Chemical Composition, Physical Properties, and Aroma Profile of Ethanol Macerates of Mistletoe (Viscum album)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mistletoe (V. album) Macerates
2.2. pH Assay, Total Acidity, and Total Extract
2.3. HS-SPME and GC/MS Conditions and Analysis
2.4. Calculation of Odor Activity Value (OAV)
2.5. Chromatic Characteristics
2.6. Statistical Analysis
3. Results
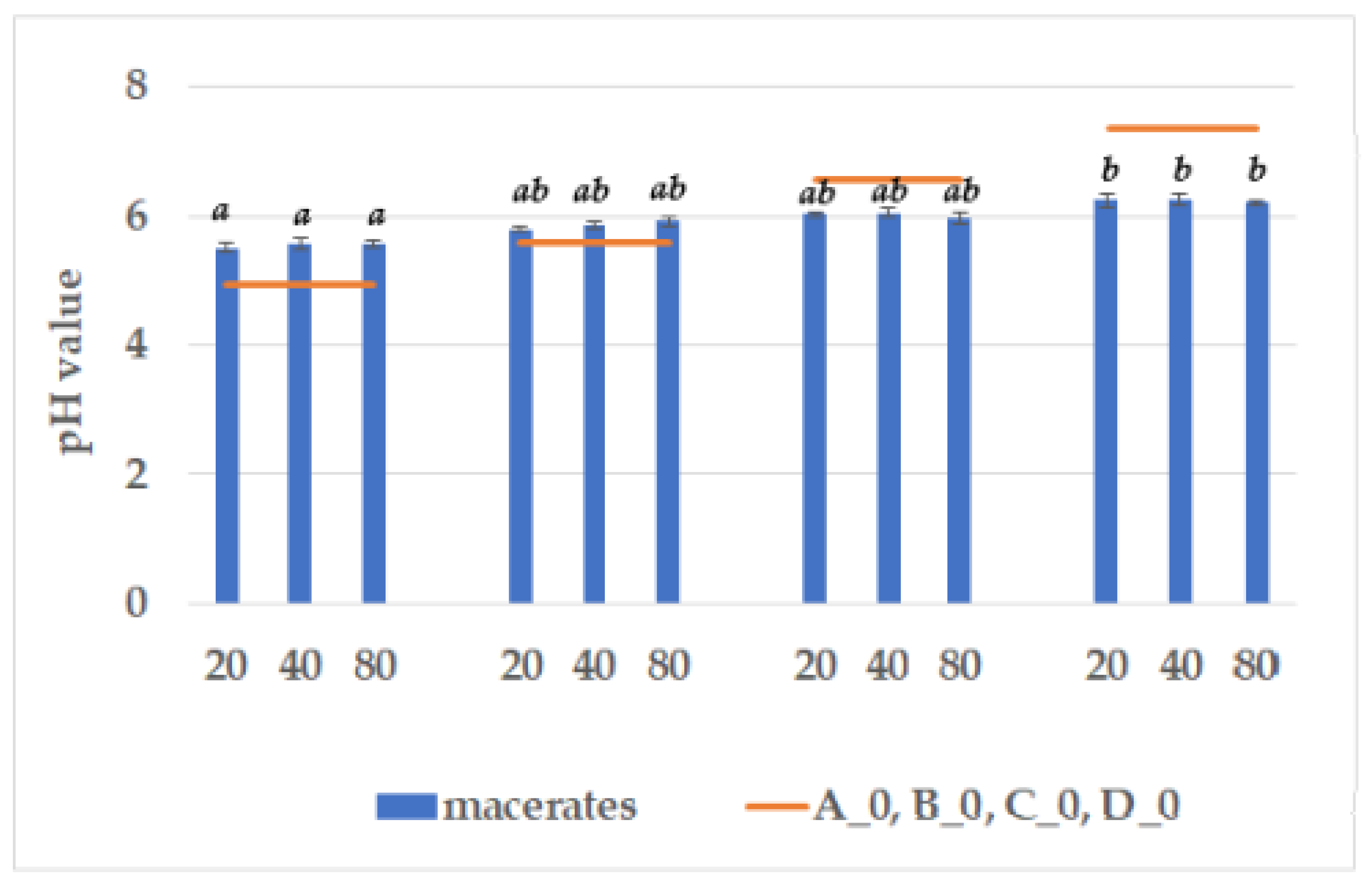
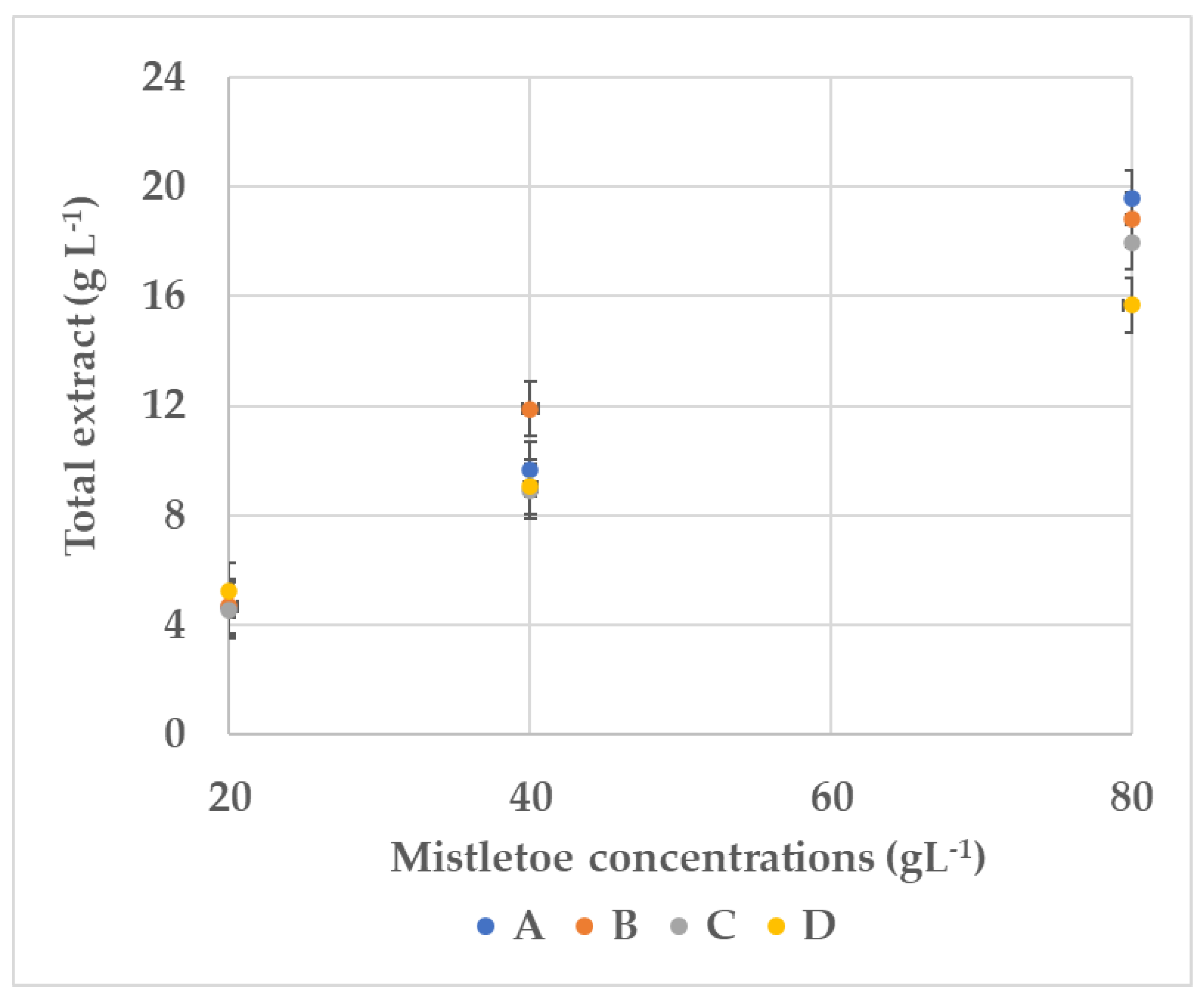
3.1. pH Assay, Total Extract, and Total Acidity
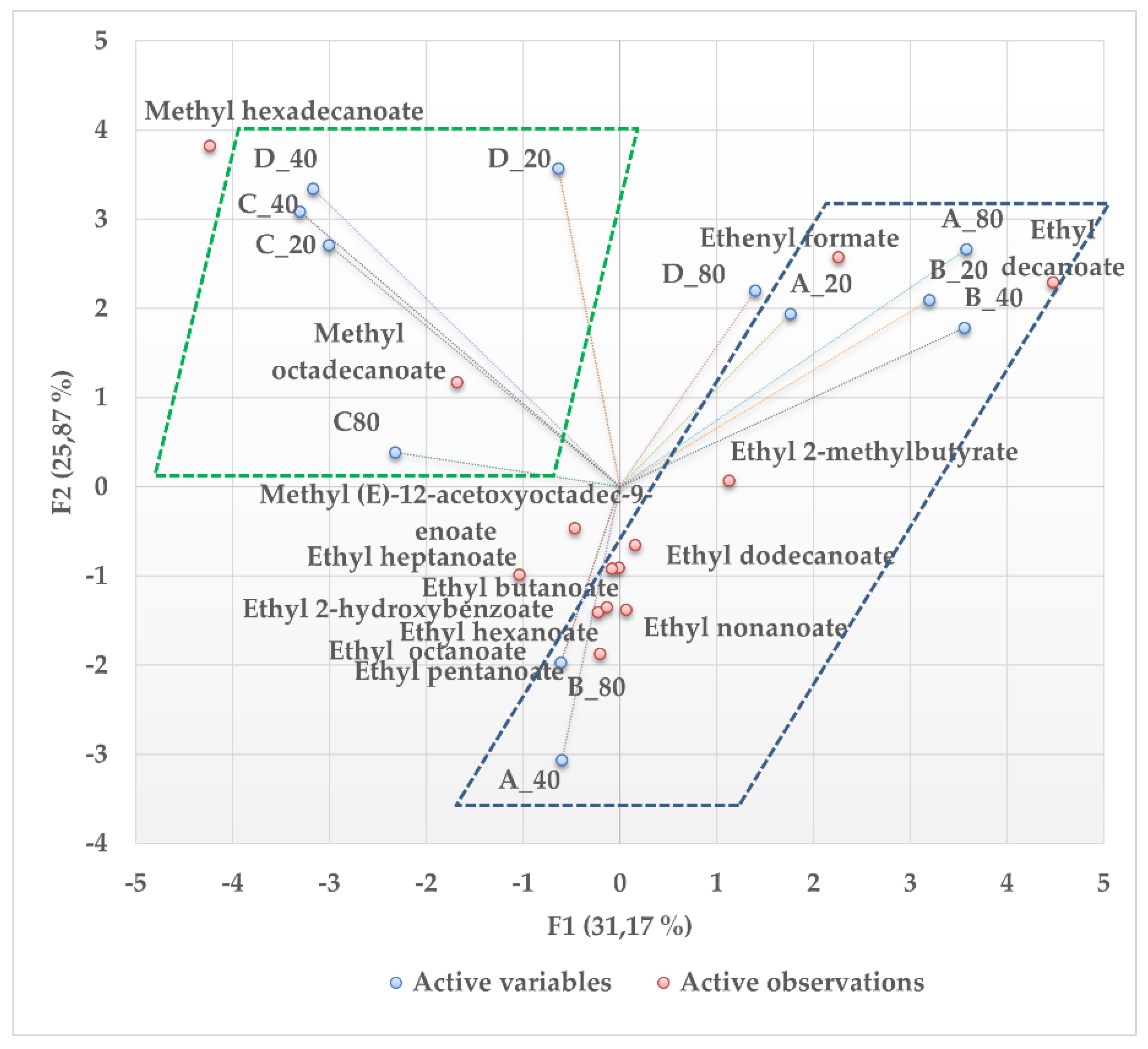
3.2. Macerate Volatile Compounds
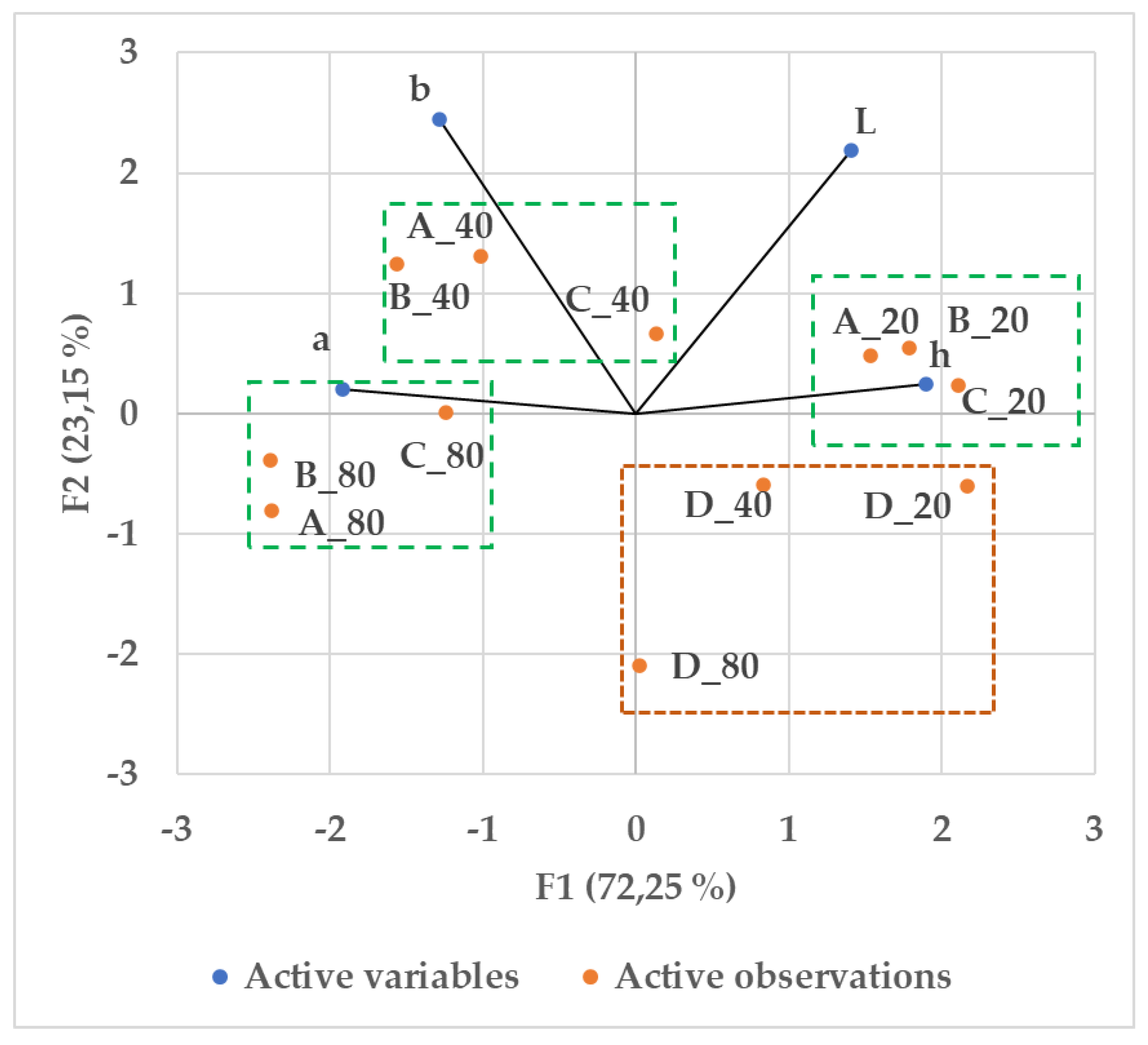
3.3. Macerate Color Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanousek Čiča, K.; Mrvčić, J.; Srečec, S.; Filipan, K.; Blažić, M.; Stanzer, D. Physicochemical and aromatic characterization of carob macerates produced by different maceration conditions. Food Sci. Nutr. 2020, 8, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Solana, R.; Carlier, J.D.; Costa, M.C.; Romano, A. Multi-element characterisation of carob, fig and almond liqueurs by MP-AES. J. Inst. Brew. 2018, 124, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Łuczaj, Ł.; Jug-Dujaković, M.; Dolina, K.; Vitasović-Kosić, I. Plants in alcoholic beverages on the Croatian islands, with special reference to rakija travarica. J. Ethnobiol. Ethnomed. 2019, 15, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrzak, W.; Nowak, R. Impact of Harvest Conditions and Host Tree Species on Chemical Composition and Antioxidant Activity of Extracts from Viscum album L. Molecules 2021, 26, 3741. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Orlikowski, P. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Prod. Res. 2016, 30, 373–385. [Google Scholar] [CrossRef]
