Thunderbird Int. Bus. Rev. 2018, 60, 771–782. [Google Scholar] [CrossRef]
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Biomass-Based Biorefineries: An Important Architype towards a Circular Economy. Fuel 2021, 288, 119622. [Google Scholar] [CrossRef]
- Chiaraluce, G. Circular Economy in the Agri-Food Sector: A Policy Overview. Ital. Rev. Agric. Econ. 2021, 76, 53–60. [Google Scholar]
- Širá, E.; Kravčáková Vozárová, I.; Kotulič, R.; Dubravská, M. EU27 Countries’ Sustainable Agricultural Development toward the 2030 Agenda: The Circular Economy and Waste Management. Agronomy 2022, 12, 2270. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Lancellotti, L.; Marchetti, A.; Pincelli, L.; Strani, L.; Tassi, L. Candying Process for Enhancing Pre-Waste Watermelon Rinds to Increase Food Sustainability. Future Foods 2022, 6, 100182. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Maletti, L.; Marchetti, A.; Roncaglia, F.; Tassi, L. Volatile Aroma Compounds of Gavina® Watermelon (Citrullus lanatus L.) Dietary Fibers to Increase Food Sustainability. AppliedChem 2023, 3, 6. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Durante, C.; Marchetti, A.; Tassi, L. VOCs Analysis of Three Different Cultivars of Watermelon (Citrullus lanatus L.) Whole Dietary Fiber. Molecules 2022, 27, 8747. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Bertacchini, L.; Marchetti, A.; Mariani, M.; Pastorelli, S.; Silvestri, M.; Tassi, L. Rosaceae Nut-Shells as Sustainable Aggregate for Potential Use in Non-Structural Lightweight Concrete. Waste 2023, 1, 33. [Google Scholar] [CrossRef]
- Bakan, B.; Bernet, N.; Bouchez, T.; Boutrou, R.; Choubert, J.-M.; Dabert, P.; Duquennoi, C.; Ferraro, V.; García-Bernet, D.; Gillot, S.; et al. Circular Economy Applied to Organic Residues and Wastewater: Research Challenges. Waste Biomass Valorizat. 2022, 13, 1267–1276. [Google Scholar] [CrossRef]
- Palmieri, N.; Suardi, A.; Alfano, V.; Pari, L. Circular Economy Model: Insights from a Case Study in South Italy. Sustainability 2020, 12, 3466. [Google Scholar] [CrossRef]
- Sehnem, S.; Vazquez-Brust, D.; Pereira, S.C.F.; Campos, L.M.S. Circular Economy: Benefits, Impacts and Overlapping. Supply Chain Manag. Int. J. 2019, 24, 784–804. [Google Scholar] [CrossRef]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and Its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of Winery Waste vs. the Costs of Not Recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- García-Galindo, D.; López, E.; Gómez, M.; Sebastián, F.; Gebresenbet, G.; Jirjis, R.; Kern, J.; Germer, S.; Pari, L.; Suardi, A.; et al. Europruning Project: Summary of Final Results. Available online: http://Www.Etaflorence.It/Proceedings/?Detail=13021 (accessed on 20 March 2023).
- Morone, P.; Koutinas, A.; Gathergood, N.; Arshadi, M.; Matharu, A. Food Waste: Challenges and Opportunities for Enhancing the Emerging Bio-Economy. J. Clean. Prod. 2019, 221, 10–16. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Malferrari, D.; Marchetti, A.; Roncaglia, F.; Tassi, L. Waste By-Product of Grape Seed Oil Production: Chemical Characterization for Use as a Food and Feed Supplement. Life 2023, 13, 326. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- FAO Faostat: FAO Statistical Databases. Available online: https://www.fao.org/faostat/en/#data (accessed on 20 February 2023).
