The Effect of Cinnamon and Ginger Spices on Anthocyanins in Sweetened Roselle Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Beverage Preparations
2.2. Real-Time Storage
2.3. Chemical Kinetics
2.4. Accelerated Storage
2.5. Analysis of Samples
2.5.1. Chemical Tests
Total Monomeric Anthocyanin
Total Phenolic Content
Antioxidant Capacity
2.5.2. Physical Tests
2.6. Statistics
3. Results and Discussions
3.1. Anthocyanins
3.1.1. Kinetics of Anthocyanins Degradation
3.1.2. Accelerated Study on Anthocyanins
3.2. Total Phenolic Content
3.3. FRAP Activity
3.4. Colour
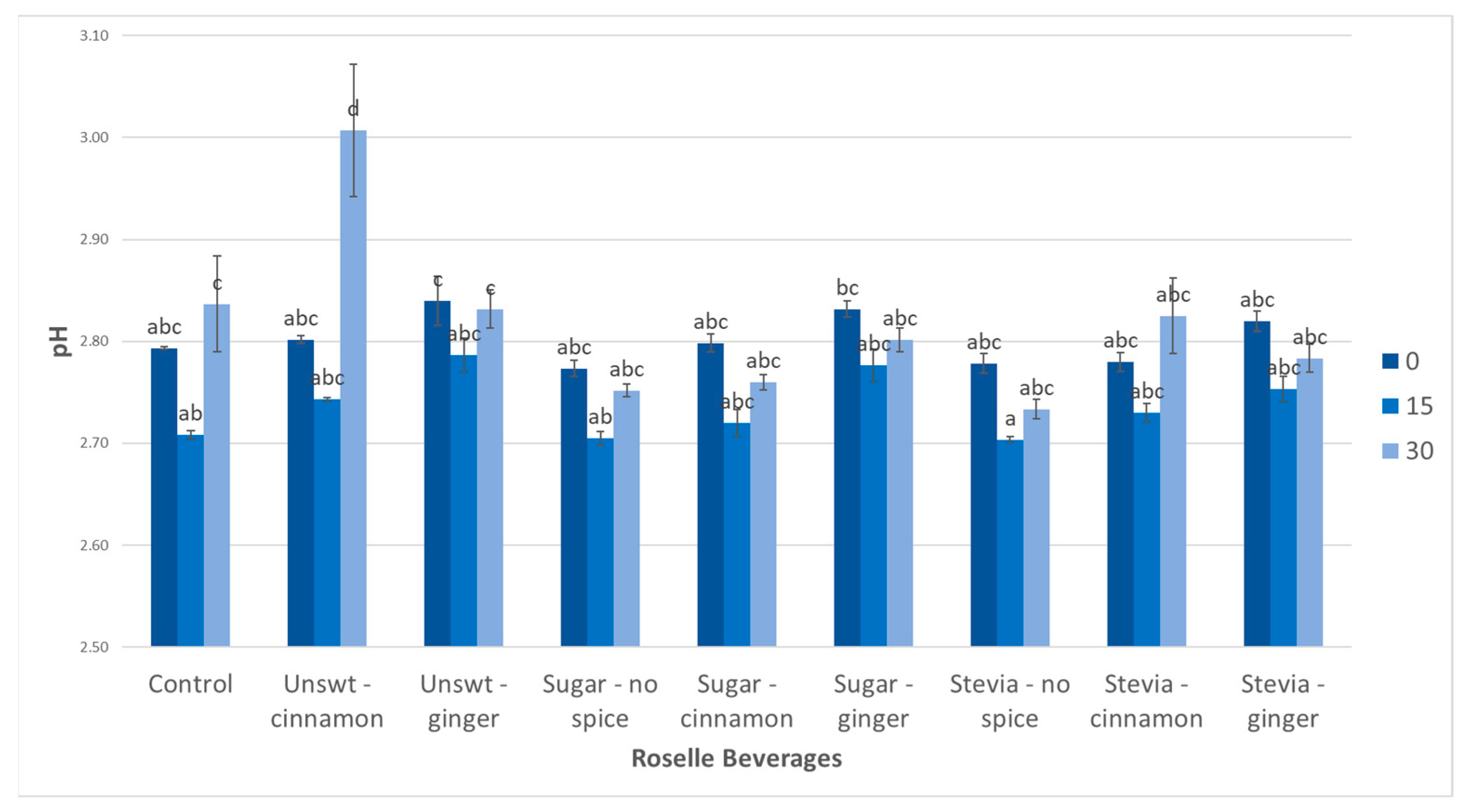
3.5. pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Source | Numerator df | Denominator df | F | Sig. |
|---|---|---|---|---|
| Intercept | 1 | 278.822 | 25,178.241 | 0.000 |
| sweetener | 2 | 278.822 | 39.277 | 0.000 |
| spice | 2 | 278.822 | 164.626 | 0.000 |
| sweetener * spice | 4 | 277.706 | 5.134 | 0.001 |
| Day | 12 | 39.903 | 2.296 | 0.025 |
| sweetener * Day | 24 | 39.903 | 1.739 | 0.060 |
| Day * spice | 24 | 39.903 | 0.529 | 0.950 |
| Source | Numerator df | Denominator df | F | Sig. |
|---|---|---|---|---|
| Intercept | 1 | 173.482 | 4535.259 | 0.000 |
| sweetener | 2 | 173.482 | 1.048 | 0.353 |
| spice | 2 | 173.482 | 37.617 | 0.000 |