- Yousefvand, S.; Fattahi, F.; Hosseini, S.M.; Urech, K.; Schaller, G. Viscotoxin and lectin content in foliage and fruit of Viscum album L. on the main host trees of Hyrcanian forests. Sci. Rep. 2022, 12, 10383. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Saha, C.; Galun, D.; Upreti, D.K.; Bayry, J.; Kaveri, S.V. European Viscum album: A potent phytotherapeutic agent with multifarious phytochemicals pharmacological properties and clinical evidence. RSC Adv. 2016, 6, 23837–23857. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, D.; Zhang, Q.; Qin, D.; Jiang, X.; Li, H.; Fang, K.; Cao, J.; Wu, H. Volatile components and nutritional qualities of Viscum articulatum Burm.f. parasitic on ancient tea trees. Food Sci. Nutr. 2019, 7, 3017–3029. [Google Scholar] [CrossRef] [Green Version]
- Ćebović, T.; Spasić, S.; Popović, M. Cytotoxic effects of the Viscum album L. extract on Ehrlich tumour cells in vivo. Phytother. Res. 2008, 22, 1097–1103. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Vázquez-Araújo, L.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Optimization of the process of aromatic and medicinal plant maceration in grape marc distillates to obtain herbal liqueurs and spirits. J. Sci. Food Agric. 2016, 96, 4760–4771. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, A.; Petrović, P.; Đorđević, V.; Zdunić, G.; Šavikin, K.; Bugarski, B. Polyphenols extraction from plant sources. Lek. Sirovine 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Caldeira, I.; Lopes, D.; Delgado, T.; Canas, S.; Anjos, O. Development of blueberry liquor: Influence of distillate, sweetener and fruit quantity. J. Sci. Food Agric. 2018, 98, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Solana, R.; Salgado, J.M.; Pérez-Santín, E.; Romano, A. Effect of carob variety and roasting on the antioxidant capacity, and the phenolic and furanic contents of carob liquors. J. Sci. Food Agric. 2019, 99, 2697–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, A.; Gouvinhas, I.; Machado, N.; Pinto, J.; Cunha, M.; Rosa, E.; Domínguez-Perles, R. New grape stems-based liqueur: Physicochemical and phytochemical evaluation. Food Chem. 2016, 190, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, H.R.; da Cruz de Assis, D.; Lafourcade Prada, A.; Carrera Silva, J.O.; de Sousa, M.B.; Ferreira, A.M.; Rodríguez Amado, J.R.; de Oliveira Carvalho, H.; Tavares de Lima Teixeira dos Santos, A.V.; Tavares Carvalho, J.C. Obtaining and characterization of anthocyanins from Euterpe oleracea (açaí) dry extract for nutraceutical and food preparations. Rev. Bras. Farmacogn. 2019, 29, 677–685. [Google Scholar] [CrossRef]
- Tian, T.T.; Sun, J.Y.; Wu, D.H.; Xiao, J.B.; Lu, J. Objective measures of greengage wine quality: From taste-active compound and aroma-active compound to sensory profiles. Food Chem. 2021, 340, 128179. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Spirituous Beverages of Vitivinicultural Origin. In Determination of Chromatic Characteristics; OIV-MA-BS-27; International Organisation of Vine and Wine: Paris, France, 2014. [Google Scholar]