- Miglietta, P.P.; Morrone, D.; Lamastra, L. Water Footprint and Economic Water Productivity of Italian Wines with Appellation of Origin: Managing Sustainability through an Integrated Approach. Sci. Total Environ. 2018, 633, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Christ, K.L.; Burritt, R.L. Critical Environmental Concerns in Wine Production: An Integrative Review. J. Clean. Prod. 2013, 53, 232–242. [Google Scholar] [CrossRef]
- Pari, L.; Alfano, V.; Garcia-Galindo, D.; Suardi, A.; Santangelo, E. Pruning Biomass Potential in Italy Related to Crop Characteristics, Agricultural Practices and Agro-Climatic Conditions. Energies 2018, 11, 1365. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV) World Wine Production Outlook. 2022. Available online: https://www.oiv.int/ (accessed on 30 January 2023).
- OIV. State of the World Vine and Wine Sector 2021. Available online: https://Www.Oiv.Int/Sites/Default/Files/Documents/Eng-State-of-the-World-Vine-and-Wine-Sector-April-2022-V6_0.Pdf (accessed on 10 April 2023).
- Segré, A.; White, G.B. Marketing Italian Wine in the U.S. Market a Case Study of Cantine Riunite. In Developments in Agricultural Economics; Vine and Wine Economy; Botos, E.P., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 8, pp. 245–259. [Google Scholar]
- Mura, R.; Chiriacò, M.; Vicentini, F. Economic and Environmental Outcomes of a Sustainable and Circular Approach: Case Study of an Italian Wine-Producing Firm. J. Bus. Res. 2022, 154, 113300. [Google Scholar] [CrossRef]
- Callejón, R.M.; Torija, M.J.; Mas, A.; Morales, M.L.; Troncoso, A.M. Changes of Volatile Compounds in Wine Vinegars during Their Elaboration in Barrels Made from Different Woods. Food Chem. 2010, 120, 561–571. [Google Scholar] [CrossRef]
- Herrero, P.; Sáenz-Navajas, M.P.; Avizcuri, J.M.; Culleré, L.; Balda, P.; Antón, E.C.; Ferreira, V.; Escudero, A. Study of Chardonnay and Sauvignon Blanc Wines from D.O.Ca Rioja (Spain) Aged in Different French Oak Wood Barrels: Chemical and Aroma Quality Aspects. Food Res. Int. 2016, 89, 227–236. [Google Scholar] [CrossRef]
- Lancellotti, L.; Ulrici, A.; Sighinolfi, S.; Marchetti, A. Chemical Characterization Of Commercial Balsamic Vinegar Glaze. J. Food Compos. Anal. 2020, 94, 103620. [Google Scholar] [CrossRef]
- Dumitriu, G.-D.; De Lerma, N.L.; Cotea, V.V.; Zamfir, C.-I.; Peinado, R.A. Effect of Aging Time, Dosage and Toasting Level of Oak Chips on the Color Parameters, Phenolic Compounds and Antioxidant Activity of Red Wines (Var. Fetească Neagră). Eur. Food Res. Technol. 2016, 242, 2171–2180. [Google Scholar] [CrossRef]
- Kayaoğlu, M.; Bayram, M.; Topuz, S. Effect of Oak Chips Addition on the Phenolic Composition of Grape Vinegar in Fermentation Process. J. Food Meas. Charact. 2022, 16, 3106–3116. [Google Scholar] [CrossRef]
- Vichi, S.; Santini, C.; Natali, N.; Riponi, C.; López-Tamames, E.; Buxaderas, S. Volatile and Semi-Volatile Components of Oak Wood Chips Analysed by Accelerated Solvent Extraction (ASE) Coupled to Gas Chromatography–Mass Spectrometry (GC–MS). Food Chem. 2007, 102, 1260–1269. [Google Scholar] [CrossRef]