| sweetener * spice | 4 | 166.017 | 13.917 | 0.000 |
| Day | 12 | 55.728 | 149.595 | 0.000 |
| sweetener * Day | 24 | 55.728 | 0.765 | 0.761 |
| Day * spice | 24 | 55.728 | 2.316 | 0.005 |
| Source | Numerator df | Denominator df | F | Sig. |
|---|---|---|---|---|
| Intercept | 1 | 226.260 | 10,547.282 | 0.000 |
| sweetener | 2 | 226.260 | 15.606 | 0.000 |
| spice | 2 | 226.260 | 61.105 | 0.000 |
| sweetener * spice | 4 | 235.015 | 2.564 | 0.039 |
| Day | 12 | 39.798 | 13.519 | 0.000 |
| sweetener * Day | 24 | 39.798 | 0.374 | 0.994 |
| Day * spice | 24 | 39.798 | 0.540 | 0.944 |
References
- Vattem, D.A.; Maitin, V. Functional Foods, Nutraceuticals and Natural Products: Concepts and Applications; DEStech Publications Inc.: Lancaster, PA, USA, 2016. [Google Scholar]
- Carvajal-Zarrabal, O.; Barradas-Dermitz, D.M.; Orta-Flores, Z.; Hayward-Jones, P.M.; Cirilo, N.-H.; Guadalupe, A.-U.M.; Anilú, M.-M. Hibiscus sabdariffa L., roselle calyx, from ethnobotany to pharmacology. J. Exp. Pharmacol. 2012, 4, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al-Wabel, N.; Blunden, G. Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: A review. Phytother. Res. 2005, 19, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Makhsudova, B.; Pakudina, Z.P.; Sadykov, A.S. Flavonols of hibiscus and a hybrid of hibiscus with the cotton plant. Chem. Nat. Compd. 