- Senica, M.; Mikulic-Petkovsek, M. Changes in beneficial bioactive compounds in eight traditional herbal liqueurs during a one-month maceration process. J. Sci. Food Agric. 2019, 100, 343–353. [Google Scholar] [CrossRef]
- Soursouri, A.; Hosseini, S.M.; Fattahi, F. Biochemical analysis of European mistletoe (Viscum album L.) foliage and fruit settled on Persian ironwood (Parrotia persica C. A. Mey.) and hornbeam (Carpinus betulus L.). Biocatal. Agric. Biotechnol. 2019, 22, 101360. [Google Scholar] [CrossRef]
- Spaho, N. Distillation Techniques in the Fruit Spirits Production. In Distillation—Innovative Applications and Modeling; Mendes, M.F., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Burdock, G.A. Fenaroli’s Hand Book of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Gironés-Vilaplana, A.; Calín-Sánchez, Á.; Moreno, D.A.; Carbonell Barrachina, Á.A.; García-Viguera, C. Novel maqui liquor using traditional pacharán processing. Food Chem. 2015, 173, 1228–1235. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Rupert, M.; Koczoń, P.; Derewiaka, D.; Gajdoš-Kljusurić, J.; Petravić-Tominac, V.; Mrvčić, J.; Stanzer, D. Characterisation of flavour compounds in Biska—A herbal spirit produced with mistletoe. J. Inst. Brew. 2019, 125, 143–154. [Google Scholar] [CrossRef]