- O’Sullivan, M. Sensory Properties of Beverage Products (Alcoholic and Nonalcoholic). In Handbook for Sensory and Consumer-Driven New Poduct Development Innovative Technologies for the Food and Beverage Industry; Woodhead Publishing: Sawston, UK, 2017; Volume 13, pp. 281–304. [Google Scholar]
- D’Eusanio, V.; Genua, F.; Marchetti, A.; Morelli, L.; Tassi, L. Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis Vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture. Molecules 2023, 28, 4074. [Google Scholar] [CrossRef]
- Gorena, T.; Saez, V.; Mardones, C.; Vergara, C.; Winterhalter, P.; von Baer, D. Influence of Post-Pruning Storage on Stilbenoid Levels in Vitis Vinifera L. Canes. Food Chem. 2014, 155, 256–263. [Google Scholar] [CrossRef]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Mérillon, J.-M.; Cluzet, S. Comparative Analyses of Stilbenoids in Canes of Major Vitis Vinifera L. Cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef]
- Sah, R.N.; Miller, R.O. Spontaneous Reaction for Acid Dissolution of Biological Tissues in Closed Vessels. Anal. Chem. 1992, 64, 230–233. [Google Scholar] [CrossRef]
- Durante, C.; Cocchi, M.; Lancellotti, L.; Maletti, L.; Marchetti, A.; Roncaglia, F.; Sighinolfi, S.; Tassi, L. Analytical Concentrations of Some Elements in Seeds and Crude Extracts from Aesculus Hippocastanum, by ICP-OES Technique. Agronomy 2021, 11, 47. [Google Scholar] [CrossRef]
- Official Journal of the European Union Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC 2009. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0001:0050:en:PDF (accessed on 13 July 2023).
- Dorosh, O.; Fernandes, V.C.; Moreira, M.M.; Delerue-Matos, C. Occurrence of Pesticides and Environmental Contaminants in Vineyards: Case Study of Portuguese Grapevine Canes. Sci. Total Environ. 2021, 791, 148395. [Google Scholar] [CrossRef]
- Rayne, S.; Karacabey, E.; Mazza, G. Grape Cane Waste as a Source of Trans-Resveratrol and Trans-Viniferin: High-Value Phytochemicals with Medicinal and Anti-Phytopathogenic Applications. Ind. Crops Prod. 2008, 27, 335–340. [Google Scholar] [CrossRef]
- Mendívil, M.A.; Muñoz, P.; Morales, M.P.; Juárez, M.C.; García-Escudero, E. Chemical Characterization of Pruned Vine Shoots from La Rioja (Spain) for Obtaining Solid Bio-Fuels. J. Renew. Sustain. Energy 2013, 5, 033113. [Google Scholar] [CrossRef]
- Courregelongue, M.; Shinkaruk, S.; Prida, A.; Darriet, P.; Pons, A. Identification and Distribution of New Impact Aldehydes in Toasted Oak Wood (Quercus Petraea). J. Agric. Food Chem. 2022, 70, 11667–11677. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M.; del Castillo, M.D. Browning Reactions. In Food Biochemistry and Food Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 56–83. ISBN 978-1-118-30803-5. [Google Scholar]
- Pereira, V.; Albuquerque, F.M.; Ferreira, A.C.; Cacho, J.; Marques, J.C. Evolution of 5-Hydroxymethylfurfural (HMF) and Furfural (F) in Fortified Wines Submitted to Overheating Conditions. Food Res. Int. 2011, 44, 71–76. [Google Scholar] [CrossRef]
- U.S. FDA (Food and Drug Administration) Exploratory Data on Furan in Food: Individual Food Products. 2008. Available online: https://www.fda.gov/food/process-contaminants-food/exploratory-data-furan-food (accessed on 21 March 2023).