1967, 3, 291–292. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Hsieh, Y.-Y.; Huang, T.-C. Effect of Sugar on Anthocyanin Degradation and Water Mobility in a Roselle Anthocyanin Model System Using 17O NMR. J. Agric. Food Chem. 2004, 52, 3097–3099. [Google Scholar] [CrossRef] [PubMed]
- Cissé, M.; Bohuon, P.; Sambe, F.; Kane, C.; Sakho, M.; Dornier, M. Aqueous extraction of anthocyanins from Hibiscus sabdariffa: Experimental kinetics and modeling. J. Food Eng. 2012, 109, 16–21. [Google Scholar] [CrossRef]
- DU, C.T.; Francis, F.J. Anthocyanins of roselle (Hibiscus sabdariffa, L.). J. Food Sci. 1973, 38, 810–812. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez De Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Barajas-Ramírez, J.A.; Gutiérrez-Salomón, A.L.; Sáyago-Ayerdi, S.G. Concentration of roselle (Hibiscus sabdariffa L) and sucrose in beverages: Effects on physicochemical characteristics and acceptance. Food Sci. Technol. Int. 2020, 27, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, M.; Piližota, V. Prevention of thermal degradation of anthocyanins in blackberry juice with addition of different sugars Prevención de degradación termal de antocianinas en zumo de mora con adición de diferentes azúcares. CyTA-J. Food 2011, 9, 237–242. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B.A.; Afkhami-Ardekani, M.; Fatehi, F.; Noori-Shadkam, M. The effects of sour tea (Hibiscus sabdariffa) on hypertension in patients with type II diabetes. J. Hum. Hypertens. 2009, 23, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B.-A.; Afkhami-Ardekani, M.; Fatehi, F. Effects of Sour Tea (Hibiscus sabdariffa) on Lipid Profile and Lipoproteins in Patients with Type II Diabetes. J. Altern. Complement. Med. 2009, 15, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S. Influence of Steviol Glycosides on the Stability of Vitamin C and Anthocyanins. J. Agric. Food Chem. 2014, 62, 11264–11269. [Google Scholar] [CrossRef] [PubMed]
- Abidoye, A.; Ojedokun, F.; Fasogbon, B.; Bamidele, O. Effects of sweet basil leaves (Ocimum basilicum L) addition on the chemical, antioxidant, and storage stability of roselle calyces (Hibiscus sabdariffa) drink. Food Chem. 2021, 371, 131170. [Google Scholar] [CrossRef] [PubMed]
- Suseno, R.; Surhaini; Rahmi, S.L.; Yanti, F. Characteristics and sensory properties of lemongrass, roselle, and ginger formulation herbal tea. IOP Conf. Ser. Earth Environ. Sci. 2022, 951, 012096. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Tohma, H.; Gülçin, İ.; Bursal, E.; Gören, A.C.; Alwasel, S.H.; Köksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several Culinary and Medicinal Herbs Are Important Sources of Dietary Antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Omoarukhe, E.D. Natural Processing for Beverages: From a Hibiscus sabdariffa (Roselle) Beverage Perspective. Ph.D. Thesis, University of Reading, Reading, UK, 2017. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Kopjar, M.; Tiban, N.N.; Pilizota, V.; Babic, J. Stability of anthocyanins, phenols and free radical scavenging activity through sugar addition during frozen storage of blackberries. J. Food Process. Preserv. 2009, 33, 1–11. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Castaño-Tostado, E.; Ramírez-de León, J.A.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015, 172, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Korir, M.; Wachira, F.; Wanyoko, J.; Ngure, R.; Khalid, R. The fortification of tea with sweeteners and milk and its effect on in vitro antioxidant potential of tea product and glutathione levels in an animal model. Food Chem. 2014, 145, 145–153. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, Y.-S. Antioxidant activity of Maillard reaction products derived from aqueous glucose/glycine, diglycine, and triglycine model systems as a function of heating time. Food Chem. 2009, 116, 227–232. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Huang, H.-P. Effect of polymerization on the antioxidant capacity of anthocyanins in Roselle. Food Res. Int. 2004, 37, 313–318. [Google Scholar] [CrossRef]
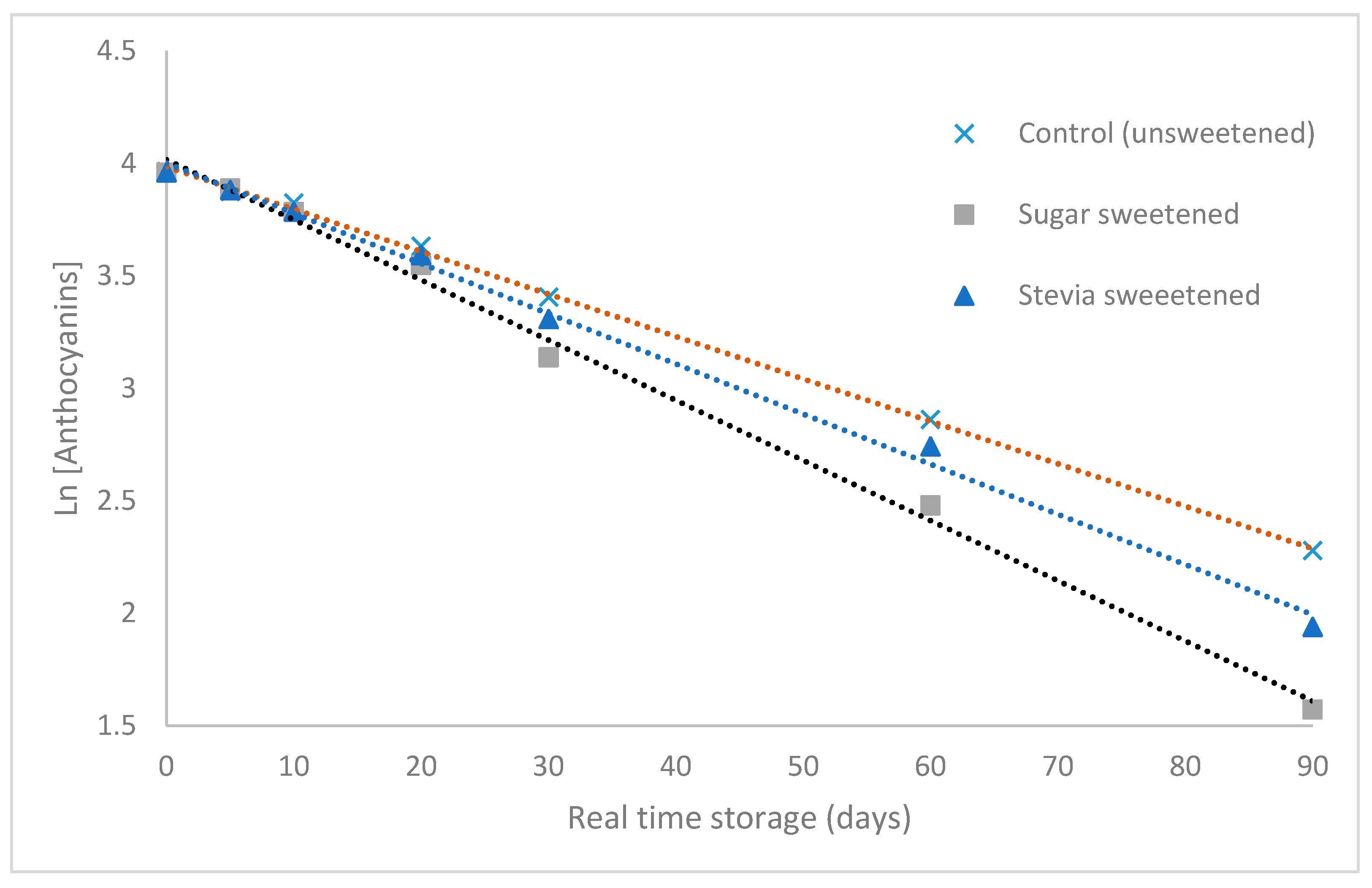
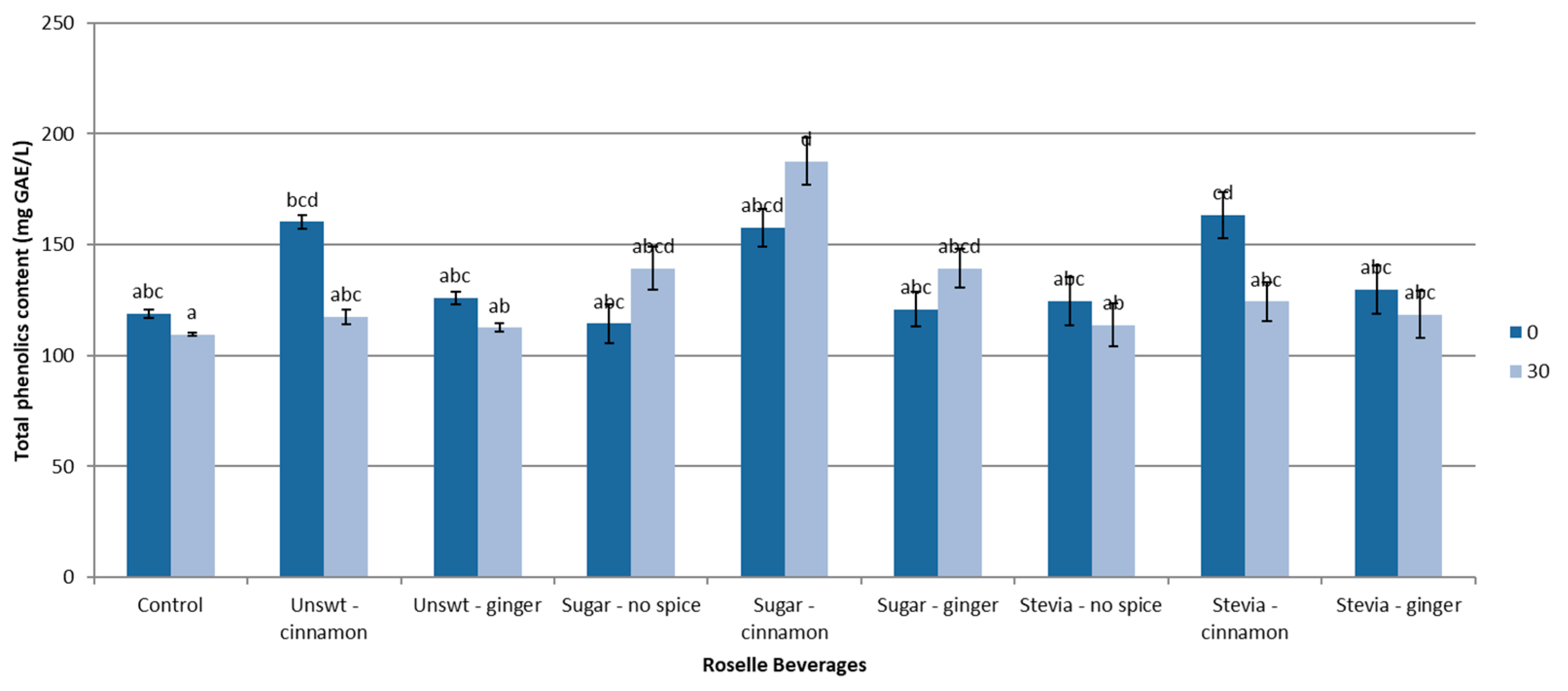
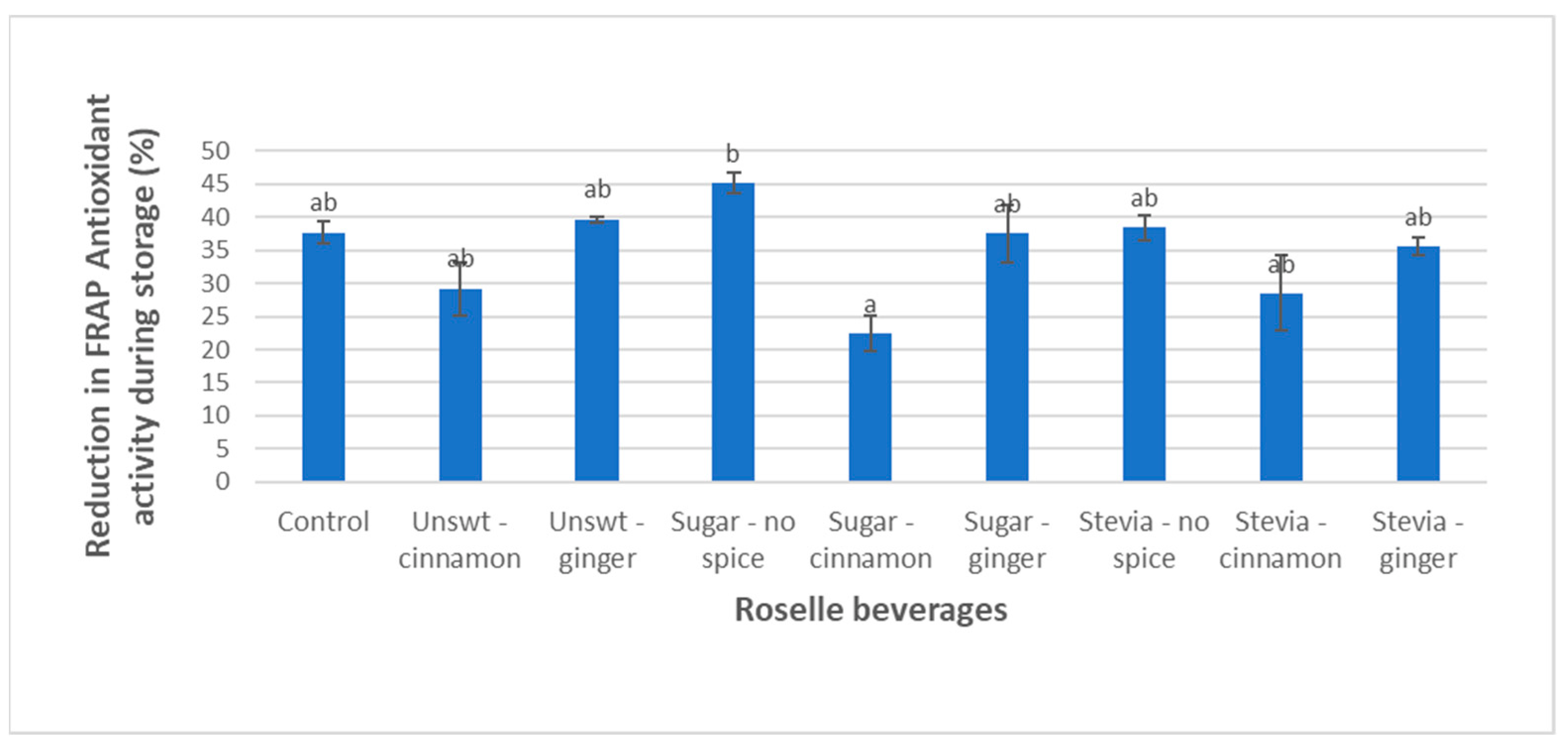
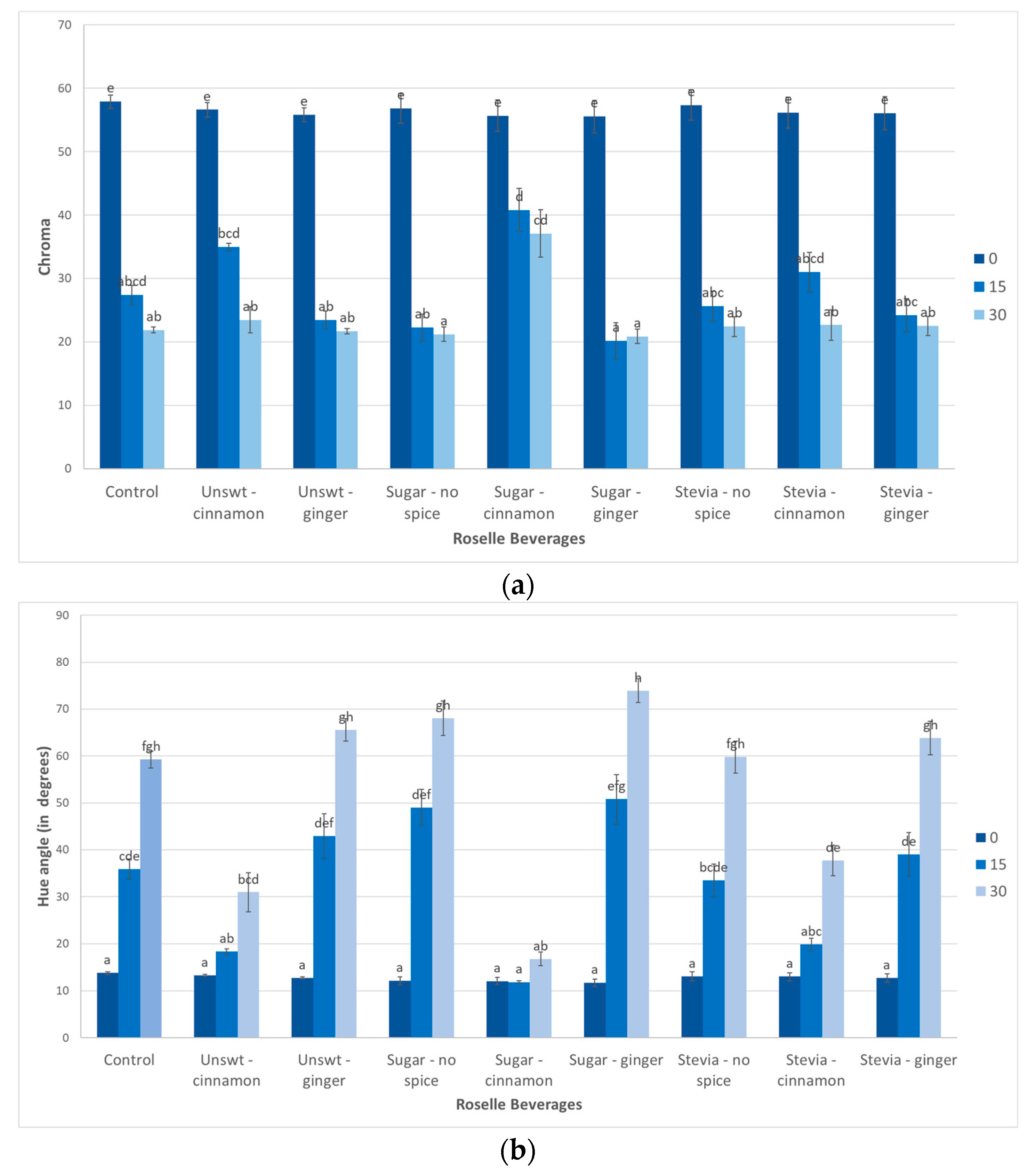
| Beverage | Rate Constant (Day−1) ± SD | Coefficient of Determination (R2) ± SD | Half-Life t1/2 (Days) ± SD |
|---|---|---|---|
| Control (unsweetened) | 0.0181 ± 0.0039 a | 0.9774 ± 0.0325 | 39 ± 7 a |
| Sugar-sweetened | 0.0240 ± 0.0048 a | 0.9830 ± 0.0226 | 30 ± 7 a |
| Stevia sweetened | 0.0208 ± 0.0025 a | 0.9908 ± 0.0043 | 34 ± 4 a |
| Sample | Initial Total Anthocyanin * (mg C3GE/L) | Rate Constant (Day−1) | Coefficient of Linear Correlation (R2) | Half-Life t1/2 (Days) |
|---|---|---|---|---|
| Control | 34 ± 4 a | 0.1022 | 0.9811 | 7 b |
| Unswt_cinnamon | 31 ± 4 a | 0.0532 | 0.9652 | 13 a |