- Snoussi, A.; Hayet, B.H.K.; Essaidi, I.; Zgoulli, S.; Moncef, C.M.; Thonart, P.; Bouzouita, N. Improvement of the Composition of Tunisian Myrtle Berries (Myrtus communis L.) Alcohol Extracts. J. Agric. Food Chem. 2012, 60, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Escher, P.; Eiblmeier, M.; Hetzger, I.; Rennenberg, H. Seasonal and spatial variation of carbohydrate sin mistletoes (Viscum album) and the xylems apofits hosts (Populus x euamericana and Abies alba). Physiol. Plant. 2004, 120, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wei, X.-Y.; Qiu, Z.-D.; Gong, L.; Chen, Z.-Y.; Ma, Y.; Shen, Y.; Zhao, Y.-J.; Wang, W.-H.; Lai, C.-J.-S.; et al. Exploring the resources of the genus Viscum for potential therapeutic applications. J. Ethnopharmacol. 2021, 277, 114233. [Google Scholar] [CrossRef]
- Galvão, A.C.; Robazza, W.S.; Sarturi, G.N.; Goulart, F.C.; Conte, D. Sucrose Solubility in Binary Liquid Mixtures Formed by Water–Methanol, Water–Ethanol, and Methanol–Ethanol at 303 and 313. K. J. Chem. Eng. Data 2016, 61, 2997–3002. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Pucek, K.; Szymczak, G.; Kocjan, R.; Luchowski, P. Evaluation of seasonal changes of triterpenic acid contents in Viscum album from different host trees. Pharm. Biol. 2017, 55, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deliorman Orhan, D.; Orhan, I. Fatty acid composition of Viscum album subspecies from Turkey. Chem. Nat. Compd. 2006, 42, 641–644. [Google Scholar] [CrossRef]
- Hayashi, S.; Miyamoto, E.; Kudo, K.; Kameoka, H.; Hanafusa, M. Comparison of the volatile components of three mistletoes. J. Essent. Oil Res. 1996, 8, 619–626. [Google Scholar] [CrossRef]