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al.; EFSA Panel on Contaminants in the Food Chain (CONTAM) Risks for Public Health Related to the Presence of Furan and Methylfurans in Food. EFSA J. 2017, 15, e05005. [Google Scholar] [CrossRef] [PubMed]
- EU. Council Regulation (EC) No 479/2008 of 29 April 2008 on the Common Organisation of the Market in Wine, Amending Regulations (EC) No 1493/1999 and Following. Off. J. Eur. Union 2008, 148, 1–61. [Google Scholar]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical Composition of Grape Canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Delgado-Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Pérez-Juan, P.M.; Luque de Castro, M.D. Comparison of Accelerated Methods for the Extraction of Phenolic Compounds from Different Vine-Shoot Cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Gaillet, S.; Carillon, J.; Vidé, J.; Ramos, J.; Izard, J.-C.; Cristol, J.-P.; Rouanet, J.-M. Vineatrol and Cardiovascular Disease: Beneficial Effects of a Vine-Shoot Phenolic Extract in a Hamster Atherosclerosis Model. J. Agric. Food Chem. 2012, 60, 11029–11036. [Google Scholar] [CrossRef]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of Increased Potassium Intake on Cardiovascular Risk Factors and Disease: Systematic Review and Meta-Analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef]
- WHO. WHO Guideline: Potassium Intake for Adults and Children; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Dong, J.-Y.; Xun, P.; He, K.; Qin, L.-Q. Magnesium Intake and Risk of Type 2 Diabetes: Meta-Analysis of Prospective Cohort Studies. Diabetes Care 2011, 34, 2116–2122. [Google Scholar] [CrossRef]
- Kim, D.J.; Xun, P.; Liu, K.; Loria, C.; Yokota, K.; Jacobs, D.R., Jr.; He, K. Magnesium Intake in Relation to Systemic Inflammation, Insulin Resistance, and the Incidence of Diabetes. Diabetes Care 2010, 33, 2604–2610. [Google Scholar] [CrossRef]
- Fan, X.; Rudel, R.; Berger, M.; Hershman, M.; Seres, D.S. General Nutritional Principles. In Yamada’s Textbook of Gastroenterology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 376–407. ISBN 978-1-119-60020-6. [Google Scholar]
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium Intake and Risk of Fracture: Systematic Review. BMJ 2015, 351, h4580. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-Shoot Waste Aqueous Extracts for Re-Use in Agriculture Obtained by Different Extraction Techniques: Phenolic, Volatile, and Mineral Compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef]
- Du Toit, W.J. Micro-Oxygenation, Oak Alternatives and Added Tannins and Wine Quality. In Managing Wine Quality; Woodhead Publishing Series in Food Science Technology and Nutrition; Reynolds, A.G., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 226–254. ISBN 978-1-84569-798-3. [Google Scholar]
- Durguti, V.Y.; Aliu, S.; Laha, F.; Feka, F. Determination of Iron, Copper and Zinc in the Wine by FAAS. Emerg. Sci. J. 2020, 4, 411–417. [Google Scholar] [CrossRef]
- Malavolti, M.; Fairweather-Tait, S.J.; Malagoli, C.; Vescovi, L.; Vinceti, M.; Filippini, T. Lead Exposure in an Italian Population: Food Content, Dietary Intake and Risk Assessment. Food Res. Int. 2020, 137, 109370. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, C.; Vesey, A.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2019, 11, 2. [Google Scholar] [CrossRef]
- Volpe, M.G.; La Cara, F.; Volpe, F.; De Mattia, A.; Serino, V.; Petitto, F.; Zavalloni, C.; Limone, F.; Pellecchia, R.; De Prisco, P.P.; et al. Heavy Metal Uptake in the Enological Food Chain. Food Chem. 2009, 117, 553–560. [Google Scholar] [CrossRef]
- Krstić, B.; Stanković, D.; Igić, R.; Nikolic, N. The Potential of Different Plant Species for Nickel Accumulation. Biotechnol. Biotechnol. Equip. 2007, 21, 431–436. [Google Scholar] [CrossRef]
- Prabagar, S.; Dharmadasa, R.M.; Lintha, A.; Thuraisingam, S.; Prabagar, J. Accumulation of Heavy Metals in Grape Fruit, Leaves, Soil and Water: A Study of Influential Factors and Evaluating Ecological Risks in Jaffna, Sri Lanka. Environ. Sustain. Indic. 2021, 12, 100147. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A Review of Its Sources and Environmental Toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Fernández-Roldán, F.; Alonso, G.L.; Salinas, R.M. Classification of Vine-shoots for Use as Enological Additives. J. Sci. Food Agric. 2022, 102, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of Toasting on Non-Volatile and Volatile Vine-Shoots Low Molecular Weight Phenolic Compounds. Food Chem. 2016, 204, 499–505. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).