| Unswt_ginger | 32 ± 4 a | 0.1095 | 0.9622 | 6 b |
| Sugar_no spice | 34 ± 8 a | 0.1295 | 0.9775 | 5 b |
| Sugar_cinnamon | 35 ± 9 a | 0.0334 | 0.8132 | 21 a |
| Sugar_ginger | 34 ± 8 a | 0.1479 | 0.9303 | 5 b |
| Stevia_no spice | 35 ± 9 a | 0.106 | 0.9773 | 7 b |
| Stevia_cinnamon | 33 ± 9 a | 0.0575 | 0.9153 | 12 a |
| Stevia_ginger | 33 ± 10 a | 0.1073 | 0.9373 | 6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omoarukhe, E.D.; Harbourne, N.; Jauregi, P. The Effect of Cinnamon and Ginger Spices on Anthocyanins in Sweetened Roselle Beverages. Beverages 2023, 9, 24. https://doi.org/10.3390/beverages9010024
Omoarukhe ED, Harbourne N, Jauregi P. The Effect of Cinnamon and Ginger Spices on Anthocyanins in Sweetened Roselle Beverages. Beverages. 2023; 9(1):24. https://doi.org/10.3390/beverages9010024
Chicago/Turabian StyleOmoarukhe, Esereosa D., Niamh Harbourne, and Paula Jauregi. 2023. "The Effect of Cinnamon and Ginger Spices on Anthocyanins in Sweetened Roselle Beverages" Beverages 9, no. 1: 24. https://doi.org/10.3390/beverages9010024
APA StyleOmoarukhe, E. D., Harbourne, N., & Jauregi, P. (2023). The Effect of Cinnamon and Ginger Spices on Anthocyanins in Sweetened Roselle Beverages. Beverages, 9(1), 24. https://doi.org/10.3390/beverages9010024