- Miller, G.H. Whiskey Science, 1st ed.; Springer: Cham, Switzerland, 2019; pp. 421–468. [Google Scholar]
- Vlad, D.C.; Popescu, R.; Dumitrascu, V.; Cimporescu, A.; Vlad, C.S.; Vágvölgyi, C.; Krisch, J.; Dehelean, C.; Horhat, F.G. Phytocomponents identification in mistletoe (Viscum album) young leaves and branches, by GC-MS and antiproliferative effect on HEPG2 and McF7 cell lines. Farmacia 2016, 64, 82–87. [Google Scholar]
- Thamkaew, G.; Sjöholm, I.; Gómez Galindo, F. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]


| Group | Compounds Detected | Relative Percentages |
|---|---|---|
| Acid | Decanoic acid | 0.317 ± 0.01 |
| Alcohols | 1-Butanol, 3-methyl-, acetate | 0.359 ± 0.01 |
| Cyclohexanol, 1-methyl-4-(1-ethylethenyl)- | 1.152 ± 0.02 | |
| 1,8-Cineole | 1.587 ± 0.06 | |
| 1-Butanol, 3-methyl | 0.282 ± 0.02 | |
| 1-Hexanol | 0.317 ± 0.01 | |
| 3-Hexen-1-ol | 0.021 ± 0.00 | |
| 1-Octen-3-ol | 0.269 ± 0.00 | |
| Cyclooctanol, acetate | 0.372 ± 0.01 | |
| 2-Furanmethanol | 0.531 ± 0.00 | |
| Oxime-, methoxy-phenyl- | 3.187 ± 0.10 | |
| Benzenemethanol | 0.435 ± 0.01 | |
| 1,2,3-Propanetriol | 2.270 ± 0.09 | |
| Aldehydes | Acetaldehyde | 0.552 ± 0.01 |
| Hexanal | 1.607 ± 0.02 | |
| Octanal | 0.593 ± 0.02 | |
| Benzaldehyde | 2.208 ± 0.05 | |
| Esters | Formic acid, ethenyl ester | 9.928 ± 0.22 |
| Butanoic acid, ethyl ester | 1.621 ± 0.31 | |
| Butanoic acid, 2-methyl-, ethyl ester | 5.678 ± 0.05 | |
| Pentanoic acid, ethyl ester | 2.049 ± 0.03 | |
| Hexanoic acid, ethyl ester | 7.651 ± 0.21 | |
| Heptanoic acid, ethyl ester | 0.690 ± 0.03 | |
| Octanoic acid, ethyl ester | 4.070 ± 0.22 | |
| Nonanoic acid, ethyl ester | 3.036 ± 0.02 | |
| Decanoic acid, ethyl ester | 14.956 ± 0.30 | |
| Methyl Salicylate | 1.649 ± 0.02 | |
| Dodecanoic acid, ethyl ester | 1.725 ± 0.02 | |
| Hexadecanoic acid, methyl ester | 0.538 ± 0.00 | |
| Hexadecanoic acid, ethyl ester | 0.359 ± 0.02 | |
| Hydrocarbons | Undecane | 1.980 ± 0.04 |
| Ketones | 2,2-Dimethyl-3-heptanone | 0.338 ± 0.01 |
| Fenchone | 1.283 ± 0.05 | |
| alpha-Thujone | 3.615 ± 0.09 | |
| beta-Thujone | 2.532 ± 0.06 | |
| Cyclohexanone,5-methyl-2-(1-methylethyl)-, cis- | 4.250 ± 0.13 | |
| Theaspirane | 4.629 ± 0.12 | |
| 2,6,10,10-Tetramethyl-1-oxa-spiro [4.5]dec-6-ene | 1.877 ± 0.04 | |
| Others | l-Alanine ethylamide, (S)- | 0.973 ± 0.02 |
| Methane, tetranitro- | 1.690 ± 0.03 | |
| Butane, 1,1-diethoxy-3-methyl- | 5.775 ± 0.30 | |
| Benzene, 1-methyl-3-(1-methylethyl)- | 0.772 ± 0.02 | |
| Sum | 100.00 ± 2.80 | |
| Terpene | Odor Description | Concentration * (mg/L) | Threshold (mg/L) | OAV |
|---|---|---|---|---|
| β-pinene | turpentine | 0.56 | 0.752 | 0.745 |
| p-cymene | slightly woody, citrus, green pepper and oregano | 0.56 | 10 | 0.056 |
| l-limonene | lemon-like | 1.23 | 0.2 | 6.15 |
| m-cymene | hints of citrus, earth, and wood | 2.06 | 0.12 | 17.16 |
| trans-menthone | minty-refreshing | 6.50 | 0.476 | 13.65 |
| ylangene | pepper, spicy | 1.41 | - | - |
| β-bourbonene | herbal, woody, floral, balsamic | 1.58 | - | - |
| Camphor | warm, minty | 0.70 | 1 | 0.70 |
| α-humulene | woody | 0.84 | 0.39 | 2.15 |
| α-bourbonene | - | 7.50 | - | - |
| isomenthone | peppermint | 1.14 | 0.63 | 1.81 |
| α-bergamotene | pepper with woody and spicy undertones | 0.13 | - | - |
| trans-caryophyllene | sweet, woody, spice, clove | 0.11 | 0.15 | 0.73 |
| 1,8-cineole/eucalyptol | mint, eucalyptus, sweet, cooling, fresh, chemical pine | 0.201 | 0.064 | 3.14 |
| linalool | orange flowers, floral, rose, aniseed | 0.059 | 0.05 | 1.18 |
| borneol | pine, woody, camphor, balsamic | 0.134 | 0.08 | 1.675 |
| menthol | menthol | 0.29 | 1 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanousek Čiča, K.; Lukin, P.; Derewiaka, D.; Mrvčić, J.; Stanzer, D. Chemical Composition, Physical Properties, and Aroma Profile of Ethanol Macerates of Mistletoe (Viscum album). Beverages 2022, 8, 46. https://doi.org/10.3390/beverages8030046
Hanousek Čiča K, Lukin P, Derewiaka D, Mrvčić J, Stanzer D. Chemical Composition, Physical Properties, and Aroma Profile of Ethanol Macerates of Mistletoe (Viscum album). Beverages. 2022; 8(3):46. https://doi.org/10.3390/beverages8030046
Chicago/Turabian StyleHanousek Čiča, Karla, Priska Lukin, Dorota Derewiaka, Jasna Mrvčić, and Damir Stanzer. 2022. "Chemical Composition, Physical Properties, and Aroma Profile of Ethanol Macerates of Mistletoe (Viscum album)" Beverages 8, no. 3: 46. https://doi.org/10.3390/beverages8030046
APA StyleHanousek Čiča, K., Lukin, P., Derewiaka, D., Mrvčić, J., & Stanzer, D. (2022). Chemical Composition, Physical Properties, and Aroma Profile of Ethanol Macerates of Mistletoe (Viscum album). Beverages, 8(3), 46. https://doi.org/10.3390/beverages8030046







